Abstract
Purpose of Review
For 30 years, ultrasound has been investigated as a means to evaluate salivary gland abnormalities in patients with autoimmune disease. We aim to review the test characteristics of ultrasound for diagnosing Sjögren’s syndrome, the scoring systems used for this purpose, and the ultrasound similarities and differences between Sjögren’s syndrome and some of its potential salivary gland mimics.
Recent Findings
Hypo/anechoic glandular lesions are the major ultrasound characteristic found in Sjögren’s syndrome. Most studies have reported such ultrasound abnormalities to have a sensitivity and specificity in the range of 65–85% and 85–95%, respectively, as well as a positive likelihood ratio between 4 and 12. However, similar findings can also be seen in sarcoidosis, amyloidosis, IgG4-related disease, HIV, and lymphoma. A “nodal” pattern of involvement or the ultrasound artifact of “through transmission” can help distinguish some of these mimics from Sjogren’s syndrome.
Summary
Ultrasound can substantially influence the diagnosis of Sjögren’s syndrome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sjögren’s syndrome is a systemic autoimmune condition that typically damages exocrine glands with resulting symptoms of dryness in the mouth, eyes, and other body regions. In about a third of affected individuals, Sjögren’s also affects extra-exocrine tissues causing arthritis, Raynaud’s, nerve damage, renal tubular acidosis, interstitial lung disease, vasculitis, as well as other possible manifestations [1]. Diagnosing this condition can be challenging since it shares many of its features with other autoimmune diseases on one hand, and can itself be a feature of other autoimmune diseases—so called secondary Sjögren’s syndrome. Furthermore, dryness of the eyes and mouth, also called sicca syndrome, can be caused by many conditions other than Sjögren’s syndrome. The research classification of Sjögren’s syndrome has passed through many iterations but typically depends on a combination of symptoms of dryness in the eyes and mouth, clinical exam features objectively confirming exocrine dryness, auto-antibody test abnormalities such as anti-SSA/SSB, and salivary gland biopsy features of focal lymphocytic sialadenitis [2]. However, the auto-antibody tests miss 30% of patients with Sjögren’s [3], while the biopsy of a minor salivary gland can be as sensitive as 82% [4], but is invasive.
Due to the challenges in diagnosing Sjögren’s syndrome, ultrasonography has been extensively investigated as a diagnostic tool since 1988 [5]. The most characteristic ultrasound feature of salivary glands affected by Sjögren’s syndrome is a diffuse cyst-like heterogeneity with involvement of both parotid and submandibular glands. Glands can be evaluated sonographically for homogeneity, echogenicity, hypoechoic areas, hyperechoic areas, and border clarity. Based on these features, numerous ultrasound scoring systems have been developed, and systematic reviews have estimated their testing characteristics. Recently, ultrasound scoring has been proposed as part of the Sjögren’s classification criteria [6•]. Doppler and elastography are recent additions which may have a role in salivary gland investigation. We will review the state of the ultrasound features and scoring systems for salivary glands as well as the potential mimics of Sjögren’s syndrome and whether ultrasound can help distinguish these conditions.
Sonographic Features of Salivary Glands in Sjögren’s Syndrome
The most characteristic feature of Sjögren’s syndrome in the salivary gland on ultrasound are hypoechoic or anechoic lesions producing tissue inhomogeneity [7] (Fig. 1). The cause of these changes has been proposed to be either foci of lymphocytic infiltrates or due to ductal dilatation. Ductal dilation is a typical histologic finding in chronic obstructive submandibular sialadenitis, but not in Sjögren’s syndrome [8•], where as lymphoplasmacytic infiltrate and acinar atrophy are typical.
Ultrasound images (in gray scale) of submandibular glands from different patients. Panel (a) shows a normal submandibular gland. Panel (b) shows a submandibular gland from patient with Sjögren’s syndrome. Small arrowheads point to small hypoechoic lesions with hazy margins which represent plasmalymphocytic infiltrates. Panel (c) shows a submandibular gland from a patient with IgG4-related disease. Note the larger “nodal” pattern producing a bulging on the surface of the gland (larger arrowhead). Panel (d) is a gland from a patient with HIV. The cystic lesions are significantly larger, with septation (arrow) and producing through transmission (asterisk)
Similarly, Hashimoto’s thyroiditis results in focal deposits throughout the thyroid parenchyma or within germinal centers producing a “giraffe pattern” on ultrasound [9]. Since the salivary gland echogenicity and homogeneity is graded in reference to the thyroid tissue, it is also important to ensure that the thyroid tissue is in fact normal as Hashimoto’s thyroiditis is a co-morbid condition with Sjögren’s syndrome in a third of cases [10].
Investigators have found that the salivary gland ultrasound score (SGUS) correlates with the focus score [11] in minor salivary gland biopsies, as well as in the parotid [12]. In contrast, there are no reports of ductal dilatation on gland biopsy of Sjögren’s syndrome. Furthermore, some research studies describe improvement in parotid echostructure and a trend toward improvement in submandibular echostructure after rituximab therapy [13]. If cystic changes were due to ductal dilation, there would be less reason to expect the lesions to improve with B cell depletion than if the lesions were due to lymphocytic infiltration.
