Abstract
Purpose of Review
Immunosuppressive therapy for connective tissue diseases (CTDs) is steadily becoming more intense. The resultant impairment in cell-mediated immunity has been accompanied by an increasing risk for opportunistic infection (OI). Pneumocystis pneumonia (PCP) has been recognized as an OI in patients with CTDs, but specific risk factors and precise indications for PCP prophylaxis remain poorly defined. This review was undertaken to update information on the risk of PCP in patients with CTDs and to examine current guidelines for PCP prophylaxis in this population.
Recent Findings
Data on the occurrence of PCP and indications for prophylaxis in patients with CTDs is sparse. Large systematic reviews did not incorporate patients with CTD secondary to the lack of randomized control trials. Upon reviewing guidelines published since 2015, prophylaxis for PCP is recommended only for patients with ANCA-positive vasculitis, specifically granulomatosis with polyangiitis (GPA), who are undergoing intense induction therapy.
Summary
Evidence-based recommendations for the prophylaxis of PCP in patients with CTDs cannot be provided. There is expert consensus that PCP prophylaxis is warranted in patients with GPA undergoing induction therapy. Prophylaxis should perhaps also be considered for other CTD patients who are receiving similar intense immunosuppressive therapy especially if they are lymphopenic or have a low CD4 count.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pneumocystis jirovecii, formally P. carinii, is an opportunistic fungal pathogen which rarely causes symptomatic infections in the immunocompetent population but can cause severe infections, most commonly pneumonia, in immunocompromised hosts [1, 2]. Although recognized as an opportunistic pathogen in those with primary defects in cell-mediated immunity for decades, Pneumocystis pneumonia (PCP) came to prominence in the 1980s with the rise of HIV, eventually becoming an AIDs-defining illness [3]. With the advent of anti-retrovirals and prophylaxis with agents like trimethoprim-sulfamethoxazole (TMP-SMX), the incidence of PCP in the HIV-infected population has decreased significantly. Concurrent with its decline in HIV-infected populations, PCP has become an increasingly recognized complication in patients whose immune system has been compromised by immunosuppressant agents such as chemotherapy or disease-modifying anti-rheumatic drugs (DMARDS), especially with the development of biologic therapy [2, 4]. While there are established guidelines for PCP prophylaxis for HIV-infected patients as well as for other non-HIV-infected patients—including those with hematologic malignancies and organ transplants—there are no consensus guidelines for the prophylaxis of PCP in connective tissue diseases (CTDs) [5••, 6]. Previous studies have reported high mortality associated with PCP in patients with CTD prompting the question as to whether PCP prophylaxis is needed in this specific population [7, 8]. Surveys of practicing rheumatologists have shown wide discrepancy in the use of PCP prophylaxis as well as the specific indications for initiating prophylaxis [9, 10]. In this paper, the literature addressing PCP prophylaxis in patients with CTD will be reviewed.
Challenges in Making Guidelines
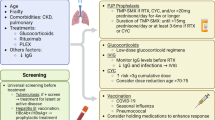
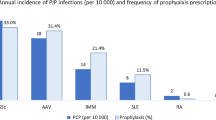
Several challenges exist when proposing guidelines for PCP prophylaxis in CTD. First, CTDs are relatively uncommon and the frequency of PCP in this population is low; consequently, the absolute number of cases of PCP in CTD is even smaller [4, 7]. Second, there is wide diversity in the diseases under the general category of CTD. A risk of PCP infection greater than 3.5% has been proposed as a cut off for PCP prophylaxis in any particular disease which balances the number needed to treat against the number needed to harm [5••]. Another Cochrane review has a suggested higher risk cut off (6%) but excluded studies involving CTDs [1]. While there are a large number of studies that support this level of risk in patients with solid organ transplants, allogeneic bone marrow transplants, and other select groups, there are only retrospective and prospective cohort studies that address the issue of PCP infection and PCP prophylaxis in CTD. When taken as a whole, the risk of PCP in CTD fails to meet this benchmark number; however, the frequency of PCP varies greatly from disease to disease. Ward et al. determined a frequency of PCP infections for granulomatosis with polyangiitis (GPA) to be 89 cases per 10,000 hospitalizations/year versus 2 per 10,000 in rheumatoid arthritis (RA) [7]. Green et al. determined that there was sufficient evidence to support a PCP risk of >3.5% in GPA but not in systemic lupus erythematosus (SLE), inflammatory myositis, or RA. The number needed to treat (NNT) for GPA was 32 which is the same as the number needed to harm (NNH); in comparison, the NNT for SLE was 110 and, for RA, 1099 [5••]. Thus, a recommendation for prophylaxis for the various CTDs likely needs to be tailored to specific diseases rather than advocating a broad “one size fits all” guideline given the wide variability of the occurrence of PCP in each given population.
Risk Factors for Infection
Determining specific risk factors for the development of PCP in patients with CTD would facilitate the development of prophylaxis guidelines. Risk factors are difficult to evaluate in such a heterogeneous population as both the immunosuppressive therapies used in treatment of these diseases and the pathophysiology of the disease (including the underlying aberrant immunity) contribute to the increased risk of infection [11, 12••, 13]. Several established, probable, and possible risk factors are noted in Table 1.
