Abstract
Purpose of Review
DNA methylation has emerged as an important contributing factor in the pathogenesis of systemic lupus erythematosus (SLE). Here, we describe the DNA methylation patterns identified in SLE and how these epigenetic changes can influence disease susceptibility, clinical heterogeneity, and disease flares.
Recent Findings
Several genome-wide DNA methylation studies have been recently completed in SLE. Important observations include robust demethylation of interferon-regulated genes, which is consistent across all cell types studied to date, and is independent of disease activity. This interferon epigenetic signature was shown to precede interferon transcription signature in SLE, suggesting it might be an early event in the disease process. Recent studies also revealed DNA methylation changes specific for renal and skin involvement in SLE, providing a proof of principle for a value of DNA methylation studies in exploring mechanisms of specific disease manifestations, and potentially as prognostic biomarkers. Inherited ethnicity-specific DNA methylation patterns have also been shown to possibly contribute to differences in SLE susceptibility between populations. Finally, a recent study revealed that DNA methylation levels at IFI44L can accurately distinguish SLE patients from healthy controls, and from patients with other autoimmune diseases, promising to be the first epigenetic diagnostic marker for SLE.
Summary
Genome-wide DNA methylation studies in SLE have provided novel insights into disease pathogenesis, clinical heterogeneity, and disease flares. Further studies promise to reveal novel diagnostic, prognostic, and therapeutic targets for SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epigenetic mechanisms regulate chromatin accessibility and gene expression patterns in cells and tissues. Each cell type presents a specific epigenome profile characterized by a related set of histone modifications and DNA methylation patterns alongside an associated transcriptome. Epigenetic dysfunctions can lead to dysregulation of the cellular processes and cause diseases [1, 2]. The importance of epigenetic dysfunctions in human complex diseases has been widely demonstrated in both cancer [3, 4] and autoimmune diseases [1, 2, 5, 6••]. In addition, recent interest comes from the fact that epigenetic alterations are potentially reversible, and therefore, characterizing epigenetic dysfunction in disease has the potential to identify new biomarkers and novel therapeutic targets [4, 7].
In this context, systemic lupus erythematosus (SLE) might be the autoimmune disease most deeply studied at the epigenetic level [5]. SLE is a systemic autoimmune disease that affects multiple organs and is characterized by autoantibody production and immune complex deposition, producing inflammation and damage in the affected tissue. As with all human complex diseases, the etiology of SLE is incompletely understood. Environmental triggers such as viral infections, exposure to chemicals and radiation, vitamin D deficiency, or sex hormones together with genetic factors are involved. Regarding the genetic factors, SLE has been extensively studied in the last decade with more than 60 risk loci identified across populations [8•]. However, in spite of these findings, a substantial proportion of disease heritability remains unexplained. In addition, most of these genetic susceptibility variants fall into non-coding regions within the genome, suggesting that they might have a regulatory role [6••, 8•]. On the other hand, the high discordance rate found among monozygotic twins in SLE [9, 10] and evidence for environmental triggers in the development of the disease also suggest that other biological mechanisms are involved in its etiology.
DNA methylation is the best characterized epigenetic modification in humans. In vertebrates, DNA methylation occurs on the 5′ carbon position of the pyrimidine ring of cytosine residues from CpG dinucleotides, although it was recently observed to occur on other motifs, i.e., CHG or CHH, in embryonic tissue and induced pluripotent stem cells [11]. Briefly, methylation status represses gene expression, while unmethylation status is associated with active transcription. An early study in autoimmunity showed that DNA hypomethylation in T cells is associated with rheumatoid arthritis and SLE [12]. Indeed, DNA methylation has emerged as an important contributing factor in the pathogenesis of SLE. An early study in SLE demonstrated the conversion to autoreactivity in CD4+ T cells when treated with 5-azacytidine, procainamide, or hydralazine, all of them drugs that suppress DNA methylation and can cause a lupus-like syndrome [13]. In the same way, the introduction of synthetically demethylated CD4+ T cells into mouse models is sufficient to produce lupus-like syndrome [14, 15]. In addition, the loss of methylation in the promoters of many specific genes such as ITGAL, CD40LG, and CD70 observed in T cells has been linked with SLE pathogenesis and progression [16,17,18]. This hypomethylation might be due to inhibition of the MAPK/ERK pathway signaling in CD4+ T cells which leads to a reduced expression of DNMT1, the enzyme responsible for maintaining methylation status during mitosis, and therefore a loss of DNA methylation resulting finally in autoimmunity [19, 20].
The development of high-throughput microarrays based on bead technology allowed for the investigation of the global DNA methylation landscape in immune cells involved in SLE pathology, providing new insights into the pathogenesis of the disease. Indeed, recent genome-wide DNA methylation studies have identified several differentially methylated genes and pathways involved in SLE susceptibility. In the current review, we have described the main changes in DNA methylation patterns associated with SLE, focusing on the most relevant findings identified by genome-wide DNA methylation approaches performed in the last 5 years.