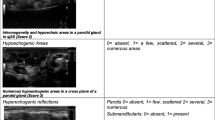
Scoring Systems
The hypo/anechoic glandular lesions tend to have indistinct borders, no through transmission, to be spread diffusely through all glandular areas, and to be small, typically less than 6 mm. Scoring systems of Salafii [14] and Jousse-Joulin [15] both utilize lesion size as part of their scoring system with measurable lesions of < 2 mm resulting in a grade of 2, lesions from 2 to 6 mm resulting in grade 3, and those > 6 mm resulting in the highest grade of 4. Presence of echogenic bands (thought to represent fibrotic septa) tissue calcifications, and posterior gland border visibility also increase the ultrasound score (0–16 total score). The scoring system of Milic [16] is the most simplified, grading the glands from 0 to 3 based on degree of homogeneity only (0–12 total score). While the scoring system on Hočevar [17] is the most complicated, grading on parenchymal echogenicity, homogeneity, hypoechoic areas (without specific measurements), hyperechoic reflections, and glandular border clarity (0–48 total score). These comprise the most commonly used scoring systems, although many more have been described. These four scoring systems were compared in the same cohort of patients with primary Sjögren’s, secondary Sjögren’s, and non-autoimmune sicca controls resulting in similar ranges of sensitivity and specificity with areas under the curve of 0.915 (Salaffi), 0.897 (Joussee-Joulin), 0.891 (Hočevar), and 0.885 (Milic) for primary Sjögren’s and 0.851 (Joussee-Joulin), 0.844 (Salaffi), 0.824 (Hočevar), and 0.808 (Milic) for secondary Sjögren’s [18•].
Doppler
Sjögren’s syndrome salivary glands show significantly more vascularity than healthy controls. Abnormal vascularity has been found to correlate with minor salivary gland histopathological grades, and Doppler grading improved the sensitivity, specificity, and accuracy of SGUS from 44%, 97%, and 65%, to 63%, 90%, and 74%, respectively [19]. However, increased Doppler has been described in many other pathologic processes that affect the salivary glands [20], and some have shown that Doppler does not discriminate between various pathological salivary gland lesions [21]. Doppler signal may also vary with disease duration, higher than normal signal in early active disease and lower than normal in late “burned out” disease [22].
Elastography
One of the newest ultrasonographic measures applied to salivary glands is elastography, which can measure the stiffness of a structure. In fact, glandular stiffness [23] in patients with Sjögren’s syndrome was determined to be significantly higher than in patients with non-autoimmune sicca. Furthermore, in cases where gray scale ultrasound was inconclusive in distinguishing patients with primary Sjögren’s syndrome from sicca controls, elastographic measurements resulted in a sensitivity of 67% and specificity of 86% for distinguishing the groups [24].
Gray-Scale Ultrasound Sensitivity and Specificity for Sjögren’s Syndrome
Through 2019, 47 studies have reported ultrasound test characteristics for salivary glands in Sjögren’s syndrome (Fig. 2). These studies have varied in many respects, including the gold standard for comparison, with most using one of the validated classification criteria and some using a biopsy result. In reference to classification criteria, parotid gland biopsy has been found to have a sensitivity of 75% and specificity of 88%, almost identical to that for labial salivary gland biopsy with sensitivity of 72% and specificity of 85%. The difference in gold standard may not matter much as optimal Hočevar ultrasound score agreed equally with parotid biopsy and classification criteria [12].
Sensitivity (a), specificity (b), and positive likelihood ratio (c) estimates for salivary gland ultrasound assessment of Sjögren’s syndrome. Circles represent point estimates reported by 47 studies on this topic published through 2019 ordered by study size, with circle areas representing relative study size
However, these results do not mean that ultrasound can reasonably substitute for salivary gland biopsy. In 22 patients with sicca and US score < 15 as well as negative anti-SSA, five (23%) still had positive labial salivary gland biopsy, and five patients (11%) fulfilled the AECG criteria [12]. Thus, a negative US, even if the serology is also negative, will miss ACR-EULAR criteria positive Sjögren’s patient 11% of the time. However, a combination of a positive serology with a positive ultrasound predicts the fulfillment of the ACR-EULAR classification criteria 97% of the time. Van Nimwegan et al. [6•] have showed that the validity of the ACR-EULAR criteria remains high after incorporation of SGUS, but that substitution of SGUS score for either minor salivary gland biopsy, parotid gland biopsy, or anti-SSA antibody testing hinders test accuracy mainly through effects on sensitivity. Despite this caveat, Cornec et al. [4] found that inclusion of SGUS in the ACR/EULAR criteria improves its sensitivity from 64.4% to 84.4%, without changing its specificity (89.3% vs. 91.0%).
The most recent meta-analysis by Carottii et al. in 2019 assessed 37 studies and found a pooled specificity 91% (CI 88–93) and a pooled sensitivity 83% (CI 78–87) [25•]. Other recent meta-analyses found similar results without significant difference in sensitivity or specificity based on scoring system used (75% sensitivity for 0–4 and 0–48 point systems vs. 84% for 0–16 point system, while specificity was 93% for 0–4, 88% for 0–16, and 95% for 0–48) [26]. However, differences in study populations and control groups as well as publication bias [27] may falsely increase the ultrasound test performance characteristics. Figure 2 demonstrates that positive likelihood ratio for salivary gland ultrasound testing ranges from 4 to 12 in the five largest studies to date, and thus, a positive salivary gland ultrasound substantially increases the probability of a patient having Sjögren’s syndrome.