A well-established risk factor for PCP in the HIV population is a CD4+ count less than 200 cells/mm3 [4]. There is little data to suggest such a defined level in CTDs. In part, many studies do not address this particular issue because they are retrospective in nature and this particular data point is not available. In a study examining non-HIV-infected patients at high risk for PCP, a subsequent subgroup analysis of CD4+ counts showed counts of <250 cells/mm3 captured all patients with underlying autoimmune disease who developed PCP [2, 16]. Of those patients, 6 of the 8 had a CD4+ count less than 200 cells/mm3. In a retrospective review of PCP in CTD by Li et al., all patients who developed PCP had a CD4 count of less than 250/μL (87 ± 78/μL) [8]. In both of these reviews, there was no comparison of CD4+ count in other patients with CTD. While low CD4+ counts are associated with increased risk of PCP, further studies need to be done to evaluate this level as marker for initiation of PCP prophylaxis in patients with CTD.
Several studies describe decreased peripheral absolute lymphocyte counts in CTD patients who acquire PCP [7, 8, 13, 14, 17,18,19,20,21,22,23]. Even when controlling for the specific CTD, lymphopenia persists as a risk factor [15•]. However, the exact cut off value for when prophylaxis should be initiated is up for debate. Godeau and colleagues found in a retrospective evaluation of 44 patients with GPA (12 with PCP, 32 without PCP) who were treated with glucocorticoids (GCs) and cyclophosphamide that not only did the lymphocyte count during treatment confer risk but also the presence of pre-treatment lymphopenia [24]. A pre-treatment count of less than 800 cells/mm3 and a 3-month treatment level of less than 600 cells/mm3 were associated with increased risk of developing PCP. In a heterogeneous group of CTD patients, a lymphocyte count of <500/μl at 2 weeks after the initiation of GC therapy (>30 mg/day) was found to be an independent risk factor for development of PCP [22]. Porges et al. found that a lymphocyte count of <350 cells/mm3 would capture those most at risk for PCP in patients with SLE on GC and cytotoxic therapy [25]. While lymphopenia is often seen in those patients who develop PCP, there is no consensus lymphocyte count to determine when prophylaxis would be appropriate.
The immunosuppressive regimens used in the treatment of CTD have been implicated as a risk factor for the development of all infections including opportunistic infections like PCP [12••]. Glucocorticoids (GCs) are the most often implicated agent but there is conflicting data on whether it is mean daily dose, cumulative dose, and/or pulse dosing that gives rise to the risk [12••, 13, 26]. The daily dose that confers risk has been noted to be between >15 mg/day of prednisone equivalent and >40 mg/day of prednisone equivalent depending on the case series [2, 22, 27, 28]. In an analysis from the Mayo Clinic, a dose of 30 mg daily of prednisone equivalent was identified as a key risk factor but up to 25% of their population with PCP were on lower doses (16 mg daily of prednisone equivalent) [29]. Even though GC use seems to increase the risk of PCP, there are populations, such as patients with giant cell arteritis, which require long-term use of high-dose GC monotherapy yet the rate of PCP remains very low [30]. Thus, while GC may increase the risk in certain populations, glucocorticoid use alone likely does not fully explain the risk of PCP as underlying disease and concomitant therapies likely influence risk.
The use of non-glucocorticoid immunosuppressive agents during the first 2 weeks after GCs were started was found to be an independent risk factor for the development of PCP in one retrospective study; interestingly, there were no significant differences between the individual therapies which included cyclophosphamide, methotrexate, and azathioprine [22]. Despite this data, in a survey of practicing rheumatologists, 68.8% of those who prescribe PCP prophylaxis indicated that the therapeutic regimen was the most important determining factor to initiate prophylaxis [9].
Cyclophosphamide has been broadly implicated in the development of infection [2], and higher rates of PCP were found in larger cumulative dosing of cyclophosphamide (manifested by oral therapy over pulse IV dosing) [31]. Another study examining patients with GPA found higher cumulative doses of cyclophosphamide in those that developed PCP than in those that did not; yet, it was not found to be an independent risk factor [24]. Another study found no cases of PCP in patients administered cyclophosphamide alone [21]. Thus, it is difficult to determine if the risk with cyclophosphamide is independent of the other immunosuppressant agents being used concurrently (namely glucocorticoids). Demoruelle et al. concluded that the apparent lack (or very low rate) of PCP in CTD patients on cytotoxic agents in the absence of GC casts doubt on cyclophosphamide as a definitive risk factor for the development of PCP [32]. Other agents like methotrexate, azathioprine, mycophenolate mofetil, rituximab, and anti-tumor necrosis factor inhibitors (anti-TNFs) have all been observed in patients who develop PCP, but causality has not been established [15•, 23, 25, 26, 33, 34]. Additionally, some patients develop PCP at the onset of diagnosis of the CTD prior to any GC or immunosuppressive therapy which argues that the risk of PCP is not only driven by drug-mediated immunosuppression but, perhaps also, by the disease itself [13, 20]. While there is little doubt that immunosuppressive therapy increases the risk of infection, there is no sufficient evidence to give prophylaxis for specific medication use in isolation.