Epigenome-Wide DNA Methylation Studies in SLE
The genome-wide DNA methylation approach in SLE was first used by Javierre et al. in 2010 for testing differences in the DNA methylation patterns in five couples of monozygotic twins discordant for SLE [21]. Direct comparison of identical twins is an excellent experimental approach to test possible epigenetic changes associated with complex diseases, as it minimizes the influence of many cofounders, including age and genetic background. Interestingly, a decrease was observed in the DNA methylation level of genes related to functional processes potentially relevant in SLE pathology, such as immune response, cell activation, cell proliferation, and cytokine production. The difference in DNA methylation patterns observed by comparing monozygotic twins with and without SLE reinforced the idea that environmental factors acting on particular genetic backgrounds can induce SLE. Despite the relevance and critical insights derived from the findings of this study, one important limitation was that it was performed in whole white blood cells. Therefore, taking into account specific epigenetic profiles of each tissue or cell type, further studies focusing on specific affected tissues or cells might uncover more insights into the implication of DNA methylation changes in the pathogenesis of SLE.
Subsequently, the majority of the epigenome-wide studies performed in autoimmune diseases, and more especially in SLE, have been focused on specific immune cells, defined by their pivotal role in the pathogenesis of the disease. This is due to the obvious implication of the immune system in the pathology of autoimmune diseases and the ability to collect and separate the different immune cells from peripheral blood samples. In SLE, previous studies showed that T-cell DNA methylation defects play an important role in its pathogenesis [12,13,14, 16,17,18,19], and therefore, many of the genome-wide DNA methylation studies have been performed in this type of cells.
The first screening of 27,578 CpG sites spanning the promoter regions of 14,495 genes in CD4+ T cells from 12 female SLE patients and 12 female healthy controls identified 336 genes differentially methylated [22]. Many of them were found to be hypomethylated, which is consistent with the global hypomethylation previously described in T cells from SLE patients [12]. Further, hypomethylated genes were significantly involved in the development of the connective tissue, which is consistent with SLE pathology. Hypermethylated genes were mainly overrepresented in nutrient metabolism pathways, with special relevance to folate biosynthesis, a pathway related to maintenance of DNA methylation [23]. Some of the differentially methylated genes correlated significantly with disease activity, suggesting a possible use as biomarkers for diagnosis and prognosis in SLE patients.
A genome-wide DNA methylation study using the HumanMethylation450 BeadChip in naïve CD4+ T cells and with a larger sample size revealed a more specific epigenetic landscape in SLE [24]. A significant hypomethylation of interferon-regulated genes, including IFIT1, IFIT3, MX1, STAT1, IFI44L, USP18, TRIM22, and BST2, was observed. These epigenetic changes did not imply change in the gene expression in naïve CD4+ T cells; however, they were differentially expressed in total CD4+ T cells. This interferon epigenetic signature in naïve CD4+ T cells of SLE patients might permit a rapid type I interferon response in SLE. Further, demethylation of interferon-regulated genes in SLE was independent of disease activity, and was detected in both active and inactive disease [24].
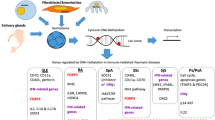
This finding was confirmed in a subsequent study and hypomethylation of interferon-regulated genes was also observed in memory and regulatory T cells [25]. Interestingly, demethylation of loci involved in type I interferon signaling have also been found in CD19+ B cells and CD14+ monocytes, suggesting that this pattern may be an earlier event in an ancestral lymphoid and myeloid precursor [25]. Some of these genes were found to be demethylated in the three types of cells, i.e., T and B cells and monocytes, such as IRF7, while others were specific of each cell type such as IKZF4 that was demethylated only in T cells (Fig. 1). Similar to the findings by Coit et al. in naïve CD4+ T cells, the interferon signature was present in patients during flares and remission stages, with no statistically significant difference between both groups of patients in any of these cell types, suggesting that this epigenetic state persists beyond stages when circulating interferon levels might be at their highest, or that demethylation of interferon-regulated genes is an early and persistent phenomenon in SLE [24, 25].
Venn diagrams showing the number of differentially methylated genes in SLE compared to healthy controls among different immune cell subsets. The size of the circles corresponds with the total number of genes significantly hypo- or hypermethylated between SLE cases and controls in each immune cell subset. Each color represents one cell subset, i.e., T cells (blue), B cells (red), monocytes (green), and neutrophils (yellow). Data are from the Supplementary Materials of Coit et al. 2013 [24], Absher et al. 2013 [25], and Coit et al. 2015 [26]
More recently, global hypomethylation has been also observed in neutrophils and low-density granulocytes (LDG) from SLE patients, with MX1 and IFI44L being the most hypomethylated genes [26]. In fact, a robust demethylation of interferon-regulated genes was detected in SLE neutrophils compared to controls. The importance of neutrophils, and LDG in particular, in the pathogenesis of SLE has been recently demonstrated [27]. LDG are pro-inflammatory neutrophils with a lower density and produce high levels of type I interferon, interferon-gamma, and TNF-alpha. These cells seem to be more prone to NETosis, i.e., extracellular chromatin traps, related to a form of cell death of neutrophils. NETs contribute to the pathogenesis of SLE by stimulating interferon-alpha production [27]. In addition, hypomethylation of transposon LINE1 has been also found in SLE neutrophils consistent with global hypomethylation. Indeed, increased expression of LINE1 is associated with increased type I interferon production and has been described in SLE [28, 29].