Reliability
Inter-rater reliability for salivary gland scoring has generally been good to excellent, with Hočevar reporting an overall kappa of 0.9, with kappa in the 0.88 to 0.9 range for echogenicity, inhomogeneity, and presence of hypoechoic areas and lower kappa of 0.5–0.52 for gland boarders and hyperechoic foci [28]. Since then, a number of other investigators have also reported an overall salivary gland ultrasound score kappa in the 0.8 to 0.95 range [29, 30]. A more recent study from 2018 [31] also showed an overall inter-rater kappa in the 0.7–0.84 range, confirming that glandular homogeneity and hypoechoic areas were much more reliable than assessments of glandular border, hyperechoic areas, or echogenicity. The number of hypoechoic/anechoic areas had inter-observer reliability of 0.53 in the submandibular gland and 0.74 in the parotid. Abnormal lymph nodes, hyperechoic bands, calcifications, and posterior border visibility showed low inter observer reliability (kappa = 0.38–0.01) [32].
Discriminating Sjögren’s from Other Diseases of the Salivary Glands
ACR-EULAR classification criteria specifically exclude patients with conditions that can be confused with Sjögren’s, namely AIDS, Hepatitis C, amyloidosis, sarcoidosis, IgG4-related disease, graft-versus-host disease, history of head and neck radiation treatment, etc. Thus, it is of particular interest whether ultrasound assessment can differentiate Sjögren’s from these conditions as well as from other autoimmune conditions that affect the salivary glands.
A study comparing salivary gland ultrasound findings in patients with systemic sclerosis in comparison to primary Sjögren’s and healthy controls found abnormal ultrasound scores in 75% of 48 patients with Sjogren’s, 28% of 25 patients with systemic sclerosis, and 9% of 35 healthy controls [33]. The abnormalities were not different in patients with systemic sclerosis than in Sjogren’s; thus, the authors conclude that ultrasound can detect Sjögren’s overlap with systemic sclerosis, rather than a salivary gland fibrotic disease specific to systemic sclerosis. A similar study compared Sjögren’s with other connective tissue diseases including SLE, systemic sclerosis, mixed connective tissue disease, and undifferentiated connective tissue disease and found an ultrasound score ≥ 2 in 78% of the Sjögren’s patients compared to 28% of the connective tissue disease patients. While the Sjögren’s patients had a similar degree of involvement of parotid and submandibular glands (62% and 64%, respectively), the non-Sjögren’s connective tissue disease cohort had a higher proportion of submandibular gland involvement than parotid involvement (28% and 14%, respectively) [34].
Sarcoidosis and amyloidosis are infiltrative diseases that have been reported to affect the salivary glands. However, there have been few descriptions of the ultrasound appearance of these diseases. A recent study [21] comparing cohorts of patients with Sjögren’s, AL amyloidosis, sarcoidosis, and healthy controls did not detect a “nodal” pattern of involvement between the groups as there is between Sjögren’s and IGG4. The overall median Hočevar US score was higher in Sjögren’s than in the other groups, and both the amyloid and sarcoid groups had higher median scores than the healthy control group. Notably, 27% of AL amyloidosis and 19% of sarcoidosis groups scored above the ultrasound score previously described as being specific for Sjögren’s syndrome. Despite prior studies suggesting a greater degree of parotid than submandibular gland involvement in sarcoidosis, this study did not confirm such a pattern.
Exclusion of underlying malignancy drives some salivary gland biopsies in patients with Sjögren’s, and ultrasound can increase suspicion of underlying MALT lymphoma. Sjögren’s patients with MALT lymphoma had average US scores almost twice as high as Sjögren’s patients without MALT lymphoma or at high risk factors for MALT lymphoma [35]. In a case series of MALT lymphomas of the head and neck, 7 of 15 cases affected the salivary glands while 8 affected the thyroid gland [36]. Of the 7 salivary gland cases, all but one affected the parotid glands. The typical sonographic pattern was that of either “linear echogenic strands pattern” also referred to as “multiple small hypoechoic nodules” or “tortoiseshell pattern”, or “segmental pattern”/ “multiple larger hypoechoic masses”. These patterns may resemble IgG4-related disease, or advanced Sjögren’s. The authors also describe diffuse large B cell lymphoma in 12 cases, where the glands are typically diffusely hypoechoic and have associated lymph node abnormalities.
Similar to the ultrasound results for MALT lymphoma, IgG4-related disease also produced higher salivary gland ultrasound scores on the Hočevar 0–48 point system than matched Sjögren’s patients (26 for IgG4-related disease compared to 21.5 for Sjögren’s group). The difference was accounted for by higher scores in the submandibular glands (18 vs 11), while in the parotid glands, the scores were essentially the same. They also found a correlation between serum IgG4 levels and SGUS in the IgG4-related disease patients (r = 0.331, p < 0.05) [37]. In a separate study of 30 patients with IgG4-related disease compared to 38 with Sjögren’s and 36 healthy controls, a reticular pattern was found in both IgG4 and Sjögren’s, but a “nodal” pattern was found in the submandibular glands in IgG4 much more commonly than Sjögren’s or controls (Fig. 1). Unfortunately, the authors did not present data on nodal pattern specificity in distinguishing the two conditions [38]. The “nodal” pattern was defined in another article as hypoechoic, homogenous areas with relatively high vascularization, and bulging from the surface of the submandibular glands [39]. This article also found submandibular gland “nodal” regions in 8 of 9 patients with IgG4-related disease, but in none of the parotid glands. Similarly, other authors described a “nodal” pattern in 10/15 cases [40], 31/42 cases [41], and 25/30 cases [42]. There are three retrospective, case-control trials, and four case series of ultrasound use for IgG4-related disease of the salivary glands which comprise a total of 160 patients. In 108 cases where submandibular glands involvement was assessed specifically, there were ultrasound abnormalities in 99 (92%) [20, 38, 40,41,42,43,44]. This is in distinction to the parotid glands where 20 out of 60 (33%) were affected. Submandibular glands are also typically longer and thicker in IgG4-related disease than in controls and tend to have rough, irregular contour [20].