In concept, lymphopenia, low CD4+ count, and immunosuppression are contributors to the development of infectious complications including PCP. Yet, interpretation of these factors has led to a wide array of recommendations. Sowden et al. proposed checking CD4+ counts after 1 month of immunosuppression for patients who meet the following criteria: (1) steroid dose >15 mg prednisolone (or equivalent) per day, (2) >3 months of treatment, and (3) total lymphocyte count <600 cells/mm3 [2]. If the CD4+ count is <200, they recommend prophylaxis if annual risk of PCP exceeds 9%. Zhang and colleagues routinely prescribe PCP prophylaxis for all patients taking immunosuppressive agents or those with a history of PCP [23]. Others recommend considering prophylaxis if patients are on high dose GC with the dose ranging from >10 to >30 mg of prednisone equivalent daily [22, 26, 29]. Demoruelle et al. proposed PCP prophylaxis in patients with CTD and 2 or more of the following: (1) steroids ≥20 mg/day for >4 weeks, (2) current use of ≥2 DMARDs, (3) absolute lymphocyte count ≤350 cells/mm3, or (4) underlying lung parenchymal disease [32]. There is little consensus on when to initiate prophylaxis in patients with CTDs.
Mortality
Mortality associated with PCP in patients with CTD is consistently reported as high, especially in comparison to HIV-infected patients [29]. The reported mortality ranges from 9 to 85% which varies by disease and patient population [8, 21]. However, it is difficult to determine the causality of increased mortality (i.e., attributable mortality) for many of the same reasons that it is difficult to determine risk factors—namely, the underlying pathophysiology of the disease itself as well as the immunosuppressive agents being used to treat the disease [23]. Further complicating interpretation of the reported mortality rates is the high rate of co-infection with other opportunistic pathogens including CMV, Aspergillus and Candida species [29]. In a frequently cited article reporting a mortality rate of 32% in CTD patients with PCP, only 3 of the 11 deaths were solely attributable to PCP with other opportunistic infections accounting for 4 deaths, gram-negative rod bacterial infections for 3 deaths, and a non-infectious process for the remaining death [20].
Li et al. showed a very high mortality for PCP-infected CTD patients with only 1 survivor among the 7 patients included in this series, all of whom had a CD4+ count less than 250 cells/mm3; however, rates of co-infection were also high in that cohort [8]. Co-infection may suggest a more severe underlying disease and/or the use of more aggressive therapeutic immunosuppression [35]. Other factors associated with increased mortality in other reviews have included the following: decreased FIO2 at diagnosis, the need for mechanical ventilation, decreased albumin [17], older age, male sex, and private insurance [7]. Interestingly, in one study, there was a trend towards higher lymphocyte counts and CD4+ counts in survivors versus non-survivors but that observation was not statistically significant [17]. As such, no definitive risk factors for mortality due to PCP in patients with CTD have been established. Poorer outcomes in CTD populations as compared to HIV patients may relate to the presence of more chronic co-morbid diseases and the more frequent occurrence of co-infections with other opportunistic pathogens, both of which may contribute to increased mortality.
Agents for Prophylaxis
Trimethoprim-Sulfamethoxazole
Benefit
Prophylaxis in the HIV population with TMP-SMX has consistently shown reduction in PCP infection rate [36]. In non-HIV-infected patients (either post-transplantation or with a hematologic malignancy), a meta-analysis found a 91% reduction in occurrence of PCP with a combined NNT of 15 as well as a reduction in PCP-related mortality [5••]. An updated review found 85% reduction in PCP infections with TMP-SMX prophylaxis [1]. There was no difference in NNT or NNH for daily versus three times weekly dosing nor was there a difference in reduction of the incidence of PCP or all-cause mortality [1].
There have been no randomized controlled trials to evaluate primary prophylaxis for PCP in patients with CTD. A retrospective cohort analysis of patients with SLE, Behcet’s disease, dermatomyositis, and vasculitis found a substantial but not statistically significant reduction in the frequency of PCP in patients given TMP-SMX prophylaxis with a NNT of 14 [36]. Risk reduction has been confirmed in other retrospective studies as well [22, 27]. In RCTs evaluating the treatment of ANCA-associated vasculitis which mandated or had high rates of PCP prophylaxis, PCP rates were low, potentially signaling benefit despite the use of cytotoxic or anti-CD20 therapy [37, 38]. In a retrospective review of patients who developed PCP and survived, there was no recurrence of PCP in patients on secondary prophylaxis versus those not given TMP-SMX prophylaxis after an initial episode of PCP [20].
Side Effects
In both HIV-infected and non-HIV-infected populations, TMP-SMX prophylaxis may be associated with a wide range of side effects, some of which may be serious or even life-threatening [2]. Potential side effects of TMP-SMX (Table 2) include nausea; diarrhea; hypersensitivity reactions (rash, fever); elevated creatinine; transaminitis; leucopenia; and rarely, severe immune-mediated reactions such as Stevens-Johnson syndrome or aseptic meningitis [40]. Green and colleagues found that in non-HIV-infected, non-CTD patients, the adverse event rate was 15.2% in adults, with 3.1% of those considered severe, but all were reversible with cessation of therapy [5••]. A study looking at CTD patients taking TMP-SMX at prophylactic doses showed an adverse event rate of 8.5% including hypersensitivity, rash, and hepatitis [36]. In two trials looking at the use of co-trimoxazole as a treatment for GPA, 20% of patients in each trial had to stop therapy secondary to side effects, most of which were classified as minor with the exception of one renal complication which resolved with cessation of therapy [41, 42]. Two other studies reported similar adverse event rates and resolution of side effects with cessation of therapy [27, 43]. SLE comprises a unique subset of CTD patients as sulfonamide drugs may have higher rates of adverse reactions in this population and potentially may precipitate flares of the underlying disease. This risk will be discussed in more detail in the SLE section below.