In summary, global hypomethylation and demethylation of interferon-regulated genes are the main features in all immune cells of SLE patients studied to date [24,25,26]. In addition, many of the hypomethylated genes are shared between different subtypes of cells. In contrast, hypermethylated genes in SLE tend to be more specific of each immune cell studied (see Fig. 1). Interestingly, Gene Ontology analyses using the most significant differentially methylated genes in each immune cell type studied to date show that hypomethylated genes are enriched in two processes of the innate immune response, the interferon pathway, especially type I interferon signaling pathway (GO:0060337), and negative regulation of viral life cycle (GO:1903901) (Fig. 2). These common pathways reflect the overall common functional enrichment among hypomethylated genes shared between all immune cells included in our analysis. More specifically, hypomethylated genes in CD4+ T cells show a signal to response in interferon-alpha (GO:0035455) and metabolic process, whereas hypomethylated genes in CD19+ B cells were specifically associated with response to interferon-beta (GO:0035456), regulation of immune system process (GO:0002682), and antigen processing and presentation of endogenous peptide antigen (GO:0002483) (Fig. 2). Taken together, demethylation of interferon-regulated genes in SLE patients may be triggered by an early event in the hematopoietic cell lineages, and provides a mechanism to explain type I interferon hyper-responsiveness in SLE patients. On the contrary, hypermethylated genes in SLE are involved in different immunological pathways (Fig. 3). Regulation of granulocyte differentiation (GO:0030852) is a common pathway between CD4+ T cells and CD19+ B cells due to shared hypermethylated genes in SLE between both cell types. No significant Gene Ontology enrichment was detected among hypermethylated genes in monocytes and neutrophils. Despite these interesting observations based on Gene Ontology analyses, future replication studies will be necessary to confirm these results.
Gene Ontology (GO) functional annotation analysis showing the main biological processes associated with hypomethylated genes in SLE. Results from Panther GO term analysis for the most significant hypomethylated genes in each immune cell subset are shown. The y-axis represents fold enrichment measured as the percent of hypomethylated genes in each Gene Ontology term. Data are from the Supplementary Materials of Coit et al. 2013 [24], Absher et al. 2013 [25], and Coit et al. 2015 [26]
Gene Ontology (GO) functional annotation analysis showing the main biological processes associated with hypermethylated genes in SLE. Results from Panther GO term analysis for the most significant hypermethylated genes in each immune cell subset are shown. The y-axis represents fold enrichment measured as the percent of hypermethylated genes in each Gene Ontology term. Data are from the Supplementary Materials of Coit et al. 2013 [24], Absher et al. 2013 [25], and Coit et al. 2015 [26]
DNA Methylation Changes and Disease Activity in SLE
To investigate the earliest T-cell epigenetic changes that occur in SLE disease flares, Coit et al. designed a genome-wide DNA methylation analysis in naïve CD4+ T cells from 74 female SLE patients with different degrees of disease activity, measured by SLE Disease Activity Index (SLEDAI) score [30••]. The study revealed that increased disease activity in SLE is associated with progressive hypomethylation of pro-inflammatory pathways and progressive hypermethylation of TGF-beta signaling pathway genes. These methylation changes occur prior to gene expression changes and differentiation of CD4+ T cells, and suggest an epigenetic pro-inflammatory activation coupled with an epigenetic suppression of immune-inhibitory signaling, as early events in SLE flares. Indeed, the genes that get hypomethylated with disease activity showed a cytokine-related pattern more consistent with an epigenetic profile of the Th2, Th17, and Tfh responses, and suggest a role of these cells during the early progression to a disease flare. The fact that no epigenetic changes were detected in the gene encoding interferon-gamma rejects the early contribution of the Th1 cells in SLE flares [30••]. The epigenetic landscape changes correlated with disease activity in SLE might be due to increased activity of EZH2, which encodes a histone methyltransferase that plays an important role in X chromosome inactivation and T-cell plasticity and differentiation. Hypermethylated regions correlated with higher disease activity were found to be enriched in binding sites for the repressive transcription factor EZH2, while hypomethylated genes were significantly depleted in EZH2 binding sites [30••]. However, no correlations in mRNA expression levels were found between EZH2 and disease activity in naïve CD4+ T cells from SLE patients [30••]. In contrast, a negative correlation between disease activity and miR-26a expression, and possibly also miR-101 expression, was detected. MiR-26a and miR-101 are both upregulated by glucose restriction and repress EZH2 expression [31]. In fact, glycolysis has been recently demonstrated to be increased in CD4+ T cells from SLE patients [32]. Therefore, we postulate that EZH2 upregulation, possibly induced by increased glucose availability in SLE T cells resulting in downregulation of miR-26a and miR-101, induces a pro-inflammatory epigenetic landscape shift in naïve CD4+ T cells that leads to disease flares. The proposed T-cell epigenetic model of disease flare in SLE patients is shown (Fig. 4). Additional work is needed to comprehensively understand the role of EZH2 dysregulation in SLE and whether inhibiting EZH2 is a potential therapeutic target for the disease.