Unlike IgG4-related disease, which tends to target submandibular glands more than parotid glands, parotid involvement in HIV has been reported to occur in 6–10% of cases and increases to 51% in AIDS [45,46,47]. Of 200 patients in Uganda with HIV presenting for hospital care, 195 had parotid abnormalities by ultrasound. Forty two percent of the patients had lymphoepithelial cysts, 20% had fatty aggregates defined as whole gland hypoechoic appearance with posterior attenuation, while another 20% had lymphocytic aggregates, and 16% had lymphadenopathy alone [48]. As previously noted, lymphocytic aggregates have a size usually less than 6 mm, ill-defined margins, and lack posterior acoustic enhancement, while lymphoepithelial cysts are the opposite in these three respects and tend to also have internal septations (Fig. 1). Unfortunately, the degree of submandibular involvement was not described, but others have noted that the submandibular glands are usually spared [49].
Conclusions
Ultrasound detects small hypo/anechoic lesions spread throughout the major salivary glands. These findings strongly correlate with histology findings and are sensitive and specific for discriminating Sjögren’s from sicca symptoms due to medication or age-related causes. Salivary gland ultrasound findings may increase diagnostic certainty when other items in the ACR-EULAR classification criteria are equivocal, but similar findings can be encountered in other conditions that can affect the salivary glands such as IgG4-related disease, lymphoma, sarcoidosis, amyloidosis, and HIV. Detection of a nodal pattern of involvement or whole gland hypoechogenicity with gland surface bulging suggests IgG4-related disease in the submandibular glands and lymphoma or HIV in the parotid glands, while large cystic lesions in the parotid glands would be most typical of HIV. Ultrasound also holds a tantalizing opportunity for identification of both early gland involvement via Doppler imaging and detection of ultrastructural glandular healing in response to therapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Ramos-Casals M, Brito-Zeron P, Seror R, Bootsma H, Bowman SJ, Dorner T, et al. Characterization of systemic disease in primary Sjogren's syndrome: EULAR-SS task force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford). 2015;54(12):2230–8. https://doi.org/10.1093/rheumatology/kev200.
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69(1):35–45. https://doi.org/10.1002/art.39859.
Garberg H, Jonsson R, Brokstad KA. The serological pattern of autoantibodies to the Ro52, Ro60, and La48 autoantigens in primary Sjogren's syndrome patients and healthy controls. Scand J Rheumatol. 2005;34(1):49–55. https://doi.org/10.1080/03009740510017940.
Cornec D, Jousse-Joulin S, Marhadour T, Pers JO, Boisrame-Gastrin S, Renaudineau Y, et al. Salivary gland ultrasonography improves the diagnostic performance of the 2012 American College of Rheumatology classification criteria for Sjogren's syndrome. Rheumatology (Oxford). 2014;53(9):1604–7. https://doi.org/10.1093/rheumatology/keu037.
de Clerck LS, Corthouts R, Francx L, Brussaard C, de Schepper A, Vercruysse HA, et al. Ultrasonography and computer tomography of the salivary glands in the evaluation of Sjogren's syndrome. Comparison with parotid sialography. J Rheumatol. 1988;15(12):1777–81.
• van Nimwegen JF, Mossel E, Delli K, van Ginkel MS, Stel AJ, FGM K, et al. Incorporation of salivary gland ultrasonography into the ACR-EULAR criteria for primary Sjogren's syndrome. Arthritis Care Res. 2019. https://doi.org/10.1002/acr.24017The most comprehensive assessment of integrating salivary gland ultraasound into the ACR-EULAR classification criteria for primary Sjogren's syndrome.
De Vita S, Lorenzon G, Rossi G, Sabella M, Fossaluzza V. Salivary gland echography in primary and secondary Sjogren's syndrome. Clin Exp Rheumatol. 1992;10(4):351–6.
• Hong X, Li W, Xie XY, Zhang ZY, Chen Y, Gao Y, et al. Differential diagnosis of IgG4-related sialadenitis, primary Sjogren’s syndrome, and chronic obstructive submandibular sialadenitis. Br J Oral Maxillofac Surg. 2017;55(2):179–84. https://doi.org/10.1016/j.bjoms.2016.10.021This study describes differences between IGG4 disease and Sjogren's syndrome on salivary gland ultrasound.
Bonavita JA, Mayo J, Babb J, Bennett G, Oweity T, Macari M, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193(1):207–13. https://doi.org/10.2214/AJR.08.1820.
Jara LJ, Navarro C, Brito-Zeron Mdel P, Garcia-Carrasco M, Escarcega RO, Ramos-Casals M. Thyroid disease in Sjogren's syndrome. Clin Rheumatol. 2007;26(10):1601–6. https://doi.org/10.1007/s10067-007-0638-6.
Baldini C, Luciano N, Tarantini G, Pascale R, Sernissi F, Mosca M, et al. Salivary gland ultrasonography: a highly specific tool for the early diagnosis of primary Sjogren's syndrome. Arthritis Res Ther. 2015;17:146. https://doi.org/10.1186/s13075-015-0657-7.