Methotrexate is a commonly used medication in the treatment of CTD. Unfortunately, the combination of TMP-SMX and methotrexate can cause profound cytopenias and bone marrow suppression even with low doses of methotrexate and short duration (2 days) of treatment with TMP-SMX [44]. The increased toxicity is likely secondary to the fact that both medications inhibit dihydrofolate reductase. Interestingly, this adverse interaction has not been reported when TMP-SMX is used at prophylactic doses [32]. Regardless, it is a consideration when starting prophylactic therapy with TMP-SMX in patients with CTD.
Alternatives to Trimethoprim-Sulfamethoxazole
If patients have a pre-existing allergy to sulfa drugs or if there is intolerance to TMP-SMX, other options for PCP prophylaxis include dapsone, atovaquone, or inhaled pentamidine [11]. These alternative regimens can be expensive and also have serious side effects as reviewed in Table 2. Additionally, these medications have not been well studied in CTD patients [36].
Specific Rheumatic Diseases and the Role for PCP Prophylaxis
Granulomatosis with Polyangiitis
Among all the rheumatic diseases, GPA has the most robust data to support use of PCP prophylaxis. Older retrospective studies have shown the frequency of PCP in GPA to be between 60 and 120 cases/10,000 patients/year with 11 of 180 patients in one series and 12 of 44 in the other developing PCP [21, 24]. In a meta-analysis of 529 patients with GPA, 12% developed PCP with an accompanying 47% mortality rate [12••]. While PCP in GPA is more frequent than in the other CTDs reviewed, the absolute frequency of PCP still remains low [7, 45].
No RCTs specifically evaluating PCP prophylaxis in GPA have been done, but trials done for the treatment of the vasculitis itself may provide some insight into the benefits, or lack thereof, of PCP prophylaxis. In an RCT from France evaluating efficacy of oral cyclophosphamide versus IV pulse cyclophosphamide in GPA, PCP was documented in 10 of the 50 patients included in the study [31]. The rate of PCP pneumonia was so high in the initial 12 months of the study that TMP-SMX prophylaxis was added to the treatment protocol for the duration of the study. Larger trials looking at rituximab in the treatment of GPA either included mandatory, or had high rates of, PCP prophylaxis in the protocol. The RITUXVAS trial had no cases of PCP pneumonia with 30 of the total 44 patients receiving prophylaxis while being treated with immunosuppressive therapy [46]. The RAVE trial had mandatory PCP prophylaxis (TMP-SMX single strength daily) and, although the exact infections acquired during the study are not published, the overall infection rate was low [47]. The extension of the RAVE trial had the same mandatory TMP-SMX prophylaxis and reported only one case of PCP (who was noted to be non-compliant with PCP prophylaxis) out of the 197 patients enrolled [37]. A French-based RCT comparing azathioprine to rituximab for maintenance therapy of GPA restricted PCP prophylaxis to patients with CD4+ counts less than 250/mm3 and had only 1 case of PCP out of 115 patients [38]. A large review examining infectious complications occurring during the treatment of ANCA-associated vasculitis from multiple trials (including those mentioned above) found that PCP was an infrequent but significant complication of treatment of GPA and recommended TMP-SMX prophylaxis during induction therapy until the GC dose was less than 10 mg daily or if the lymphocyte count declined below 300/mm3 [45]. European League Against Rheumatism (EULAR) has also encouraged the use of PCP prophylaxis with TMP-SMX in GPA patients being treated with cyclophosphamide [48•].
Trimethoprim-sulfamethoxazole has been shown to decrease relapse rates in GPA although it is not clear if the benefit is from suppression of infectious triggers of relapse and/or immunosuppression from the folic acid inhibition property of TMP-SMX [41, 42]. Not surprisingly, the rate of PCP is low when TMP-SMX is given in the treatment of GPA. A South American retrospective study with 134 patients with GPA did not have any cases of PCP pneumonia during the 3 years of the study with 61% of the patients on TMP-SMX as adjunctive GPA treatment [49].
A cost analysis of TMP-SMX prophylaxis in patients with GPA demonstrated an improvement in quality-adjusted life years and cost savings of over $1000 [50]. However, if the patient developed an adverse event to TMP-SMX and transitioned to inhaled monthly pentamidine, there was minimal improvement in quality-adjusted life years and cost rose significantly. From a cost-effective standpoint, prophylaxis with TMP-SMX was beneficial as long as the incidence of PCP was greater than 0.2%. As discussed earlier, an occurrence rate of ≥3.5% justified prophylaxis with TMP-SMX in regard to benefit versus harm. The NNT to prevent one PCP infection in a patient with GPA was 32 and was equal to the number needed to harm [5••].