Postulated epigenetic model of SLE flares based on genome-wide DNA methylation data and associated bioinformatics analyses in naïve CD4+ T cells in SLE. Reproduced from “Epigenetic reprogramming in naïve CD4+ T cells favoring T cell activation and non-Th1 effector T cell immune response as an early event in lupus flares” by Patrick Coit, Mikhail G. Dozmorov, Joan T. Merrill, W. Joeseph McCune, Kathleen Maksimowics-McKinnon, Jonathan D. Wren, Amr H. Sawalha, September 1, 2016, with permission from BMJ Publishing Group Ltd.
DNA Methylation in Disease-Specific Manifestations
SLE is a heterogeneous multi-system disease and can present with a large spectrum of symptoms and manifestations including dermatological, musculoskeletal, renal, cardiovascular, nervous system disease, and others. The understanding of the epigenetic mechanisms involved in the pathogenesis of specific disease manifestation is essential to better understand SLE and develop novel more specific therapeutic approaches and disease biomarkers.
Lupus nephritis is a main cause of morbidity and mortality in SLE patients. Sixty percent of SLE patients develop renal damage, being more frequent in African, Asian, and Hispanic ethnicities [33]. Its incidence and severity also vary between patients. Regarding DNA methylation changes, a significantly more robust demethylation of the type I interferon-regulated genes has been observed in naïve CD4+ T cells from SLE patients with renal involvement [34, 35]. In fact, a number of interferon-regulated genes have been observed to be specifically hypomethylated in SLE patients with a history of renal involvement, including IRF7, a well-known risk locus for SLE susceptibility [36] and IFI44. The mRNA expression levels of both IRF7 and IFI44 were previously observed to be increased in CD4+ T cells from SLE patients [37]. IRF7 is an activating transcription factor of the type I interferon response. Also, an SLE-associated genetic variant in this locus confers elevated IRF7 activity and downstream interferon pathway [38]. Therefore, both genetic and epigenetic alterations support a relevant role of IRF7 in SLE susceptibility and more especially in the development of renal involvement [25, 34, 39]. Indeed, it has been hypothesized that inhibitors of IRF7 might provide a potential therapeutic benefit for lupus nephritis [34]. However, further studies may be required. On the other hand, IFI44 is an interferon-inducible gene with an unknown function, but it has been found to be hypomethylated across multiple leukocyte subsets in SLE patients [24,25,26]. Its hypomethylation in lupus nephritis has been recently confirmed in an independent study [35]. Outside of the interferon pathway, other genes such as TNK2, DUSP5, CD47, or CD247 were found to be differentially methylated only in SLE patients with but not without renal involvement [34].
Cutaneous manifestations such as photosensitivity, malar rash, or discoid rash occur in the majority of SLE patients. Specific DNA methylation patterns associated with malar rash and discoid rash were identified in naïve CD4+ T cells of SLE patients, although the consistent hypomethylation of interferon signature genes was common to both groups of patients as expected [40]. Interestingly, the methylation changes associated with specific skin damage were related to immune functions such as environmental stress response, apoptosis, proliferation, and antigen processing and presentation. More specifically, the most extensive hypomethylated promoter associated with malar rash was VTRNA2-1 (previously annotated as miR-886) that regulates eIF2α and NF-κB signaling pathways, both involved in cell survival and apoptosis. In SLE patients with discoid rash, genes such as TRIM69, RHOJ, and HZF also implied in cell survival and apoptosis were found to be hypomethylated. Further, in this last set of patients, PSMB8 and TAP1 were also hypomethylated, suggesting a relevant role of the processing and presentation of antigens in cutaneous lupus manifestations.
The relationship between DNA methylation and the production of autoantibodies that are of great importance in SLE pathogenesis has also been investigated by a genome-wide DNA methylation approach in a large case–control cohort using whole blood samples [41]. The study showed a stronger differential methylation of CpG sites within major histocompatibility complex (MHC) genes associated with anti-Sm and anti-RNP autoantibody production in SLE. Demethylation in interferon signature genes was also observed, consistent with previous reports. Traditional therapies used to treat SLE were not shown to have any effect on the methylation status of the interferon signature genes [41].
DNA Methylation as a Potential Diagnostic Biomarker
As described above, an important feature of SLE is the presence of autoantibodies, such as anti-nuclear antibodies, anti-double stranded DNA antibody, anti-Sm antibody, and others, which have been used as conventional serological markers in SLE patients [42]. However, the low specificity or sensitivity of some of these serological tests can be challenging. Despite progress in characterizing the genetic basis of SLE, genetic risk score estimates remain of limited diagnostic or prognostic utility [8•, 43,44,45]. This is in part due to the fact that SLE is a complex polygenic disease, with most genetic risk variants being common among populations, and that only a small fraction of SLE heritability can be explained by the genetic susceptibility loci identified to date.