Mossel E, Delli K, van Nimwegen JF, Stel AJ, Kroese FGM, Spijkervet FKL, et al. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjogren's syndrome. Ann Rheum Dis. 2017;76(11):1883–9. https://doi.org/10.1136/annrheumdis-2017-211250.
Jousse-Joulin S, Devauchelle-Pensec V, Cornec D, Marhadour T, Bressollette L, Gestin S, et al. Brief report: ultrasonographic assessment of salivary gland response to rituximab in primary Sjogren's syndrome. Arthritis Rheumatol. 2015;67(6):1623–8. https://doi.org/10.1002/art.39088.
Salaffi F, Argalia G, Carotti M, Giannini FB, Palombi C. Salivary gland ultrasonography in the evaluation of primary Sjogren's syndrome. Comparison with minor salivary gland biopsy. J Rheumatol. 2000;27(5):1229–36.
Cornec D, Jousse-Joulin S, Pers JO, Marhadour T, Cochener B, Boisrame-Gastrin S, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjogren's syndrome: toward new diagnostic criteria? Arthritis Rheumatol. 2013;65(1):216–25. https://doi.org/10.1002/art.37698.
Milic VD, Petrovic RR, Boricic IV, Radunovic GL, Pejnovic NN, Soldatovic I, et al. Major salivary gland sonography in Sjogren's syndrome: diagnostic value of a novel ultrasonography score (0-12) for parenchymal inhomogeneity. Scand J Rheumatol. 2010;39(2):160–6. https://doi.org/10.3109/03009740903270623.
Hocevar A, Ambrozic A, Rozman B, Kveder T, Tomsic M. Ultrasonographic changes of major salivary glands in primary Sjogren's syndrome. Diagnostic value of a novel scoring system. Rheumatology (Oxford). 2005;44(6):768–72. https://doi.org/10.1093/rheumatology/keh588.
• Martel A, Coiffier G, Bleuzen A, Goasguen J, de Bandt M, Deligny C, et al. What is the best salivary gland ultrasonography scoring methods for the diagnosis of primary or secondary Sjogren's syndromes? Joint Bone Spine. 2019;86(2):211–7. https://doi.org/10.1016/j.jbspin.2018.06.014This study compares the test charecteristiscs of various salivary gland ultrasound scoring systems in the same patient cohort.
Shimizu M, Okamura K, Yoshiura K, Ohyama Y, Nakamura S. Sonographic diagnosis of Sjogren syndrome: evaluation of parotid gland vascularity as a diagnostic tool. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(4):587–94. https://doi.org/10.1016/j.tripleo.2007.11.007.
Omotehara S, Nishida M, Satoh M, Inoue M, Kudoh Y, Horie T, et al. Sonographic findings of immunoglobulin G4-related sclerosing sialadenitis. J Med Ultrason (2001). 2016;43(2):257–62. https://doi.org/10.1007/s10396-015-0693-6.
Law ST, Jafarzadeh SR, Govender P, Sun X, Sanchorawala V, Kissin EY. Comparison of ultrasound features of major salivary glands in Sarcoidosis, amyloidosis, and Sjogren's syndrome. Arthritis Care Res. 2019. https://doi.org/10.1002/acr.24029.
Lee KA, Lee SH, Kim HR. Diagnostic and predictive evaluation using salivary gland ultrasonography in primary Sjogren's syndrome. Clin Exp Rheumatol. 2018;36 Suppl 112(3):165–72.
Hofauer B, Mansour N, Heiser C, Gahleitner C, Thuermel K, Bas M, et al. Sonoelastographic modalities in the evaluation of salivary gland characteristics in Sjogren's syndrome. Ultrasound Med Biol. 2016;42(9):2130–9. https://doi.org/10.1016/j.ultrasmedbio.2016.04.011.
Dejaco C, De Zordo T, Heber D, Hartung W, Lipp R, Lutfi A, et al. Real-time sonoelastography of salivary glands for diagnosis and functional assessment of primary Sjogren's syndrome. Ultrasound Med Biol. 2014;40(12):2759–67. https://doi.org/10.1016/j.ultrasmedbio.2014.06.023.
• Carotti M, Salaffi F, Di Carlo M, Barile A, Giovagnoni A. Diagnostic value of major salivary gland ultrasonography in primary Sjogren's syndrome: the role of grey-scale and colour/power Doppler sonography. Gland Surg. 2019;8(Suppl 3):S159–S67. https://doi.org/10.21037/gs.2019.05.03The most recent meta-analysis of salivary gland ultrasound for Sjogren's syndrome.
Zhou M, Song S, Wu S, Duan T, Chen L, Ye J, et al. Diagnostic accuracy of salivary gland ultrasonography with different scoring systems in Sjogren's syndrome: a systematic review and meta-analysis. Sci Rep. 2018;8(1):17128. https://doi.org/10.1038/s41598-018-35288-5.
Delli K, Dijkstra PU, Stel AJ, Bootsma H, Vissink A, Spijkervet FK. Diagnostic properties of ultrasound of major salivary glands in Sjogren's syndrome: a meta-analysis. Oral Dis. 2015;21(6):792–800. https://doi.org/10.1111/odi.12349.
Hocevar A, Rainer S, Rozman B, Zor P, Tomsic M. Ultrasonographic changes of major salivary glands in primary Sjogren's syndrome. Evaluation of a novel scoring system. Eur J Radiol. 2007;63(3):379–83. https://doi.org/10.1016/j.ejrad.2007.02.003.
