Abstract
Gout is characterized by the deposition of monosodium urate crystals and by acute and chronic inflammation in response to crystals so deposited. Multiple case reports and series describe the deposition of monosodium urate in the spine as a rare manifestation of gout, but the actual prevalence of spinal involvement is unknown and likely to be higher than generally anticipated. Here we review the characteristics of 131 previously reported cases of spinal involvement in gout. We focus in particular on the use of imaging modalities and the extent to which they correlate with presenting symptoms and tissue diagnoses. The recent innovation of using dual-energy computerized tomography to identify urate crystal deposition holds promise for reducing the need for surgical intervention and for establishing a true prevalence rate for spinal gout.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of gout, a monosodium urate crystal deposition disease, has been increasing, and gout now affects almost 4 % of all adults in the USA [1]. Gout most commonly affects the first metatarsophalangeal joint, knees, ankles, wrists, and hand joints. With unknown frequency, gout may also affect the spine. The first case of gout in the spine was published in 1950 [2]. Since then, an accruing series of cases has been reported in the literature, but limitations in diagnostic methodology, and lack of studies assessing for spinal gout in asymptomatic or mildly symptomatic patients, have made an accurate assessment of incidence and prevalence difficult to attain. Several authors have speculated that the incidence of spinal gout has been, and remains, more common than previously believed [3–5].
Comprehensive reviews have disagreed on the best approach to diagnosis and treatment of this condition. Although some authors have advocated for computerized tomography (CT) as the best method to identify spinal urate deposition [4, 6], others have asserted that imaging was inherently nonspecific. These authors believed that only a tissue sample could provide a correct diagnosis, despite the risks of surgery and low yield of biopsies [7]. In this manuscript, we review the literature concerning spinal gout, including symptomatology, abnormal laboratory results, pertinent imaging findings, diagnostic methods, and treatments. We then examine cutting-edge methods to diagnose spinal gout and possibly uncover the prevalence of this condition.
Methods
A literature search using the terms “spine,” “vertebral,” “tophus,” and “gout” was carried out using PubMed. Articles that discussed cases of gout involving the spinal column, including case reports, retrospective trials, and review articles, were included. Additional cases were added after cross-referencing previously published review articles. Articles describing gout affecting only the sacroiliac joints (37 cases) [5, 18, 66] were excluded, in order to focus specifically on cases involving the spinal column.
A total of 133 cases of spinal gout were identified. Two cases were excluded (one was in another language and the other could not be accessed), leaving 131 total cases that were included in the review [1–132]. Ours is the most comprehensive review of spinal gout to date.
Results
Demographics
Of the 131 cases reported, 129 included a gender. Ninety-eight (76 %) of those 129 were males and 31 (24 %) were females. In two cases, the gender was not reported. The ages ranged from 17–87, with a median age of 58.9 (with most cases occurring between 44 and 74 years).
Symptoms
Patients with spinal gout presented variably, with acute, subacute, or chronic symptoms. In two cases, patients had no symptoms and the diagnosis was made incidentally on autopsy for other reasons [43, 73]. Symptom chronicity ranged from 1 day to 6 years [92, 112]. One patient had new MRI lesions develop over only 3 months, concomitantly with her symptoms. [33]
Of the total 131 cases, 130 mentioned presenting symptoms of the patient. The most common symptom was back pain, which was present in 89 (68.5 %) cases. When present, back pain was usually but not consistently reported in the general area of the spinal level affected by urate deposition. Tkach et al. found a frequent coexistence of primary osteoarthritis and gout in patients who had pain in their spines, but whether the pain was due to gout, osteoarthritis, or both was not made clear [113]. The second most common complaint was neurological impairment. Patients variously presented with radiculopathy, loss of sensation, motor weakness, bowel/bladder dysfunction, or quadriparesis. Not surprisingly, back pain and neurologic impairment were not mutually exclusive. Eighty-five (65.4 %) of the 130 cases where the presenting symptom was mentioned had neurological findings, either as a symptom or as a sign on physical exam. These neurologic symptoms or signs were frequently concerning for compression of the spinal cord or exiting spinal nerves by the mass (ultimately identified as tophus), prompting the pursuit of tissue diagnosis, often in the context of surgical management. In many cases, diagnosis of spinal urate deposition was accomplished in the course of a workup over concern for a mass or tumor. Aside from back pain and/or neurologic involvement, no other indications for referral were reported.
Of the 131 total cases, 129 included mention of the spinal level where the gouty tophus was found. Most cases (80.6 %) only involved one region of the spine. In 32 (24.8 %) cases, the tophus affected only the cervical spine. The lumbar spine alone was affected in 49 (38.0 %) cases. The thoracic spine alone was involved in 23 (17.8 %) cases. Eighteen (14.0 %) cases affected both the lumbar and upper sacral (S1) spine. A single (0.7 %) case involved both cervical and thoracic spine. Six (4.7 %) cases affected both thoracic and lumbar spine. There were two cases where the spinal level was not mentioned. When including the patients with multiple spinal regions involved, 33 patients had gouty tophi in the cervical spine, 73 patients had lumbar spine pathology, and 29 cases had gout in their thoracic spine.
Prior History of Gout
Of the 131 cases, 122 addressed a past history of gout. The majority of patients (75.4 %) had a history of gout or the known gout risk factor of hyperuricemia. Eighty-seven patients (71.3 %) had pre-existing gout, 5 (4.1 %) had recognized hyperuricemia without gouty attacks, and 30 (24.6 %) had no prior history of either gout or hyperuricemia. However, it is likely that many patients without a known previous history of gout had not had a prior serum urate level, resulting in underestimation of the prevalence of pre-existing hyperuricemia. Nine of the case reports made no mention of any prior history. Among the 100 gout patients for whom the presence or absence of visible peripheral tophi was reported, 59 had tophi on exam and 41 did not have tophi; there was no mention in the remaining 31.