Taking into account the complex heterogeneity of SLE, the development of effective biomarkers is necessary. In this regard, DNA methylation marks have been suggested as possible robust biomarkers for diseases that can be potentially more objective and developed using more standardized protocols than serological tests [7, 46].
Zhao et al. performed a genome-wide DNA methylation study in whole blood to investigate whether specific gene methylation changes could meet sensitivity and specificity criteria for a robust diagnostic biomarker in SLE [47••]. Significant hypomethylation of a CpG site within IFI44L promoter was detected in both active and inactive SLE patients compared to healthy controls. Subsequent pyrosequencing in several large case–control cohorts of different ethnicities confirmed IFI44L demethylation in peripheral blood samples from SLE patients compared to healthy controls. Indeed, IFI44L methylation levels can distinguish SLE patients from healthy controls and from patients with rheumatoid arthritis or primary Sjögren’s syndrome, with a high sensitivity and specificity. These data suggest that IFI44L methylation can be potentially developed as a novel diagnostic test for SLE and provide a proof of principle for direct possible clinical applications of epigenetic studies in autoimmunity.
Genetic–Epigenetic Interaction
Findings linking genetic risk of SLE and epigenetic modifications have been also reported. In particular, the possible relationship between SLE-associated genetic variants within MECP2 and DNA methylation patterns in stimulated human CD4+ T cells from healthy women was investigated. MECP2 encodes a master epigenetic and transcriptional regulator of gene expression and can alter chromatin accessibility and induce either transcriptional repression or activation. Genetic variants in this locus are known to affect SLE susceptibility [48,49,50], and the SLE-associated genetic variant in this locus has been shown to regulate the expression of several genes, including a number of interferon-regulated genes in SLE patients [49]. Indeed, the SLE risk variant of MECP2 correlates with higher relative mRNA expression of MECP2 isoform 2 (MECP2B) in stimulated T cells. In order to further evaluate the possible effect of the SLE risk variant in MECP2 on DNA methylation patterns, genome-wide DNA methylation was assessed in stimulated T cells from healthy women with either the disease risk or non-risk MECP2 alleles. No evidence of global DNA methylation change was observed, but significant widespread locus-specific DNA methylation changes were found to be associated with the risk variant [51]. In fact, significant DNA hypomethylation was detected in the HLA region, especially in HLA-DR and HLA-DQ, and in interferon-regulated genes, providing the first evidence for genetic–epigenetic interaction in an SLE genetic risk variant.
The possible role that inherent ethnicity-specific DNA methylation changes might play in SLE susceptibility has been recently evaluated. SLE prevalence varies across populations, being more frequent in African, Asian, and Latin-American ancestries than in Europeans [33]. These differences might be explained by the existence of specific risk loci detected across populations [8•]. However, it is difficult to determine if genetic factors alone or other non-genetic factors such as environmental or socioeconomic causes are also involved. Multiple studies have demonstrated the existence of differences in DNA methylation patterns between populations [52, 53], suggesting that inherent epigenetic differences might affect the development and progression of human complex diseases. Ethnicity-specific DNA methylation changes that might be involved in SLE susceptibility and pathogenesis have been evaluated in naïve CD4+ T cells from a total of 66 healthy women and 63 women with SLE from two different ancestries, African-American and European-American [54]. The results of this genome-wide DNA methylation study showed differences in DNA methylation patterns between both populations that were explained in part by the presence of genetic polymorphisms that modify CpG sites and, consequently, DNA methylation status [54]. Significant enrichment of pro-apoptotic genes and genes previously linked to autoimmunity and SLE were observed among the hypomethylated genes in African-Americans compared to European-Americans, both in healthy individuals and in SLE patients. This study demonstrated that heritable ethnicity-specific DNA methylation changes might at least in part explain differences in SLE susceptibility between populations [54]. Further studies in other populations with high prevalence of SLE are required to confirm and expand these findings.
Conclusion and Future Perspectives
The few genome-wide DNA methylation studies published to date have provided new insights into the pathogenesis of SLE. All in all, interferon-regulated genes seem to be epigenetically poised for transcription, providing a mechanism to explain type I interferon hyper-responsiveness in SLE. In addition, the persistent demethylation of interferon-regulated genes found across immune cells and disease manifestations in SLE suggests that an epigenetic interferon signature might be an early event in the disease process. Further investigations will be required to establish at what stage does this epigenetic interferon signature develop relative to the onset of clinical disease in SLE and how this can be mechanistically explained.
Genome-wide DNA methylation studies have also provided evidence that can lead to the development of novel diagnostic biomarkers for SLE, such as IFI44L methylation in whole blood. Similarly, disease subset-specific methylation changes have been identified and serve as proof of principle that specific epigenetic biomarkers might have prognostic value in SLE. Epigenetic changes might help explain differences in SLE prevalence and severity between populations, and studies to identify epigenetic biomarkers of disease flares and remission and to predict response to therapy should be pursued in the near future.