Takagi Y, Kimura Y, Nakamura H, Sasaki M, Eguchi K, Nakamura T. Salivary gland ultrasonography: can it be an alternative to sialography as an imaging modality for Sjogren's syndrome? Ann Rheum Dis. 2010;69(7):1321–4. https://doi.org/10.1136/ard.2009.123836.
Wernicke D, Hess H, Gromnica-Ihle E, Krause A, Schmidt WA. Ultrasonography of salivary glands -- a highly specific imaging procedure for diagnosis of Sjogren's syndrome. J Rheumatol. 2008;35(2):285–93.
Delli K, Arends S, van Nimwegen JF, Dijkstra PU, Stel AJ, Spijkervet FKL, et al. Ultrasound of the major salivary glands is a reliable imaging technique in patients with clinically suspected primary Sjogren's syndrome. Ultraschall Med. 2018;39(3):328–33. https://doi.org/10.1055/s-0043-104631.
Jousse-Joulin S, Nowak E, Cornec D, Brown J, Carr A, Carotti M, et al. Salivary gland ultrasound abnormalities in primary Sjogren's syndrome: consensual US-SG core items definition and reliability. RMD Open. 2017;3(1):e000364. https://doi.org/10.1136/rmdopen-2016-000364.
Couderc M, Tournadre A, Mathieu S, Pereira B, Soubrier M, Dubost JJ. Do the salivary glands of patients with systemic sclerosis show ultrasonographic modifications suggestive of Sjogren's syndrome? Ann Rheum Dis. 2019:annrheumdis-2019-215777. https://doi.org/10.1136/annrheumdis-2019-215777.
La Paglia GMC, Sanchez-Pernaute O, Alunno A, Martinez-Becerra MJ, Romero-Bueno F, Recuero S, et al. Ultrasound salivary gland involvement in Sjogren's syndrome vs. other connective tissue diseases: is it autoantibody and gland dependent? Clin Rheumatol. 2019. https://doi.org/10.1007/s10067-019-04780-2.
Jazzar A, Manoharan A, Brown JE, Shirlaw PJ, Carpenter GH, Challacombe SJ, et al. Predictive value of ultrasound scoring in relation to clinical and histological parameters in xerostomia patients. Oral Dis. 2019;25(1):150–7. https://doi.org/10.1111/odi.12959.
Orita Y, Sato Y, Kimura N, Marunaka H, Tachibana T, Yamashita Y, et al. Characteristic ultrasound features of mucosa-associated lymphoid tissue lymphoma of the salivary and thyroid gland. Acta Otolaryngol. 2014;134(1):93–9. https://doi.org/10.3109/00016489.2013.831994.
Ning XR, Wang ZQ, Zhang SS, Zhang X, Tang SM, Liu YY. Application of ultrasonography scoring system in the assessment of IgG4-related sialadenitis. Beijing Da Xue Xue Bao. 2019;51(6):1032–5.
Shimizu M, Okamura K, Kise Y, Takeshita Y, Furuhashi H, Weerawanich W, et al. Effectiveness of imaging modalities for screening IgG4-related dacryoadenitis and sialadenitis (Mikulicz's disease) and for differentiating it from Sjogren's syndrome (SS), with an emphasis on sonography. Arthritis Res Ther. 2015;17:223. https://doi.org/10.1186/s13075-015-0751-x.
Shimizu M, Moriyama M, Okamura K, Kawazu T, Chikui T, Goto TK, et al. Sonographic diagnosis for Mikulicz disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(1):105–13. https://doi.org/10.1016/j.tripleo.2009.02.032.
Asai S, Okami K, Nakamura N, Shiraishi S, Sugimoto R, Anar D, et al. Localized or diffuse lesions of the submandibular glands in immunoglobulin G4-related disease in association with differential organ involvement. J Ultrasound Med. 2013;32(5):731–6. https://doi.org/10.7863/ultra.32.5.731.
Li W, Xie XY, Su JZ, Hong X, Chen Y, Gao Y, et al. Ultrasonographic features of immunoglobulin G4-related sialadenitis. Ultrasound Med Biol. 2016;42(1):167–75. https://doi.org/10.1016/j.ultrasmedbio.2015.09.014.
Takano S, Sato T, Fukasawa M, Shindo H, Takahashi E, Yokota Y, et al. Hypoechoic lesions in submandibular glands are diagnostic markers of type 1 autoimmune pancreatitis. Pancreas. 2016;45(1):e8–9. https://doi.org/10.1097/MPA.0000000000000442.
Wang ZJ, Zheng LY, Pu YP, Zhou HH, Xie LS, Shi H, et al. Clinical features and treatment outcomes of immunoglobulin G4-related sclerosing sialadenitis. J Craniofac Surg. 2014;25(6):2089–93. https://doi.org/10.1097/SCS.0000000000001016.
Takagi Y, Nakamura H, Origuchi T, Miyashita T, Kawakami A, Sumi M, et al. IgG4-related Mikulicz's disease: ultrasonography of the salivary and lacrimal glands for monitoring the efficacy of corticosteroid therapy. Clin Exp Rheumatol. 2013;31(5):773–5.
Schiodt M, Greenspan D, Daniels TE, Nelson J, Leggott PJ, Wara DW, et al. Parotid gland enlargement and xerostomia associated with labial sialadenitis in HIV-infected patients. J Autoimmun. 1989;2(4):415–25. https://doi.org/10.1016/0896-8411(89)90170-4.