Regarding risk factors for gout, there were 21 cases with a history of kidney disease and 4 cases that had received a renal transplant after end-stage renal disease: two due to IgA nephropathy [3, 9], one due to progressive membranoproliferative glomerulonephritis [45], and one without clear reason for renal failure [94].
Laboratory Values
The most frequently abnormal values encountered in patients with spinal gout included the serum uric acid (sUA) concentration, the erythrocyte sedimentation rate (ESR), the C-reactive protein (CRP), the white blood count (WBC), and the serum creatinine (Cr). For the purposes of analyzing and unifying the data, we defined the normal value for uric acid as <7 mg/dL, the normal ESR as <20 mm/h, the normal CRP as <3 mg/L, the normal WBC as <12,000/μL, and the normal Cr as ≤1.2 mg/dL.
Of the 131 cases reviewed, the sUA was reported in 104. Of those cases, 18 (17.3 %) had a sUA <7 mg/dL and 83 (79.8 %) had a sUA >7 mg/dL. In three (2.9 %) additional cases, the sUA was reported to be normal but a numeric value was not provided. The median sUA was 9.9 mg/dL (range, 1.9–19.1 mg/dL) [29, 64]. Thus, patients with spinal gout often but not always had an elevated sUA at the time of evaluation, and a normal sUA did not exclude spinal gout from the diagnosis.
ESR and CRP were also found to be elevated in some cases. The ESR was only provided in 36 (27.5 %) cases; in 33 of those (92 %), the ESR was elevated, and the mean ESR was 75.9 mm/h. CRP was similarly mentioned in only 37 (28.2 %) cases but was elevated in 34 of those (92 %), with a mean of 117.13 mg/L. In the aforementioned cases, 15 had an elevation in both ESR and CRP.
The WBC was reported in 49 of the 131 cases. It was elevated in 14 (28.6 %) cases and normal in 27 (55.1 %), with 8 (16.3 %) additional cases listed as normal without a numeric value provided. In 82 cases, the WBC was not mentioned. The average WBC was 11.7. Eleven cases reported concurrent elevations in WBC, ESR, and CRP; in the other cases with an elevated WBC, the ESR and CRP were not reported.
The serum Cr was mentioned in 52 of the 131 cases. It was >1.2 mg/dL in 40 (76.9 %) of the 52 cases, ≤1.2 mg/dL in four (7.7 %) of those cases, not mentioned but presumed elevated in one (1.9 %) patient who had end-stage renal disease, and listed as normal without a value in seven (13.5 %) cases. The Cr was not mentioned in the remaining 79 cases.
Overall, the laboratory abnormalities were often consistent with an inflammatory state such as is often seen in acute gouty arthropathy in other joints. However, 13 of the 23 cases whose initial presentation included fever also had an elevation in ESR, CRP, or WBC, if not all three. In these cases, infection was higher on the differential than gout, making the diagnosis without a tissue sample more challenging.
Imaging Modalities
The imaging studies most often performed on patients with spinal gout were plain radiographs, magnetic resonance imaging (MRI), and computed tomography (CT) (including scans with contrast, positron emission tomography, and dual-energy formats). Much less frequently, patients had myelography or technetium bone scans. In addition to spinal imaging, many of the patients who had peripheral arthritis symptoms also had plain radiographs of those joints.
Sixty-three patients underwent plain radiography of the spine, with 51 (81.0 %) of the 63 having abnormal radiographs. The other 12 (19 %) had unremarkable findings on their X-rays. Generally speaking, many of the abnormalities reported on plain radiographs were nonspecific for spinal gout. Abnormalities reported include spondylosis [21], spondylolisthesis [115], degenerative changes [11], and diffuse spinal hyperostosis [50]. In other cases, radiographs were read as normal [116]. Patients with severe neurological symptoms were often the ones with the most abnormal X-ray findings, showing focal narrowing or destructive changes, including atlanto-axial subluxation [124]. In subsequent evaluation, these findings were typically associated with large invasive tophi. However, there was no consistent relationship between the X-ray findings and the laboratory results.
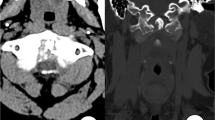
MRI with gadolinium contrast was the most common method used to evaluate patients with spinal gout; 90 of the cases received an MRI, and 89 (98.9 %) of those cases were abnormal, revealing a high degree of sensitivity. However, findings on MRI were usually nonspecific, with only 19 (21 %) of the 90 cases interpreted specifically as a gouty or tophaceous lesion (Fig. 1) [17–19, 28, 47, 75, 88, 90, 103, 104, 107, 112, 114, 116, 125]. Changes on MRI may mimic osteomyelitis or tuberculosis, creating a challenge for making a specific diagnosis. Although classically believed to be hypointense on T1 and hyperintense on T2 [114], tophi were variously reported as hypointense or isointense soft tissue densities on T1 and ranged from hypointense to hyperintense onT2 [17, 47, 66, 112]. Among patients who received gadolinium contrast as part of their MRI, tophi often demonstrated either homogenous or heterogeneous peripheral enhancement [3, 5, 7, 11, 76, 93].