When to stop prophylaxis is as equally debated as when to start. Some authors recommend prophylaxis through the duration of immunosuppressive treatment [51], while others advocate prophylaxis until the daily prednisone dose is ˂20 mg [52] or even ˂10 mg [48•]. Some advocate for prophylaxis through the duration of B-cell depletion if anti-CD20 therapy is being used [51]. Unfortunately, there is no consensus or data to help guide therapy.
Prophylaxis during induction therapy for GPA has strong support and is recommended by societies such as EULAR. There is noted cost effectiveness and, in this specific population, benefit likely outweighs harm. Additionally, there is potential benefit for treatment with TMP-SMX for decreased relapse rates but this has not been examined at prophylactic doses. Thus, we would recommend prophylaxis for PCP with TMP-SMX in patients with GPA during induction therapy with cyclophosphamide and/or rituximab for the duration of induction therapy. At that point, risk should be assessed on a case by case basis taking into consideration GC dose, immunosuppressive medication, and presence of leukopenia or low CD4+ count.
Systemic Lupus Erythematosus
Many SLE patients require high-dose GC and/or cytotoxic medications in the treatment of their disease leading to concern for increased susceptibility to infection and specifically, for PCP [10, 14, 36]. This concern is displayed in a recent survey of practicing rheumatologists where 50% reported using PCP prophylaxis in SLE patients on cyclophosphamide therapy (either oral of IV) [10]. The data for the risk of PCP is less convincing for routine prophylaxis. In a meta-analysis of 2120 patients with SLE, approximately 5% developed PCP but the mortality was high at 46% [12••]. In a larger review of 76,156 SLE patients on cyclophosphamide, only 121 cases of PCP were documented giving a frequency of 15.88 per 10,000 patients [10]. Other studies have found similar rates of PCP in the SLE population [7, 8], thus supporting the general impression that the rate of PCP remains low, even with cytotoxic therapy. As with GPA, SLE patients who develop PCP are frequently found to be lymphopenic at the diagnosis of PCP but again, the exact number and exact risk is not clear [10, 14, 25]. With a low event rate, the NNT to prevent one case of PCP in patients with SLE is 110 [5••].
Further complicating the issue of prophylaxis in patients with SLE is the increased rate of adverse events from sulfonamide antibiotics as alluded to earlier in this article. Petri and colleagues found an increase in overall drug reactions to sulfonamide medications in SLE populations with flare of the underlying SLE in 21% of those experiencing a reaction [53]. Pope et al. found an increased rate of adverse events (52%) in SLE patients exposed to sulfa antibiotics as compared to patients with inflammatory arthritis [54]. The most common adverse event was rash. A retrospective analysis of CTD patients receiving PCP prophylaxis with TMP-SMX found an adverse event rate of 7.05% which was higher than a pulmonary disease cohort (2.67%) which among others included asthma, interstitial lung disease, hypersensitivity pneumonitis, and neoplasms [55]. The event rate was even higher in the SLE subset (11.0%). Interestingly, anti-RNP positivity conferred an independent increased risk of adverse events. Vananuvat et al. found that all of their adverse events from TMP-SMX were in SLE patients but that SLE flares were not significantly different between those exposed to TMP-SMX and those not exposed [36]. The overall adverse event rate in the TMP-SMX group was 9.4% including drug hypersensitivity and reversible hepatitis. The lower adverse event rate in this last study may be secondary to prophylactic dosing (single strength daily) versus the higher doses used to treat acute infection. Another study looking at single strength daily dosing saw no adverse events or worsening of the underlying SLE in the 15 patients with SLE in their cohort [27].
With an overall low incidence of PCP in SLE patients, there is not sufficient evidence to support universal use of prophylaxis with TMP-SMX even despite the use of cytotoxic therapy especially given the concern for increased adverse event rates.
Inflammatory Myopathy
In inflammatory myopathies, which include dermatomyositis (DM) and polymyositis (PM), PCP is less common than with GPA but more common than in SLE. Ward et al. noted the frequency to be 27 per 10,000 hospitalizations [7] while Godeau et al. estimated the frequencies to be 20/10,000 patient-years [24].
The largest review that addresses PCP risk in patients with inflammatory myopathies is from Marie and colleagues; in their series of 279 patients with DM or PM, 3 patients developed PCP [34]. Only one case was fatal and occurred at onset of disease prior to any immunosuppressive or corticosteroid therapy. Severe infections (all cause) were found in 37.3% of the cohort with the percentage of pyogenic infections (68.3%) substantially higher than opportunistic infections (35.7%). Risk factors for development of any infection included muscle weakness, dysphonia, esophageal dysfunction, respiratory insufficiency, and median daily GC dose (56.4 vs 27.3 mg of prednisone equivalent). Additionally, the use of immunosuppressive therapy increased the risk. Infections were more likely in patients with lymphopenia. The presence of ANA, anti-Jo-1, and cumulative dose of GC did not confer an increased risk of infection. The absolute risk for PCP was small in this cohort with non-PCP infections being significantly more common. A meta-analysis, including the reports noted above, found that 6% (40/688) of patients with DM/PM developed PCP [12••]. Green and colleagues calculated the NNT to prevent one PCP infection in DM/PM to be 73 which is well above their calculated NNH of 32 [5••].