Soberman N, Leonidas JC, Berdon WE, Bonagura V, Haller JO, Posner M, et al. Parotid enlargement in children seropositive for human immunodeficiency virus: imaging findings. AJR Am J Roentgenol. 1991;157(3):553–6. https://doi.org/10.2214/ajr.157.3.1651645.
Vargas PA, Mauad T, Bohm GM, Saldiva PH, Almeida OP. Parotid gland involvement in advanced AIDS. Oral Dis. 2003;9(2):55–61. https://doi.org/10.1034/j.1601-0825.2003.02868.x.
Kabenge C, Ng S, Muyinda Z, Ameda F. Diagnostic ultrasound patterns of parotid glands in human immunodeficiency virus-positive patients in Mulago, Uganda. Dentomaxillofac Radiol. 2010;39(7):389–99. https://doi.org/10.1259/dmfr/23992216.
Martinoli C, Pretolesi F, Del Bono V, Derchi LE, Mecca D, Chiaramondia M. Benign lymphoepithelial parotid lesions in HIV-positive patients: spectrum of findings at gray-scale and Doppler sonography. AJR Am J Roentgenol. 1995;165(4):975–9. https://doi.org/10.2214/ajr.165.4.7677004.
Corthouts B, De Clerck LS, Francx L, De Schepper A, Vercruysse HA, Stevens WJ. Ultrasonography of the salivary glands in the evaluation of Sjogren's syndrome. Comparison with sialography. J Belg Radiol. 1991;74(3):189–92.
Napoli V, Tozzini A, Neri E, Calderazzi A, Gabriele M, Bonaretti S, et al. The imaging diagnosis of Sjogren's syndrome: echography, sialography and scintigraphy compared in the study of the salivary glands. Minerva Stomatol. 1996;45(4):141–8.
Kawamura H, Taniguchi N, Itoh K, Kano S. Salivary gland echography in patients with Sjogren's syndrome. Arthritis Rheum. 1990;33(4):505–10. https://doi.org/10.1002/art.1780330407.
Carotti M, Salaffi F, Manganelli P, Argalia G. Ultrasonography and colour Doppler sonography of salivary glands in primary Sjogren's syndrome. Clin Rheumatol. 2001;20(3):213–9. https://doi.org/10.1007/s100670170068.
Giuseppetti GM, Argalia G, Salera D, Ranaldi R, Danieli G, Cappelli M. Ultrasonographic contrast-enhanced study of sicca syndrome. Eur J Radiol. 2005;54(2):225–32. https://doi.org/10.1016/j.ejrad.2004.04.018.
Poul JH, Brown JE, Davies J. Retrospective study of the effectiveness of high-resolution ultrasound compared with sialography in the diagnosis of Sjogren's syndrome. Dentomaxillofac Radiol. 2008;37(7):392–7. https://doi.org/10.1259/dmfr/50668408.
Yoshiura K, Yuasa K, Tabata O, Araki K, Yonetsu K, Nakayama E, et al. Reliability of ultrasonography and sialography in the diagnosis of Sjogren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(3):400–7. https://doi.org/10.1016/s1079-2104(97)90249-3.
Xu. Value of sonographic score in the diagnosis of salivary gland involvement in patients with Sj(o)gren's syndrome. Chin J Ultrason. 2010;19:977–80.
Obinata K, Sato T, Ohmori K, Shindo M, Nakamura M. A comparison of diagnostic tools for Sjogren syndrome, with emphasis on sialography, histopathology, and ultrasonography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(1):129–34. https://doi.org/10.1016/j.tripleo.2009.08.033.
Shimizu M, Okamura K, Yoshiura K, Ohyama Y, Nakamura S, Kinukawa N. Sonographic diagnostic criteria for screening Sjogren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):85–93. https://doi.org/10.1016/j.tripleo.2005.09.012.
Niemela RK, Takalo R, Paakko E, Suramo I, Paivansalo M, Salo T, et al. Ultrasonography of salivary glands in primary Sjogren's syndrome. A comparison with magnetic resonance imaging and magnetic resonance sialography of parotid glands. Rheumatology (Oxford). 2004;43(7):875–9. https://doi.org/10.1093/rheumatology/keh187.
Makula E, Pokorny G, Kiss M, Voros E, Kovacs L, Kovacs A, et al. The place of magnetic resonance and ultrasonographic examinations of the parotid gland in the diagnosis and follow-up of primary Sjogren's syndrome. Rheumatology (Oxford). 2000;39(1):97–104. https://doi.org/10.1093/rheumatology/39.1.97.
Astorri E, Sutcliffe N, Richards PS, Suchak K, Pitzalis C, Bombardieri M, et al. Ultrasound of the salivary glands is a strong predictor of labial gland biopsy histopathology in patients with sicca symptoms. J Oral Pathol Med. 2016;45(6):450–4. https://doi.org/10.1111/jop.12387.
Chen S, Wang Y, Zhang G, Chen S. Combination of salivary gland ultrasonography and virtual touch quantification for diagnosis of Sjogren's syndrome: a preliminary study. Biomed Res Int. 2016;2016:2793898–6. https://doi.org/10.1155/2016/2793898.
El Miedany YM, Ahmed I, Mourad HG, Mehanna AN, Aty SA, Gamal HM, et al. Quantitative ultrasonography and magnetic resonance imaging of the parotid gland: can they replace the histopathologic studies in patients with Sjogren's syndrome? Joint Bone Spine. 2004;71(1):29–38. https://doi.org/10.1016/j.jbspin.2003.04.003.