a Presence of a mass in the lumbosacral region of the spine, as seen on MRI in coronal and sagittal views (mass indicated by white arrows). b Confirmation of mass as a tophus through surgical biopsy and examination of recovered material under polarizing microscopy. Reprinted from Samuels et al. [101] (with permission)
CT appears to be more sensitive and specific than plain radiography for the diagnosis of spinal gout, but given the fact that most studies involved case reports, an accurate denominator is lacking. Fifty-seven (96.6 %) of the 59 CT scans performed on patients with spinal gout were read as abnormal, with 11 (18.6 %) scans reported as highly suspicious for tophaceous gout [37, 42, 49, 57, 66, 75, 86, 88, 90, 108, 112]. The most common findings included bone or joint erosions with well-defined sclerotic margins, facet or intervertebral bone neoformation, or juxta- or intra-articular masses that were denser than the surrounding muscle [4, 132]. Other CT findings that were less frequently reported included degenerative changes (5 cases) [120], lytic lesions(18 cases) [17, 39, 96], and spinal stenosis (7 cases) [14, 123]. One subject underwent a PET-CT to evaluate spinal gout, which revealed increased uptake around the lesion [18], consistent with the fact that tophi are complex structures that include surrounding zones of metabolically active leukocytes and fibroblasts [22]. Variability of standard CT findings appears to depend in part upon where the uric acid crystals deposit within the joints. For example, epidural compression by a mass can easily be confused for a tumor or abscess and cannot be differentiated without a tissue diagnosis.
A newer method of imaging gout is dual-energy CT (DECT) scanning. DECT scanners capture images at two separate energy levels, compared to the standard single-energy CT. The separate energy levels allow substances of different chemical composition to appear distinct based on their differential X-ray photon energy [87], and more effectively differentiate tophi from other types of masses. Using DECT, researchers have been able to identify subclinical gouty tophi and directly measure their volume [23]. In a recent study, Hu et al. found the sensitivity and specificity of DECT in identifying gouty tophi at 91.9 and 85.4 %, respectively, when adjusting certain CT scanner settings for performing imaging and analysis [48]. More recent studies suggest that DECT may be less sensitive at identifying diffuse as opposed to dense lesions [26]. To date, most reports and studies of DECT scanning in gout have focused on tophi in peripheral appendages.
In two reported cases, DECT has been used as part of the workup of the spinal gout patient. Dhaese et al. used DECT to confirm urate deposition in the costovertebral joints of the thoracic spine and treated the patient conservatively, with complete resolution of all symptoms (Fig. 2) [29]. Similarly, Parikh et al. employed DECT on a patient who had gout with worsening back pain and discovered deposition of uric acid crystals in the lumbar facet joints [93]. While the urate depositions were clearly seen on DECT, a conventional CT image only showed nonspecific areas of mildly increased attenuation where the tophi were located. This patient was treated conservatively and improved.
Presence of monosodium urate crystal deposition at the level of thoracic vertebrae 1 and 2, on axial projection, imaged using dual-energy computed tomography. In this image, the green areas are indicative of uric acid. From Dhaese et al. [29] (reproduced with permission of Lippincott Williams & Wilkins in the format journal/magazine via Copyright Clearance Center)
The relative specificity of DECT may permit, for the first time, an assessment of the actual prevalence of spinal involvement in gout. In a report published in abstract form, Law et al. performed DECT of the thoracic and lumbar spine on 17 patients with tophaceous gout. They observed that eight (47 %) patients had DECT evidence of MSU deposits, most commonly in the lumbar spine [69]. Back pain was reported to be a common complaint, but actual prevalence of back pain was not provided. These observations suggest that, at least among gout patients with peripheral tophi, spinal deposition of urate may be more common than previously appreciated, may contribute to the total body burden of urate, and may contribute to back pain.
Diagnosis
Definitive diagnosis of the tophus was most commonly made during surgery, revealing a white chalky mass visualized macroscopically, and negatively birefringent urate crystals seen microscopically under polarizing conditions (Fig. 1). Surgery was performed in 75 cases. An additional 32 cases were diagnosed via needle aspiration or bone biopsy of the suspected lesions. Eleven cases were diagnosed without tissue by consideration of the overall clinical picture, including history, physical exam, elevated uric acid, and abnormal imaging findings. Four additional cases diagnosed the patient on autopsy with tophi found in the spine [2, 43, 67, 73]. In three cases, the patient was diagnosed indirectly by obtaining crystals from other joints, combined with abnormal findings on imaging studies and a high suspicion for spinal gout [71, 104, 125]. In six reports, the means of diagnosis was not mentioned.
Treatment
Forty-three (38 % of all treated patients) cases of spinal gout were reported to be managed with surgery alone; 26 (23 % of all treated patients) cases were managed with a combination of surgery and pharmacologic treatment. Forty-two patients (37.2 % of all treated patients) were treated conservatively (i.e., nonsurgically) with various combinations of colchicine, steroids, allopurinol, and/or febuxostat. Two (1.8 % of all treated patients) patients failed initial conservative treatment and were subsequently treated surgically with improvement in their symptoms. Four patients died of other causes prior to receiving treatment. In 14 reports, treatment information was not provided. Forty (37.7 %) patients had complete resolution of symptoms with treatment. Fifty-five (51.9 %) patients noted some improvement without complete mention of resolution. Two (1.9 %) patients did not improve after being treated, and nine (8.5 %) died of various causes (one after an initial report of gout improvement). Of those reported to be treated only surgically, 18 (47.4 %) had complete resolution of symptoms, 18 (47.4 %) improved, and 2 (5.2 %) died post-operatively (both of pneumonia). No data was available regarding symptom resolution in the remaining five patients treated only surgically. It is possible that some of these patients were subsequently treated medically but that the results were not reported. Among those subjects reported to be treated first surgically, then medically, four (15.4 %) cases completely resolved, 19 (73 %) cases improved, one (3.8 %) died from post-operative infection, one (3.8 %) did not improve, and one case had no treatment outcomes mentioned. Of those patients treated medically only, the outcome was mentioned in 38 of the 42 cases. Seventeen (44.7 %) of those 38 cases had complete resolution of symptoms, 18 (47.4 %) cases improved, one (2.6 %) initially improved but died from aspiration pneumonia, another case (2.6 %) died of pneumonia, and one (2.6 %) case did not improve. In the remaining four cases, the authors did not provide an outcome.