Although published studies show the pivotal role for DNA methylation changes in SLE, many questions still need to be answered. Examination of other cell subsets relevant to SLE, including cells derived from biopsies of affected tissues, might uncover additional epigenetic susceptibility loci for SLE manifestations. In this way, single-cell approaches for capturing DNA methylation patterns might be a novel powerful tool to study the cellular plasticity and diversity of the complex tissue samples from SLE patients. In addition, future studies and replication efforts with larger sample sizes might detect smaller DNA methylation changes between patients and controls. Large international consortium-based approaches might help to obtain more robust findings. Also, newer sequencing technologies that permit investigation of other regulatory regions and epigenetic marks in the genome might expand the epigenetic landscape associated with SLE and allow for a better understanding of disease etiology and the identification of novel therapeutic targets. Finally, integrative genetic and epigenetic epidemiological approaches will be required to refine the functional and regulatory variation associated with SLE-risk variants, and might permit the development of prediction models with potential clinical use in the diagnosis, classification, and prognosis of SLE.
Abbreviations
BST2, bone marrow stromal cell antigen 2; CD40LG, CD40 ligand; DNMT1, DNA methyltransferases 1; DNA, deoxyribonucleic acid; eIF2α, Eukaryotic Initiation Factor 2; EZH2, enhancer of zeste homolog 2; HZF, official name Ring finger protein 39 (RNF39); IFI44, interferon-induced protein 44; IFI44L, interferon-induced protein 44 like; IFI6, interferon-alpha inducible protein 6; IFIT1, interferon-induced protein with tetratricopeptide repeats 1; IFIT3, interferon-induced protein with tetratricopeptide repeats 3; IKZF4, IKAROS family zinc finger 4; IRF6, interferon regulatory factor 6; IRF7, interferon regulatory factor 7; ITGAL, integrin subunit alpha; LDG, low-density granulocytes; LINE-1, long interspersed nuclear element 1; MAPK/ERK, mitogen-activated protein kinases, originally called ERK, extracellular signal-regulated kinases; MHC, major histocompatibility complex; MX1, MX dynamin like GTPase 1; NET, extracellular chromatin traps; NF-κB, nuclear factor kappa B; PSMB, proteasome subunit beta 8; RA, rheumatoid arthritis; RNA, ribonucleic acid; RHOJ, Ras homolog family member J; SLE, systemic lupus erythematosus; SLEDAI, SLE Disease Activity Index; STAT1, signal transducer and activator of transcription 1; TAP1, transporter 1, ATP binding cassette subfamily B member; TRIM22, tripartite motif containing 22; TRIM69, tripartite motif containing 69; USP18, ubiquitin-specific peptidase 18; VTRNA2-1, vault RNA 2-1, previously annotated as miR886.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Ballestar E. Epigenetic alterations in autoimmune rheumatic diseases. Nat Rev Rheumatol. 2011;7:263–71.
Gupta B, Hawkins RD. Epigenomics of autoimmune diseases. Immunol Cell Biol. 2015;93:271–6.
Feinberg AP, Koldobskiy MA, Gondor A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet. 2016;17:284–99.
Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27.
Jeffries MA, Sawalha AH. Autoimmune disease in the epigenetic era: how has epigenetics changed our understanding of disease and how can we expect the field to evolve? Expert Rev Clin Immunol. 2015;11:45–58.
•• Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, Shoresh N, Whitton H, Ryan RJ, Shishkin AA, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–43. The aim of the study is to identify the causality of the genome-wide association variants in autoimmune diseases using epigenetic marks. The study found that ~90% of causal variants are non-coding, with ~60% mapping to immune-cell enhancers and only 10–20% directly alter recognizable transcription factor binding motifs
Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–41.
• Teruel M, Alarcon-Riquelme ME. The genetic basis of systemic lupus erythematosus: what are the risk factors and what have we learned. J Autoimmun. 2016;74:161–75. Recent review about the genetic risk factors to SLE susceptibility identified across populations
Deng Y, Tsao BP. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol. 2010;6:683–92.
Deng Y, Tsao BP. Advances in lupus genetics and epigenetics. Curr Opin Rheumatol. 2014;26:482–92.
Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22.
Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990;33:1665–73.
Quddus J, Johnson KJ, Gavalchin J, Amento EP, Chrisp CE, Yung RL, Richardson BC. Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. J Clin Invest. 1993;92:38–53.
Yung RL, Quddus J, Chrisp CE, Johnson KJ, Richardson BC. Mechanism of drug-induced lupus. I. Cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025–35.
Richardson B, Sawalha AH, Ray D, Yung R. Murine models of lupus induced by hypomethylated T cells (DNA hypomethylation and lupus...). Methods Mol Biol. 2012;900:169–80.
Lu Q, Wu A, Richardson BC. Demethylation of the same promoter sequence increases CD70 expression in lupus T cells and T cells treated with lupus-inducing drugs. J Immunol. 2005;174:6212–9.
Lu Q, Kaplan M, Ray D, Ray D, Zacharek S, Gutsch D, Richardson B. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis Rheum. 2002;46:1282–91.
Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol. 2007;179:6352–8.