Mortality due to PCP is similarly high in this patient population with some case series reporting case fatality rates as high as 100% but more commonly ranging from 33 to 56% [8, 12••, 13, 17, 18]. Much like all CTDs, the risk factors for mortality have not been established. Despite the seemingly high mortality, PCP prophylaxis is difficult to broadly recommend given the rarity of PCP in this patient population. However, consideration for prophylaxis on a case by case basis in patients with DM/PM undergoing treatment with high-dose corticosteroids and/or immunosuppressive therapies in the setting of baseline lymphopenia may be reasonable.
Rheumatoid Arthritis
Unlike the other diseases discussed in this paper, PCP is extremely rare in RA. In a large meta-analysis of several RA trials, there were only 8 total cases out of more than 30,000 patients [33]. There was no statistical difference in PCP rates between those on biologic DMARDs (6/21,916) and those on non-biologic DMARDs (2/10,588). In a retrospective cohort study of American RA patients, Baddley and colleagues also found similar rates of PCP in patients on anti-TNF agents and patients on non-biologic DMARDs [56]. The rates were 0.056/10,000 person-years for those on anti-TNFs compared to 0.051/10,000 person-years for those on non-biologic DMARDs. Although PCP was the most common opportunistic infection in this cohort, the overall frequency of pneumocystosis was low. This low frequency has been confirmed in several studies [7, 12••, 57•]. The one exception to this finding is from the Japanese literature where a higher rate of PCP in RA patients has been reported with rates as high as 8 cases/10,000 person-years [57•]. The rates in the American or European cohorts mostly remain less than 0.1 cases/10,000 person-years. Similar to outcomes in other CTDs, mortality is high in RA patients infected with PCP [12••, 18, 23]. Green and colleagues calculated the NNT to prevent one PCP infection in patients with rheumatoid arthritis to be 1099 which is over 30-fold the NNH [5••]. Thus, despite the high mortality with PCP in RA patients, the low incidence makes it impossible to recommend routine prophylaxis in this group of CTD patients.
Other Connective Tissue Diseases
There is even less data on the role of PCP prophylaxis for other CTDs such as scleroderma, polyarteritis nodosa (PAN), and microscopic polyangiitis (MPA). PCP in patients with scleroderma has been reported in the literature and in one series was estimated at 8 cases/10,000 hospitalizations/year [7]. Another case series of 117 patients reported no cases of PCP during the 310 person-year observation period [58]. Large RCTs for scleroderma (i.e., Scleroderma Lung Study) have not reported on the incidence of PCP specifically [59]. There are not enough cases in the literature (owing in part to the rarity of the disease) to determine specific risk factors [7, 17].
Examining the data for other vasculitic processes, Ward and Donald estimated the frequency of PCP in PAN to be 65 cases/10,000 hospitalizations/year [7], but these numbers have not been seen in other large series [20]. Among ANCA-positive vasculitis, there are several case reports of PCP in patients with MPA [8, 23] but most of the literature focuses only on GPA. Given the similar pathophysiology (though not identical) and treatment regimens, it would be reasonable to consider PCP prophylaxis in MPA in the same instances that one would for GPA; however, this would not be as evidence driven. EULAR guidelines are for ANCA-positive vasculitis rather than GPA specifically. As discussed earlier, rate of PCP in GCA is low and prophylaxis is not warranted [30]. No reports of PCP in Takayasu arteritis were found. Ultimately, there is not enough evidence to recommend empiric PCP prophylaxis in these and the other CTDs.
Conclusions
Pneumocystis pneumonia in patients with CTD is a serious complication with significant associated mortality. Most of the available literature on this infectious complication in CTD patients is in the form of case reports and is retrospective in nature—making it difficult to draw definitive conclusions about risk and the role for prophylaxis. In the absence of unequivocal risk data that implicate specific co-morbidities or individual immunosuppressive therapies as highly predictive of subsequent infection and in the absence of precise laboratory markers that can be incorporated into prediction rules to define infection risk, encompassing evidence-based guidelines for PCP prophylaxis cannot be proposed. Conceptually, RCTs to evaluate PCP prophylaxis in this population and inform decision-making are desperately needed. Unfortunately, no such trials presently exist and there likely will not be any future such trials for many of the reasons discussed above in this paper. The diversity of the diseases under the umbrella term “connective tissue disease” and the wide variation in frequency of PCP in those groups would make combined studies unhelpful. Yet, studies targeting only one CTD would likely not provide useful data given the relative rarity of the individual CTDs and the relative infrequency of the target event (i.e., PCP). As a consequence, any such studies would almost certainly be grossly under-powered and thus not clinically relevant.
The lack of evidence-based data and resultant lack of consensus guidelines makes it difficult to implement PCP prophylaxis effectively into current practice. Nevertheless, “common-sense” recommendations based on available data, imperfect though they are, can be cautiously proposed (Table 3). Clearly, these recommendations are open to discussion and further revision as new data come forth. In our world of ever-expanding evidence, the art of medicine is still sometimes all we have to rely upon until the evidence becomes available and/or matures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stern A, Green H, Paul M, et al. Prophylaxis for pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2014; doi:10.1002/14651858.CD005590.pub3.
Sowden E, Carmichael AJ. Autoimmune inflammatory disorders, systemic corticosteroids and pneumocystis pneumonia: a strategy for prevention. BMC Infect Dis. 2004;4:42. doi:10.1186/1471-2334-4-42.