Based on the above data, conservative medical treatment appears to be as effective as surgical treatment while avoiding post-operative complications. Often, patients who presented with focal neurologic deficits received surgery for salvage. Fifty-nine (84.3 %) patients with neurological symptoms were treated with surgery (with or without medications), compared to only 11 (15.7 %) without neurological findings who received surgery. On the other hand, patients who had no neurological findings were more likely to be treated conservatively. Twenty-five (59.5 %) patients whose chief complaint was back pain without neurologic deficits were treated surgically, in contrast to 17 (40.5 %) with neurological findings who were treated conservatively.
Discussion
Spinal gout can present in a variety of ways. Symptoms—mainly back pain, and/or nerve or spine compression—are not specific for gout and can occur within a short time frame or develop over many years. Patients most commonly have an extensive prior history of gout or hyperuricemia, but spinal involvement can also be the first manifestation of gouty symptoms. The uric acid is usually elevated, as are inflammatory markers such as ESR, CRP, and WBC. However, none of these satisfactorily excludes infection from the differential diagnosis, and vigilance is required, especially if a patient presents with fever.
Although a variety of imaging studies have been used to evaluate spinal gout, most are nonspecific and cannot definitively exclude other causes of spinal mass. Therefore, imaging usually leads to a more invasive measure to diagnose the condition, such as open surgery or needle biopsy (which may not yield diagnostic tissue or may require multiple attempts) [3], to differentiate gout from infection or tumor. DECT is a newer imaging modality that is both sensitive and specific for monosodium urate deposition in peripheral joints. Further study is warranted to determine whether the sensitivity and specificity of DECT are sufficient to obviate the need for invasive procedure and tissue diagnosis in the case of a spinal mass observed on imaging. At the present time, however, DECT is not widely available and is more expensive than conventional CT, and many radiologists will need to be trained before they can effectively read the images. [48]
In gout patients who present with back pain not responding to conservative measures, urate crystal deposition in the spine should be higher on the list of differentials, and DECT should be considered to work up the patient, if available. If DECT is not available, CT and/or MRI with biopsy (if needed) can also be used. Treatment for nonurgent cases should initially be conservative and resemble the treatment of other forms of chronic gout, with anti-inflammatory prophylaxis accompanied by urate lowering therapy, e.g., a xanthine oxidase inhibitor (allopurinol or febuxostat) with or without probenecid, or pegloticase in the case of inadequate urate lowering with the initial regimen. If symptoms do not improve, surgery may be the next step. In patients with urgent neurological symptoms, especially evidence of spinal compression, surgery may be the first treatment option in order to remove the gouty mass.
The prevalence of spinal gout is still unknown, and the use of conventional imaging modalities has shed only limited light on the question. Among a sample of patients with gout, Jajic et al. reported that 12 of 54 (22 %) had abnormal radiographs with hyperostotic spondylosis or diffuse idiopathic spinal hyperostosis [50]. The results suggest that gout patients commonly have spine disease, but the findings they report are not specific to gout and do not directly reflect the presence of urate. Konatalapalli et al. studied the prevalence of gout with a retrospective study using CT scans and reported that nine of 64 (14 %) gout patients in the study had evidence of uric acid crystal deposition on their CT scans, while the remainder did not have the characteristic findings [4]. The lumbar spine was most commonly affected, occurring in 80 % of all abnormal CT scans. Of the patients studied, only three had gotten CT scans for back pain, while the rest had gotten CT scans for other indications. Of those three patients, two had no CT findings of spinal gout, and only one had a CT consistent with axial gout. However, the study had a small sample size and had no histological confirmation making the data more difficult to interpret. Although the number of subjects was once again small, the unpublished DECT study by Law et al. suggests that spinal gout involvement may be much more common than previously appreciated, possibly approaching 50 % in patients with clinically appreciated tophi [69].
Overall, we conclude that spinal involvement in patients with gout may be common, may be an underappreciated source of axial pain, and may require consideration as a urate reservoir and a barrier to urate lowering therapy. Additional DECT studies are clearly warranted, both to better understand the modality and to better understand the incidence and prevalence of spinal gout.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41.
Kersley GD, Mandel L, Jeffrey MR. Gout: an unusual case with softening and subluxation of the first cervical vertebra and splenomegaly. Ann Rheum Dis. 1950;9:282–304.
Hou LC, Hsu AR, Veeravagu A, Boakye M. Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol. 2007;67:65–73. Case report with a literature review of axial gout. In addition to quite large number of cases reviewed, also provided a useful algorithm for approaching a patient with suspected spinal gout. The authors of this study recommended surgical intervention in all patients with neurological symptoms. They also believed that for patients without neurological symptoms, initial workup with MRI followed by biopsy for diagnosis.
Konatalapalli RM, Demarco PJ, Jelinek JS, et al. Gout in the axial skeleton. J Rheumatol. 2009;36:609–13. Review of medical records of gout patients looking at those who had CT images of the spine for any reason in the past. Results showed that 9 of 64 (14%) patients had evidence of axial gout on spinal CT. However, only one of those 9 cases had gotten a CT for back pain, raising the question of how common asymptomatic spinal gout may be.
Saketkoo LA, Robertson HJ, Dyer HR, Virk ZU, Ferreyro HR, Espinoza LR. Axial gouty arthropathy. Am J Med Sci. 2009;338:140–6.
de Mello FM, Helito PV, Bordalo-Rodrigues M, Fuller R, Halpern AS. Axial gout is frequently associated with the presence of current tophi, although not with spinal symptoms. Spine (Phila Pa 1976). 2014;39:E1531–6.