Chikui T, Okamura K, Tokumori K, Nakamura S, Shimizu M, Koga M, et al. Quantitative analyses of sonographic images of the parotid gland in patients with Sjogren's syndrome. Ultrasound Med Biol. 2006;32(5):617–22. https://doi.org/10.1016/j.ultrasmedbio.2006.01.013.
Lin D, Yang W, Guo X, Cao J, Lv Q, Jin O, et al. Cross-sectional comparison of ultrasonography scoring systems for primary Sjogren's syndrome. Int J Clin Exp Med. 2015;8(10):19065–71.
Andretta M, Sfriso P, Botsios C, Ostuni PA, Grava C, Tregnaghi A, et al. Comparison of ultrasonography and sialography vs. magnetic resonance in the diagnosis of the primary Sjogren's syndrome. Acta Otorhinolaryngol Ital. 2001;21(1):22–31.
Kim JW, Lee H, Park SH, Kim SK, Choe JY, Kim JK. Salivary gland ultrasonography findings are associated with clinical, histological, and serologic features of Sjogren's syndrome. Scand J Rheumatol. 2018;47(4):303–10. https://doi.org/10.1080/03009742.2017.1374451.
Luciano N, Baldini C, Tarantini G, Ferro F, Sernissi F, Varanini V, et al. Ultrasonography of major salivary glands: a highly specific tool for distinguishing primary Sjogren's syndrome from undifferentiated connective tissue diseases. Rheumatology (Oxford). 2015;54(12):2198–204. https://doi.org/10.1093/rheumatology/kev253.
Milic VD, Petrovic RR, Boricic IV, Marinkovic-Eric J, Radunovic GL, Jeremic PD, et al. Diagnostic value of salivary gland ultrasonographic scoring system in primary Sjogren's syndrome: a comparison with scintigraphy and biopsy. J Rheumatol. 2009;36(7):1495–500. https://doi.org/10.3899/jrheum.081267.
Ariji Y, Ohki M, Eguchi K, Izumi M, Ariji E, Mizokami A, et al. Texture analysis of sonographic features of the parotid gland in Sjogren's syndrome. AJR Am J Roentgenol. 1996;166(4):935–41. https://doi.org/10.2214/ajr.166.4.8610577.
Makula E, Pokorny G, Rajtar M, Kiss I, Kovacs A, Kovacs L. Parotid gland ultrasonography as a diagnostic tool in primary Sjogren's syndrome. Br J Rheumatol. 1996;35(10):972–7. https://doi.org/10.1093/rheumatology/35.10.972.
Salaffi F, Carotti M, Iagnocco A, Luccioli F, Ramonda R, Sabatini E, et al. Ultrasonography of salivary glands in primary Sjogren's syndrome: a comparison with contrast sialography and scintigraphy. Rheumatology (Oxford). 2008;47(8):1244–9. https://doi.org/10.1093/rheumatology/ken222.
Theander E, Mandl T. Primary Sjogren's syndrome: diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res. 2014;66(7):1102–7. https://doi.org/10.1002/acr.22264.
Zhang X, Zhang S, He J, Hu F, Liu H, Li J, et al. Ultrasonographic evaluation of major salivary glands in primary Sjogren's syndrome: comparison of two scoring systems. Rheumatology (Oxford). 2015;54(9):1680–7. https://doi.org/10.1093/rheumatology/kev103.
Zhou MSS, Chen L, Liu X, Duan T. Study on the value of salivary gland ultrasonography in Sjogren's syndrome. Chin J Rheumatol. 2016;20:317–20.
Takagi Y, Sumi M, Nakamura H, Iwamoto N, Horai Y, Kawakami A, et al. Ultrasonography as an additional item in the American College of Rheumatology classification of Sjogren's syndrome. Rheumatology (Oxford). 2014;53(11):1977–83. https://doi.org/10.1093/rheumatology/keu238.
Milic V, Petrovic R, Boricic I, Radunovic G, Marinkovic-Eric J, Jeremic P, et al. Ultrasonography of major salivary glands could be an alternative tool to sialoscintigraphy in the American-European classification criteria for primary Sjogren's syndrome. Rheumatology (Oxford). 2012;51(6):1081–5. https://doi.org/10.1093/rheumatology/ker431.
Qi X, Sun C, Tian Y, Han Y, Peng C, Jin H, et al. Comparison of the diagnostic value of four scoring systems in primary sjogren's syndrome patients. Immunol Lett. 2017;188:9–12. https://doi.org/10.1016/j.imlet.2017.05.009.
Yonetsu K, Takagi Y, Sumi M, Nakamura T, Eguchi K. Sonography as a replacement for sialography for the diagnosis of salivary glands affected by Sjogren's syndrome. Ann Rheum Dis. 2002;61(3):276–7. https://doi.org/10.1136/ard.61.3.276.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tracian James-Goulbourne, Vagishwari Murugesan, and Eugene Y. Kissin declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
James-Goulbourne, T., Murugesan, V. & Kissin, E.Y. Sonographic Features of Salivary Glands in Sjögren’s Syndrome and its Mimics. Curr Rheumatol Rep 22, 36 (2020). https://doi.org/10.1007/s11926-020-00914-7
Published:
DOI: https://doi.org/10.1007/s11926-020-00914-7