Sawalha AH, Jeffries M, Webb R, Lu Q, Gorelik G, Ray D, Osban J, Knowlton N, Johnson K, Richardson B. Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients. Genes Immun. 2008;9:368–78.
Gorelik G, Richardson B. Key role of ERK pathway signaling in lupus. Autoimmunity. 2010;43:17–22.
Javierre BM, Fernandez AF, Richter J, Al-Shahrour F, Martin-Subero JI, Rodriguez-Ubreva J, Berdasco M, Fraga MF, O'Hanlon TP, Rider LG, et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010;20:170–9.
Jeffries MA, Dozmorov M, Tang Y, Merrill JT, Wren JD, Sawalha AH. Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics. 2011;6:593–601.
Li Y, Liu Y, Strickland FM, Richardson B. Age-dependent decreases in DNA methyltransferase levels and low transmethylation micronutrient levels synergize to promote overexpression of genes implicated in autoimmunity and acute coronary syndromes. Exp Gerontol. 2010;45:312–22.
Coit P, Jeffries M, Altorok N, Dozmorov MG, Koelsch KA, Wren JD, Merrill JT, McCune WJ, Sawalha AH. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naive CD4+ T cells from lupus patients. J Autoimmun. 2013;43:78–84.
Absher DM, Li X, Waite LL, Gibson A, Roberts K, Edberg J, Chatham WW, Kimberly RP. Genome-wide DNA methylation analysis of systemic lupus erythematosus reveals persistent hypomethylation of interferon genes and compositional changes to CD4+ T-cell populations. PLoS Genet. 2013;9:e1003678.
Coit P, Yalavarthi S, Ognenovski M, Zhao W, Hasni S, Wren JD, Kaplan MJ, Sawalha AH. Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J Autoimmun. 2015;58:59–66.
Knight JS, Kaplan MJ. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr Opin Rheumatol. 2012;24:441–50.
Sukapan P, Promnarate P, Avihingsanon Y, Mutirangura A, Hirankarn N. Types of DNA methylation status of the interspersed repetitive sequences for LINE-1, Alu, HERV-E and HERV-K in the neutrophils from systemic lupus erythematosus patients and healthy controls. J Hum Genet. 2014;59:178–88.
Mavragani CP, Sagalovskiy I, Guo Q, Nezos A, Kapsogeorgou EK, Lu P, Liang Zhou J, Kirou KA, Seshan SV, Moutsopoulos HM, et al. Expression of long interspersed nuclear element 1 retroelements and induction of type I interferon in patients with systemic autoimmune disease. Arthritis & rheumatology. 2016;68:2686–96.
•• Coit P, Dozmorov MG, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, Wren JD, Sawalha AH. Epigenetic reprogramming in naive CD4+ T cells favoring T cell activation and non-Th1 effector T cell immune response as an early event in lupus flares. Arthritis & rheumatology. 2016;68:2200–9. The first genome-wide DNA methylation analysis in naïve CD4 + T cells from SLE patients with different degrees of disease activity in order to investigate the earliest T-cell epigenetic change associated with SLE disease flares
Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, Wei S, Crespo J, Wan S, Vatan L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol. 2016;17:95–103.
Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra218.
Gonzalez LA, Toloza SM, Alarcon GS. Impact of race and ethnicity in the course and outcome of systemic lupus erythematosus. Rheum Dis Clin N Am. 2014;40:433–54. vii-viii
Coit P, Renauer P, Jeffries MA, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, Sawalha AH. Renal involvement in lupus is characterized by unique DNA methylation changes in naive CD4+ T cells. J Autoimmun. 2015;61:29–35.
Mok A, Solomon O, Nayak RR, Coit P, Quach HL, Nititham J, Sawalha AH, Barcellos LF, Criswell LA, Chung SA. Genome-wide profiling identifies associations between lupus nephritis and differential methylation of genes regulating tissue hypoxia and type 1 interferon responses. Lupus science & medicine. 2016;3:e000183.
International Consortium for Systemic Lupus Erythematosus, G, Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–10.
Zhao M, Liu S, Luo S, Wu H, Tang M, Cheng W, Zhang Q, Zhang P, Yu X, Xia Y, et al. DNA methylation and mRNA and microRNA expression of SLE CD4+ T cells correlate with disease phenotype. J Autoimmun. 2014;54:127–36.
Fu Q, Zhao J, Qian X, Wong JL, Kaufman KM, Yu CY, Hwee Siew H, Tan Tock Seng Hospital Lupus Study, G, Mok MY, Harley JB, et al. Association of a functional IRF7 variant with systemic lupus erythematosus. Arthritis Rheum. 2011;63:749–54.
Kawasaki A, Furukawa H, Kondo Y, Ito S, Hayashi T, Kusaoi M, Matsumoto I, Tohma S, Takasaki Y, Hashimoto H, et al. Association of PHRF1-IRF7 region polymorphism with clinical manifestations of systemic lupus erythematosus in a Japanese population. Lupus. 2012;21:890–5.