Hasegawa EM, de Mello FM, Goldenstein-Schainberg C, Fuller R. Gout in the spine. Rev Bras Reumatol. 2013;53:296–302. Recent review of axial gout from Brazil that highlights the fact that axial gout may be more common than previously thought, and should be considered in patients with longstanding gout who present with acute back pain. The authors advocated for reserving surgery for patients who had failed medical therapy, or those with neurological impairments, and treating everyone else conservatively.
Adenwalla HN, Usman MH, Baqir M, Zulqarnain M, Shah H. Vertebral gout and ambulatory dysfunction. South Med J. 2007;100:413–4.
Ahmad I, Tejada JG. Spinal gout: a great mimicker. A case report and literature review. Neuroradiol J. 2012;25:621–5.
Alarcon GS, Reveille JD. Gouty arthritis of the axial skeleton including the sacroiliac joints. Arch Intern Med. 1987;147:2018–9.
Arnold MH, Brooks PM, Savvas P, Ruff S. Tophaceous gout of the axial skeleton. Aust N Z J Med. 1988;18:865–7.
Barrett K, Miller ML, Wilson JT. Tophaceous gout of the spine mimicking epidural infection: case report and review of the literature. Neurosurgery. 2001;48:1170–2. discussion 2-3.
Beier CP, Hartmann A, Woertgen C, Brawanski A, Rothoerl RD. A large, erosive intraspinal and paravertebral gout tophus Case repot. J Neurosurg Spine. 2005;3:485–7.
Bonaldi VM, Duong H, Starr MR, Sarazin L, Richardson J. Tophaceous gout of the lumbar spine mimicking an epidural abscess: MR features. AJNR Am J Neuroradiol. 1996;17:1949–52.
Bret P, Ricci AC, Saint-Pierre G, Mottolese C, Guyotat J. Thoracic spinal cord compression by a gouty tophus. Case report. Review of the literature. Neurochirurgie. 1999;45:402–6.
Buenzli D, So A. Inflammatory sciatica due to spinal tophaceous gout. BMJ Case Rep 2009;2009.
Cabot J, Mosel L, Kong A, Hayward M. Tophaceous gout in the cervical spine. Skelet Radiol. 2005;34:803–6.
Cardoso FN, Omoumi P, Wieers G, et al. Spinal and sacroiliac gouty arthritis: report of a case and review of the literature. Acta Radiol Short Rep 2014;3:2047981614549269.
Celik SE, Gorgulu M. Tophaceous gout of the atlantoaxial joint case illustration. J Neurosurg Spine. 2005;2:230.
Chan AT, Leung JL, Sy AN, et al. Thoracic spinal gout mimicking metastasis. Hong Kong Med J. 2009;15:143–5.
Chang IC. Surgical versus pharmacologic treatment of intraspinal gout. Clin Orthop Relat Res 2005;106-10.
Chhana A, Dalbeth N. The gouty tophus: a review. Curr Rheumatol Rep. 2015;17:19.
Choi HK, Al-Arfaj AM, Eftekhari A, et al. Dual energy computed tomography in tophaceous gout. Ann Rheum Dis. 2009;68:1609–12.
Clerc D, Marfeuille M, Labous E, Desmoulins F, Quillard J, Bisson M. Spinal tophaceous gout. Clin Exp Rheumatol. 1998;16:621.
Coulier B, Tancredi MH. Articular tophaceous gout of the cervical spine: CT diagnosis. JBR-BTR. 2010;93:325.
Dalbeth N, House ME, Aati O, et al. Urate crystal deposition in asymptomatic hyperuricaemia and symptomatic gout: a dual energy CT study. Ann Rheum Dis. 2015;74:908–11.
Das DS. Intervertebral disc involvement in gout: brief report. J Bone Joint Surg (Br). 1988;70:671.
de Parisot A, Ltaief-Boudrigua A, Villani AP, Barrey C, Chapurlat RD, Confavreux CB. Spontaneous odontoid fracture on a tophus responsible for spinal cord compression: a case report. Joint Bone Spine. 2013;80:550–1.
Dhaese S, Stryckers M, Van Der Meersch H, Terryn W, Van Laecke S. Gouty arthritis of the spine in a renal transplant patient: a clinical case report: an unusual presentation of a common disorder. Medicine (Baltimore). 2015;94:e676.
Dharmadhikari R, Dildey P, Hide IG. A rare cause of spinal cord compression: imaging appearances of gout of the cervical spine. Skelet Radiol. 2006;35:942–5.
Dhote R, Roux FX, Bachmeyer C, Tudoret L, Daumas-Duport C, Christoforov B. Extradural spinal tophaceous gout: evolution with medical treatment. Clin Exp Rheumatol. 1997;15:421–3.
Diaz A, Porhiel V, Sabatier P, et al. Tophaceous gout of the cervical spine, causing cord compression. Case report and review of the literature. Neurochirurgie. 2003;49:600–4.
Draganescu M, Leventhal LJ. Spinal gout: case report and review of the literature. J Clin Rheumatol. 2004;10:74–9.
Dubey D, Steen E, Sawhney A, Stuve O. Spinal gout: a rare cause of paraplegia. Neurol India. 2015;63:284–5.
Duprez TP, Malghem J, Vande Berg BC, Noel HM, Munting EA, Maldague BE. Gout in the cervical spine: MR pattern mimicking diskovertebral infection. AJNR Am J Neuroradiol. 1996;17:151–3.
El Sandid M, Ta H. Another presentation of gout. Ann Intern Med. 2004;140:W32.
Fenton P, Young S, Prutis K. Gout of the spine. Two case reports and a review of the literature. J Bone Joint Surg Am. 1995;77:767–71.
Fontenot A, Harris P, Macasa A, Menon Y, Quinet R. An initial presentation of polyarticular gout with spinal involvement. J Clin Rheumatol. 2008;14:188–9.