Centers for Disease Control and Prevention: Pneumoycstis pneumonia. https://www.cdc.gov/fungal/diseases/pneumocystis-pneumonia (2014). Accessed 17 Feb 2017.
Morris A, Norris K. Colonization by pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25(2):297–317.
•• Green H, Paul M, Vidal L, et al. Prophylaxis of pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82(9):1052–9. This study reviews data for PCP prophylaxis with special attention to CTD.
Avino LJ, Naylor SM, Roecker AM. Pneumocystis jirovecii pneumonia in the non-HIV-infected population. Ann Pharmacol. 2016;50(8):673–9.
Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum. 1999;42(4):780–9.
Li J, Huang XM, Fang WG, et al. Pneumocystis carinii pneumonia in patients with connective tissue disease. J Clin Rheumatol. 2006;12(3):114–7.
Cettomai D, Gelber AC, Christopher-Stine L. A survey of rheumatologists’ practice for prescribing pneumocystis prophylaxis. J Rheumatol. 2010;37(4):792–9.
Gupta D, Zachariah A, Roppelt H, et al. Prophylactic antibiotic usage for pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus on cyclophosphamide: a survey of US rheumatologist and the review of literature. J Clin Rheumatol. 2008;14:267–72.
Stamp LK, Hurst M. Is there a role for consensus guidelines for P. jiroveci pneumonia prophylaxis in immunosuppressed patients with rheumatic disease? J Rheumatol. 2010;37:686–8.
•• Falagas ME, Manta KG, Betsi GI, et al. Infection-related morbidity and mortality in patients with connective tissue diseases: a systemic review. Clin Rheumatol. 2007;26:663–70. This study details infectious complications, including PCP, for the CTDs with large numbers of patients included.
Marie I, Hachulla E, Cherin P, et al. Opportunistic infections in polymyositis and dermatomyositis. Arthritis Rheum. 2005;53(2):155–65.
Kadoya A, Okada J, Iikuni Y, et al. Risk factors for pneumocystis carinii pneumonia in patients with polymyositis/dermatomyositis or systemic lupus erythematosus [abstract]. J Rheumatol. 1996;23(7):1186–8.
• Tadros S, Teichtahl AJ, Ciciriello S, et al. Pneumocystis jirovecii in systemic autoimmune rheumatic disease: a case-control study. Semin Arthritis Rheum. 2016; doi:10.1016/j.semarthrit.2016.09.009. Case series examining risk factors for PCP while controlling for the specific rheumatic disease.
Mansharamani NG, Balachandran D, et al. Peripheral blood CD4 + T-lymphocyte counts during pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118(3):712–20.
Chen M, Tian X, Qin F, et al. Pneumocystis pneumonia in patients with autoimmune disease: a retrospective study focused on clinical characteristics and prognostic factors related to death. PLoS One. 2015;10(9):e0139144. doi:10.1371/journal.pone.0139144.
Teichtahl AJ, Morrisroe K, Ciciriello S, et al. Penumocystis jirovecci pneumonia in connective tissue diseases: comparison with other immunocompromised patients. Semin Arthritis Rheum. 2015;45:86–90.
Jarrousse B, Guillevin L, Bindi P, et al. Increased risk of pneumocystis carinii pneumonia in patients with wegener’s granulomatosis [abstract]. Clin Exp Rheumatol. 1993;11(6):615–21.
Godeau B, Coutant-Perronne V, Huong DLT, et al. Pneumocystis carinii pneumonia in the course of connective tissue disease: report of 34 cases. J Rheumatol. 1994;21(2):246–51.
Ognibene FP, Shelhamer JH, Hoffman GS, et al. Pneumocystis carinii pneumonia: a major complication of immunosuppressive therapy in patients with wegener’s granulomatosis. Am J Respir Crit Care Med. 1995;151:795–9.
Ogawa J, Harigai M, Nagasaka K, et al. Prediction of and prophylaxis against pneumocystis pneumonia in patients with connective tissue diseases undergoing medium- or high-dose corticosteroid therapy. Mod Rheumatol. 2005;15:91–6.
Zhang Y, Zheng Y. Pneumocystis jirovecii pneumonia in mycophenolate mofetil-treated patients with connective tissue disease: analysis of 17 cases. Rheumatol Int. 2014;34:1765–71.
Godeau B, Mainardi JL, Roudot-Thoraval F, et al. Factors associated with pneumocystis carinii pneumonia in wegener’s granulmatosis. Ann Rheum Dis. 1995;54:991–4.
Porges AJ, Beattie SL, et al. Patients with systemic lupus erythematosus at risk for pneumocystis carinii pneumonia. J Rheumatol. 1992;19:1191–4.
Chew LC, Maceda-Galang LM, Tan YK, et al. Pneumocystis jirovecii pneumonia in patients with autoimmune disease on high-dose glucocorticoid. J Clin Rheumatol. 2015;21:72–5.
Okada J, Kadoya A, Rana M, et al. Efficacy of sulfamethoxazole-trimthoprim administration in the prevention of pneumocystis carinii pneumonia in patients with connective tissue disease. Kansenshogaku Zasshi. 1999;73(11):1123–9.