Renauer P, Coit P, Jeffries MA, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, Sawalha AH. DNA methylation patterns in naive CD4+ T cells identify epigenetic susceptibility loci for malar rash and discoid rash in systemic lupus erythematosus. Lupus science & medicine. 2015;2:e000101.
Chung SA, Nititham J, Elboudwarej E, Quach HL, Taylor KE, Barcellos LF, Criswell LA. Genome-wide assessment of differential DNA methylation associated with autoantibody production in systemic lupus erythematosus. PLoS One. 2015;10:e0129813.
Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun. 2014;48-49:10–3.
Hughes T, Adler A, Merrill JT, Kelly JA, Kaufman KM, Williams A, Langefeld CD, Gilkeson GS, Sanchez E, Martin J, et al. Analysis of autosomal genes reveals gene-sex interactions and higher total genetic risk in men with systemic lupus erythematosus. Ann Rheum Dis. 2012;71:694–9.
Morris DL, Sheng Y, Zhang Y, Wang YF, Zhu Z, Tombleson P, Chen L, Cunninghame Graham DS, Bentham J, Roberts AL, et al. Genome-wide association meta-analysis in Chinese and European individuals identifies ten new loci associated with systemic lupus erythematosus. Nat Genet. 2016;48:940–6.
Taylor KE, Chung SA, Graham RR, Ortmann WA, Lee AT, Langefeld CD, Jacob CO, Kamboh MI, Alarcon-Riquelme ME, Tsao BP, et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 2011;7:e1001311.
Heyn H, Esteller M. DNA methylation profiling in the clinic: applications and challenges. Nat Rev Genet. 2012;13:679–92.
•• Zhao M, Zhou Y, Zhu B, Wan M, Jiang T, Tan Q, Liu Y, Jiang J, Luo S, Tan Y, et al. IFI44L promoter methylation as a blood biomarker for systemic lupus erythematosus. Ann Rheum Dis. 2016;75:1998–2006. First genome-wide DNA methylation study in whole blood to investigate whether specific gene methylation changes could meet sensitivity and specificity criteria for a robust diagnostic biomarker in SLE. The methylation level of IFI44L promoter was found to distinguish patients with SLE from healthy persons and other autoimmune diseases, and was a highly sensitive and specific diagnostic marker for SLE, suggesting this epigenetic mark as potential diagnostic biomarker of SLE
Sawalha AH, Webb R, Han S, Kelly JA, Kaufman KM, Kimberly RP, Alarcon-Riquelme ME, James JA, Vyse TJ, Gilkeson GS, et al. Common variants within MECP2 confer risk of systemic lupus erythematosus. PLoS One. 2008;3:e1727.
Webb R, Wren JD, Jeffries M, Kelly JA, Kaufman KM, Tang Y, Frank MB, Merrill J, Kimberly RP, Edberg JC, et al. Variants within MECP2, a key transcription regulator, are associated with increased susceptibility to lupus and differential gene expression in patients with systemic lupus erythematosus. Arthritis Rheum. 2009;60:1076–84.
Kaufman KM, Zhao J, Kelly JA, Hughes T, Adler A, Sanchez E, Ojwang JO, Langefeld CD, Ziegler JT, Williams AH, et al. Fine mapping of Xq28: both MECP2 and IRAK1 contribute to risk for systemic lupus erythematosus in multiple ancestral groups. Ann Rheum Dis. 2013;72:437–44.
Koelsch KA, Webb R, Jeffries M, Dozmorov MG, Frank MB, Guthridge JM, James JA, Wren JD, Sawalha AH. Functional characterization of the MECP2/IRAK1 lupus risk haplotype in human T cells and a human MECP2 transgenic mouse. J Autoimmun. 2013;41:168–74.
Zhang FF, Cardarelli R, Carroll J, Fulda KG, Kaur M, Gonzalez K, Vishwanatha JK, Santella RM, Morabia A. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics. 2011;6:623–9.
Heyn H, Moran S, Hernando-Herraez I, Sayols S, Gomez A, Sandoval J, Monk D, Hata K, Marques-Bonet T, Wang L, et al. DNA methylation contributes to natural human variation. Genome Res. 2013;23:1363–72.
Coit P, Ognenovski M, Gensterblum E, Maksimowicz-McKinnon K, Wren JD, Sawalha AH. Ethnicity-specific epigenetic variation in naive CD4+ T cells and the susceptibility to autoimmunity. Epigenetics Chromatin. 2015;8:49.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI097134 and award number U19AI110502. M.T. is supported by the Instituto de Salud Carlos III from Spain through the Sara Borrell subprogram (CD13/00316).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
A.H.S. is listed as inventor on a patent application to use IFI44L methylation as a diagnostic test for lupus.
M.T. declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Systemic Lupus Erythematosus
Rights and permissions
About this article
Cite this article
Teruel, M., Sawalha, A.H. Epigenetic Variability in Systemic Lupus Erythematosus: What We Learned from Genome-Wide DNA Methylation Studies. Curr Rheumatol Rep 19, 32 (2017). https://doi.org/10.1007/s11926-017-0657-5
Published:
DOI: https://doi.org/10.1007/s11926-017-0657-5