Fraser JF, Anand VK, Schwartz TH. Endoscopic biopsy sampling of tophaceous gout of the odontoid process. Case report and review of the literature. J Neurosurg Spine. 2007;7:61–4.
Funck-Brentano T, Salliot C, Leboime A, et al. First observation of the efficacy of IL-1ra to treat tophaceous gout of the lumbar spine. Rheumatology (Oxford). 2011;50:622–4.
Gines R, Bates DJ. Tophaceous lumbar gout mimicking an epidural abscess. Am J Emerg Med. 1998;16:216.
Gongidi P, Gough-Fibkins S. Spondyloarthritis: a gouty display. J Radiol Case Rep. 2010;4:13–8.
Hall MC, Selin G. Spinal Involvement in Gout1960.
Hasturk AE, Basmaci M, Canbay S, Vural C, Erten F. Spinal gout tophus: a very rare cause of radiculopathy. Eur Spine J. 2012;21 Suppl 4:S400–3.
Hausch R, Wilkerson M, Singh E, Reyes C, Harrington T. Tophaceous gout of the thoracic spine presenting as back pain and fever. J Clin Rheumatol. 1999;5:335–41.
Hongli W, Jianyuan J. Re. Axial gout is frequently associated with the presence of current tophi, although not with spinal symptoms. Spine (Phila Pa 1976) 2015;40:587.
Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW. Tophaceous gout of the spine: MR imaging features. Clin Radiol. 2002;57:919–25.
Hu HJ, Liao MY, Xu LY. Clinical utility of dual-energy CT for gout diagnosis. Clin Imaging 2015.
Jacobs SR, Edeiken J, Rubin B, DeHoratius RJ. Medically reversible quadriparesis in tophaceous gout. Arch Phys Med Rehabil. 1985;66:188–90.
Jajic I. Gout in the spine and sacro-iliac joints: radiological manifestations. Skelet Radiol. 1982;8:209–12.
Jegapragasan M, Calniquer A, Hwang WD, Nguyen QT, Child Z. A case of tophaceous gout in the lumbar spine: a review of the literature and treatment recommendations. Evid Based Spine Care J. 2014;5:52–6.
Kao MC, Huang SC, Chiu CT, Yao YT. Thoracic cord compression due to gout: a case report and literature review. J Formos Med Assoc. 2000;99:572–5.
Kara A, McQuillan P, Dhaliwal G. A multifaceted case. J Hosp Med. 2013;8:267–70.
Kaye PV, Dreyer MD. Spinal gout: an unusual clinical and cytological presentation. Cytopathology. 1999;10:411–4.
Kelly J, Lim C, Kamel M, Keohane C, O'Sullivan M. Topacheous gout as a rare cause of spinal stenosis in the lumbar region Case report. J Neurosurg Spine. 2005;2:215–7.
Kern A, Schunk K, Thelen M. Gout in the area of the cervical vertebrae and the sternoclavicular joint. Röfo. 1999;170:515–7.
King JC, Nicholas C. Gouty arthropathy of the lumbar spine: a case report and review of the literature. Spine (Phila Pa 1976). 1997;22:2309–12.
Ko KH, Huang GS, Chang WC. Clinical images: lumbar spondylolisthesis caused by tophaceous gout. Arthritis Rheum. 2009;60:198.
Ko KH, Huang GS, Chang WC. Tophaceous gout of the lumbar spine. J Clin Rheumatol. 2010;16:200.
Ko PJ, Huang TJ, Liao YS, Hsueh S, Hsu RW. Recurrent spinal stenosis caused by tophaceous gout: a case report and review of literature. Changgeng Yi Xue Za Zhi. 1996;19:272–6.
Komarla A, Schumacher R, Merkel PA. Spinal gout presenting as acute low back pain. Arthritis Rheum. 2013;65:2660.
Konatalapalli RM, Lumezanu E, Jelinek JS, Murphey MD, Wang H, Weinstein A. Correlates of axial gout: a cross-sectional study. J Rheumatol. 2012;39:1445–9.
Koskoff YD, Morris LE, Lubic LG. Paraplegia as a complication of gout. J Am Med Assoc. 1953;152:37–8.
Krishnakumar R, Renjitkumar J. Tophaceous gout of the spine masquerading as spondylodiscitis. Indian J Med Res. 2013;137:566–7.
Kuo YJ, Chiang CJ, Tsuang YH. Gouty arthropathy of the cervical spine in a young adult. J Chin Med Assoc. 2007;70:180–2.
Kwan BY, Osman S, Barra L. Spinal gout in a young patient with involvement of thoracic, lumbar and sacroiliac regions. Joint Bone Spine. 2013;80:667–8.
Lagier R, Mac GW. Spondylodiscal erosions due to gout: anatomico-radiological study of a case. Ann Rheum Dis. 1983;42:350–3.
Lam HY, Cheung KY, Law SW, Fung KY. Crystal arthropathy of the lumbar spine: a report of 4 cases. J Orthop Surg (Hong Kong). 2007;15:94–101.
Law G, Abufayyah M, Shojania K, Choi H, Nicolaou S, Reid G, et al. Dual energy computed tomography scans of the spine in patients with tophaceous gout. Ann Rheum Dis. 2011;70:152.
Leaney BJ, Calvert JM. Tophaceous gout producing spinal cord compression. Case report. J Neurosurg. 1983;58:580–2.
Lee YA, Lee SH, Yang HI, Hong SJ. Clinical images: aggressive gouty arthritis with concurrent involvement of the ankle and cervical spine. Arthritis Rheum. 2009;60:3581.
Levin E, Hurth K, Joshi R, Brasington R. Acute presentation of tophaceous myelopathy. J Rheumatol. 2011;38:1525–6.
Levin MH, Lichtenstein L, Scott HW. Pathologic changes in gout; survey of eleven necropsied cases. Am J Pathol. 1956;32:871–95.