Mecoli CA, Saylor D, Gelber AC, et al. Pneumocystis jiroveci pneumonia in rheumatic disease: a 20-year single-centre experience [abstract]. Clin Exp Rheumatol 2017; Accessed 2/17/17.
Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71(1):5–13.
Kermani TA, Ytterberg SR, Warrington KJ. Pneumocystis jiroveci pneumonia in giant cell arteritis: a case series. Arthritis Care Res. 2011;63(5):761–5.
Guillevin L, Cordier JF, Lhote F, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized wegener’s granulomatosis. Arthritis Rheum. 1997;40(12):2187–98.
Demoruelle MK, Kahr A, Verilhac K, et al. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res. 2013;65(2):314–23.
Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and risk of opportunistic infections: a meta-analysis. Clin Infect Dis. 2014;58(12):1649–57.
Marie I, Menard JF, Hachulla E, et al. Infectious complications in polymyositis and dermatomyositis: a series of 279 patients. Semin Arthritis Rheum. 2011;41(1):48–60.
Hardak E, Neuberger A, Yigla M, et al. Outcome of pneumocystis jirovecii pneumonia diagnosed by polymerase chain reaction in patients without human immunodeficiency virus infection. Respirology. 2012;17:681–6.
Vananuvat P, Suwannalai P, Sungkanuparph S, et al. Primary prophylaxis for pneumocystis jirovecii pneumonia in patients with connective tissue diseases. Semin Arthritis Rheum. 2011;41:497–502.
Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–27.
Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371(19):1771–80.
UpToDate: Drug information. https://www.uptodate.com (2017). Accessed 7 Mar 2017.
Smilack JD. Trimethoprim-sulfamethoxazole. Mayo Clin Proc. 1999;74:730–4.
Zycinska K, Wardyn KA, Zielonka TM, et al. Co-trimoxazole and prevention of relapses of PR3-ANCA positive vasculitis with pulmonary involvement. Eur J Med Res. 2009;14:265–7.
Stegeman CA, Tervaert JW. Trimethoprime-suflamethoxazole(co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch co-trimoxazole wegener study group. N Engl J Med. 1996;355(1):16–20.
Meuli K, Chapman P, O’Donnell J, et al. Audit of pneumocystis pneumonia in patients seen by christchurch hospital rheumatology service over a 5-year period. Intern Med J. 2007;37:687–92.
Bourre-Tessier J, Haraoui B. Methotrexate drug interactions in the treatment of rheumatoid arthritis: a systematic review. J Rheumatol. 2010;37(7):1416–21.
Kronbichler A, Jayne DRW, Mayer G. Frequency, risk factors and prophylaxis of infection in ANCA-associated vasculitis. Eur J Clin Investig. 2015;45(3):346–68.
Jones RB, Tervaert JWC, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–20.
Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32.
• Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75:1583–94. EULAR recommendations for treatment of ANCA Vasculitis which includes recommendations on PCP prophylaxis.
de Souza FH, Radu Halpern AS, et al. Wegener’s granulomatosis: experience from a Brazilian tertiary center. Clin Rheumatol. 2010;29(8):855–60.
Chung JB, Armstrong K, Schwartz S, et al. Cost-effectiveness of prophylaxis against pneumocystis carinii pneumonia in patients with wegener’s granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum. 2000;43(8):1841–8.
Hugle B, Solomon M, Harvey E, et al. Pneumocystis jiroveci pneumonia following rituximab treatment in wegener’s granulomatosis. Arthritis Care Res. 2012;62(11):1661–4.
Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34(8):1098–107.
Petri M, Allbritton J. Antibiotic allergy in systemic lupus erythematosus: a case-control study. J Rheumatol. 1992;19(2):265–9.
Pope J, Jerome D, Fenlon D, et al. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J Rheumatol. 2003;30:480–4.
Maezawa R, Kurasawa K, Arai S, et al. Positivity for anti-RNP antibody is a risk factor for adverse effects caused by trimethoprim–sulfamethoxazole, a prophylactic agent for P. jiroveci pneumonia, in patients with connective tissue diseases. Mod Rheumatol. 2013;23:62–70.
Baddley JW, Winthrop KL, Chen L, et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the safety assessment of biologic therapy (SABER) study. Ann Rheum Dis. 2014;73(11):1942–8.
• Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: implications for chemoprophylaxis. Curr Rheumatol Rep. 2014;16:445. Large meta-analysis of PCP in rheumatoid arthritis with a specific focus on biologic therapy.
Foocharoen C, Siriphannon Y, Mahakkanukrauh A, et al. Incidence rate and causes of infection in Thai systemic sclerosis patients. Int J Rheum Dis. 2012;15(3):277–83.
Tashkin DP, Elashoff R, Clements PJ. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rachel M. Wolfe and James E. Peacock, Jr. declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Infection and Arthritis
Rights and permissions
About this article
Cite this article
Wolfe, R.M., Peacock, J.E. Pneumocystis Pneumonia and the Rheumatologist: Which Patients Are At Risk and How Can PCP Be Prevented?. Curr Rheumatol Rep 19, 35 (2017). https://doi.org/10.1007/s11926-017-0664-6
Published:
DOI: https://doi.org/10.1007/s11926-017-0664-6