Litvak J, Briney W. Extradural spinal depositions of urates producing paraplegia Case report. J Neurosurg. 1973;39:656–8.
Lu F, Jiang J, Zhang F, Xia X, Wang L, Ma X. Lumbar spinal stenosis induced by rare chronic tophaceous gout in a 29-year-old man. Orthopedics. 2012;35:e1571–5.
Lumezanu E, Konatalapalli R, Weinstein A. Axial (spinal) gout. Curr Rheumatol Rep. 2012;14:161–4. Review of axial gout that discussed clinical features, imaging, pathology, characteristics of patients and treatment options. Authors argued for CT as most definitive non-surgical diagnostic method, with characteristic findings, and also mentioned dual energy CT as a sensitive tool for diagnosis.
Magid SK, Gray GE, Anand A. Spinal cord compression by tophi in a patient with chronic polyarthritis: case report and literature review. Arthritis Rheum. 1981;24:1431–4.
Mahmud T, Basu D, Dyson PH. Crystal arthropathy of the lumbar spine: a series of six cases and a review of the literature. J Bone Joint Surg (Br). 2005;87:513–7.
Marinho F, Zeitoun-Eiss D, Renoux J, Brasseur JL, Genestie C, Grenier P. Tophaceous gout of the spine: case report and review of the literature. J Neuroradiol. 2012;39:123–6.
Marsaudon E, Bouchard C, Langand D. Spinal cord compression due to tophaceous vertebral gout: a case report and literature review. Rev Med Interne. 1999;20:253–7.
Mekelburg K, Rahimi AR. Gouty arthritis of the spine: clinical presentation and effective treatments. Geriatrics. 2000;55:71–4.
Miller JD, Percy JS. Tophaceous gout in the cervical spine. J Rheumatol. 1984;11:862–5.
Miller LJ, Pruett SW, Losada R, Fruauff A, Sagerman P. Clinical image. Tophaceous gout of the lumbar spine: MR findings. J Comput Assist Tomogr. 1996;20:1004–5.
Mrabet D, Monastiri I, Sahli H, et al. Uncommon feature of gout effecting the spine. Tunis Med. 2010;88:966–7.
Murphy DJ, Shearman AL, Mascarenhas R, Haigh RC. Lucent lesions of the spine—a case of spinal gout. Age Ageing. 2010;39:660.
Murshid WR, Moss TH, Ettles DF, Cummins BH. Tophaceous gout of the spine causing spinal cord compression. Br J Neurosurg. 1994;8:751–4.
Nicolaou S, Liang T, Murphy DT, Korzan JR, Ouellette H, Munk P. Dual-energy CT: a promising new technique for assessment of the musculoskeletal system. AJR Am J Roentgenol. 2012;199:S78–86.
Niva M, Tallroth K, Konttinen YT. Tophus in the odontoid process of C2. Clin Exp Rheumatol. 2006;24:112.
Ntsiba H, Makosso E, Moyikoua A. Thoracic spinal cord compression by a tophus. Joint Bone Spine. 2010;77:187–8.
Nunes EA, Rosseti AG, Jr., Ribeiro DS, Santiago M. Gout initially mimicking rheumatoid arthritis and later cervical spine involvement. Case Rep Rheumatol 2014;2014:357826.
Nygaard HB, Shenoi S, Shukla S. Lower back pain caused by tophaceous gout of the spine. Neurology. 2009;73:404.
Paquette S, Lach B, Guiot B. Lumbar radiculopathy secondary to gouty tophi in the filum terminale in a patient without systemic gout: case report. Neurosurgery. 2000;46:986–8.
Parikh P, Butendieck R, Kransdorf M, Calamia K. Detection of lumbar facet joint gouty arthritis using dual-energy computed tomography. J Rheumatol. 2010;37:2190–1.
Peeters P, Sennesael J. Low-back pain caused by spinal tophus—a complication of gout in a kidney transplant recipient. Nephrol Dial Transplant. 1998;13:3245–7.
Pfister AK, Schlarb CA, O'Neal JF. Vertebral erosion, paraplegia, and spinal gout. AJR Am J Roentgenol. 1998;171:1430–1.
Popovich T, Carpenter JS, Rai AT, Carson LV, Williams HJ, Marano GD. Spinal cord compression by tophaceous gout with fluorodeoxyglucose-positron-emission tomographic/MR fusion imaging. AJNR Am J Neuroradiol. 2006;27:1201–3.
Reynolds Jr AF, Wyler AR, Norris HT. Paraparesis secondary to sodium urate deposits in the ligamentum flavum. Arch Neurol. 1976;33:795.
Rivero-Garvia M, Rodriguez Boto G, Gutierrez Gonzalez R, Martinez A. [Tophaceous gout causing stenosis of T11-T12]. Med Clin (Barc). 2008;130:479.
Sabharwal S, Gibson T. Cervical gout. Br J Rheumatol. 1988;27:413–4.
Sakamoto FA, Winalski CS, Rodrigues LC, Fernandes AR, Bortoletto A, Sundaram M. Radiologic case study. Orthopedics. 2012;35:353–437.
Samuels J, Keenan RT, Yu R, Pillinger MH, Bescke T. Erosive spinal tophus in a patient with gout and back pain. Bull NYU Hosp Jt Dis. 2010;68:147–8.
Sanmillan Blasco JL, Vidal Sarro N, Marnov A, Acebes Martin JJ. Cervical cord compression due to intradiscal gouty tophus: brief report. Spine (Phila Pa 1976). 2012;37:E1534–6.
Saripalli K, Baskar S. Tophaceous gouty arthropathy of the lumbar spine. Clin Med. 2014;14:683–4.
Schorn C, Behr C, Schwarting A. Fever and back pain—a case report of spinal gout. Dtsch Med Wochenschr. 2010;135:125–8.
Semlali S, El Kharras A, El Fenni J, Chaouir S, BenAmeur M, Akjouj S. Tophaceous gout of the lumbar spine simulating spondylodiscitis: imaging features: a case report. J Radiol. 2008;89:904–6.
Sequeira W, Bouffard A, Salgia K, Skosey J. Quadriparesis in tophaceous gout. Arthritis Rheum. 1981;24:1428–30.
Souza AW, Fontenele S, Carrete Jr H, Fernandes AR, Ferrari AJ. Involvement of the thoracic spine in tophaceous gout. A case report. Clin Exp Rheumatol. 2002;20:228–30.
Staub-Schmidt T, Chaouat A, Rey D, Bloch JG, Christmann D. Spinal involvement in gout. Arthritis Rheum. 1995;38:139–41.
Suk KS, Kim KT, Lee SH, Park SW, Park YK. Tophaceous gout of the lumbar spine mimicking pyogenic discitis. Spine J. 2007;7:94–9.
Taniguchi Y, Matsumoto T, Tsugita M, Fujimoto S, Terada Y. Spondylodiscitis and Achilles tendonitis due to gout. Mod Rheumatol. 2014;24:1026–7.
Thavarajah D, Hussain R, Martin JL. Cervical arthropathy caused by gout: stabilisation without decompression. Eur Spine J. 2011;20 Suppl 2:S231–4.
Thornton FJ, Torreggiani WC, Brennan P. Tophaceous gout of the lumbar spine in a renal transplant patient: a case report and literature review. Eur J Radiol. 2000;36:123–5.
Tkach S. Gouty arthritis of the spine. Clin Orthop Relat Res. 1970;71:81–6.
Tran A, Prentice D, Chan M. Tophaceous gout of the odontoid process causing glossopharyngeal, vagus, and hypoglossal nerve palsies. Int J Rheum Dis. 2011;14:105–8.
Tsai CH, Chen YJ, Hsu HC, Chen HT. Bacteremia coexisting with tophaceous gout of the spine mimicking spondylodiscitis: a case report. Spine (Phila Pa 1976). 2009;34:E106–9.
Udayakumar D, Kteleh T, Alfata S, Bali T, Joseph A. Spinal gout mimicking paraspinal abscess: a case report. J Radiol Case Rep. 2010;4:15–20.
Vaccaro AR, An HS, Cotler JM, Ahmad S, Jordan AG. Recurrent cervical subluxations in a patient with gout and endstage renal disease. Orthopedics. 1993;16:1273–6.
van de Laar MA, van Soesbergen RM, Matricali B. Tophaceous gout of the cervical spine without peripheral tophi. Arthritis Rheum. 1987;30:237–8.
Varga J, Giampaolo C, Goldenberg DL. Tophaceous gout of the spine in a patient with no peripheral tophi: case report and review of the literature. Arthritis Rheum. 1985;28:1312–5.
Vervaeck M, De Keyser J, Pauwels P, Frecourt N, D'Haens J, Ebinger G. Sudden hypotonic paraparesis caused by tophaceous gout of the lumbar spine. Clin Neurol Neurosurg. 1991;93:233–6.
Vinstein AL, Cockerill EM. Involvement of the spine in gout. A case report. Radiology. 1972;103:311–2.
Wald SL, McLennan JE, Carroll RM, Segal H. Extradural spinal involvement by gout. Case report. J Neurosurg. 1979;50:236–9.
Wang LC, Hung YC, Lee EJ, Chen HH. Acute paraplegia in a patient with spinal tophi: a case report. J Formos Med Assoc. 2001;100:205–8.
Wazir NN, Moorthy V, Amalourde A, Lim HH. Tophaceous gout causing atlanto-axial subluxation mimicking rheumatoid arthritis: a case report. J Orthop Surg (Hong Kong). 2005;13:203–6.
Wendling D, Prati C, Hoen B, et al. When gout involves the spine: five patients including two inaugural cases. Joint Bone Spine. 2013;80:656–9.
Yamamoto M, Tabeya T, Masaki Y, et al. Tophaceous gout in the cervical spine. Intern Med. 2012;51:325–8.
Yasuhara K, Tomita Y, Takayama A, Fujikawa H, Otake Y, Takahashi K. Thoracic myelopathy due to compression by the epidural tophus: a case report. J Spinal Disord. 1994;7:82–5.
Yehia B, Flynn J, Sisson S. Crystal clear. Am J Med. 2008;121:488–90.
Yen HL, Cheng CH, Lin JW. Cervical myelopathy due to gouty tophi in the intervertebral disc space. Acta Neurochir (Wien). 2002;144:205–7.
Yen PS, Lin JF, Chen SY, Lin SZ. Tophaceous gout of the lumbar spine mimicking infectious spondylodiscitis and epidural abscess: MR imaging findings. J Clin Neurosci. 2005;12:44–6.
Yoon JW, Park KB, Park H, et al. Tophaceous gout of the spine causing neural compression. Korean J Spine. 2013;10:185–8.
Zheng ZF, Shi HL, Xing Y, Li D, Jia JY, Lin S. Thoracic cord compression due to ligamentum flavum gouty tophus: a case report and literature review. Spinal Cord 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Pillinger has received grant support from Takeda Inc. and Crealta. He has also served as a consultant for AstraZeneca and Crealta.
Dr. Krasnokutsky and Dr. Toprover has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Crystal Arthritis
Rights and permissions
About this article
Cite this article
Toprover, M., Krasnokutsky, S. & Pillinger, M.H. Gout in the Spine: Imaging, Diagnosis, and Outcomes. Curr Rheumatol Rep 17, 70 (2015). https://doi.org/10.1007/s11926-015-0547-7
Published:
DOI: https://doi.org/10.1007/s11926-015-0547-7