Abstract
Calcium pyrophosphate deposition disease (CPPD) is a common and clinically heterogeneous form of arthritis caused by the deposition of calcium pyrophosphate (CPP) crystals in articular tissues. The diagnosis of CPPD is supported by the presence of radiographic chondrocalcinosis; yet, conventional radiography detects only about 40 % of clinically important CPPD. Here, we critically review the recent literature on imaging in CPPD. New studies inform our use of conventional radiographic screening methodologies for CPPD and provide additional evidence for the utility of diagnostic ultrasound. Recent work also highlights the polyarticular nature of CPPD, its association with tissue damage, and the high prevalence of tendon involvement. While dual energy CT and diffraction-enhanced synchrotron imaging remain research tools, they present potential avenues for improved visualization of CPP deposits. Advances in imaging in CPPD will increase diagnostic accuracy and eventually result in better management of this common form of arthritis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcium pyrophosphate deposition disease (CPPD) is caused by the deposition of calcium pyrophosphate (CPP) crystals in and around articular tissues. It comprises a heterogeneous group of clinical syndromes which range from an acute inflammatory mono-articular arthritis, previously known as pseudogout, to a group of chronic polyarticular degenerative processes with or without features of inflammation. CPPD can be hereditary or sporadic. While advanced age is the major risk factor for sporadic CPPD, a handful of metabolic diseases including hemochromatosis, hyperparathyroidism, hypomagnesemia, and hypophosphatasia are associated with an increased prevalence of CPPD.
CPPD is under-recognized and under-studied. There are few population-based studies of its prevalence, but conservative estimates suggest that CPPD affects up to 10 million Americans. For example, 25–30 % percent of knee specimens harvested at the time of surgery for a diagnosis of knee osteoarthritis (OA) contain CPP crystals [1–3]. Because of its clinical heterogeneity, as well as the propensity of CPPD to complicate other types of arthritis such as OA and even rheumatoid arthritis [4], rapid and accurate diagnosis can prevent unnecessary treatment when the diagnosis of CPPD is uncertain.
In the only proposed set of diagnostic criteria, the gold standard for CPPD diagnosis is chemical or structural validation of the presence of articular CPP crystals with Fourier transform infrared (FTIR) spectroscopy or x-ray diffraction [5]. These expensive research tools are not commonly used in the clinical evaluation of patients, and most clinicians rely on two clinical tests to diagnose CPPD. The first and most specific test is visualization of weakly birefringent rhomboid CPPD crystals in synovial fluid aspirates from the affected joint. Unfortunately, reliance on morphologic crystal identification is risky as crystals may not always be seen in a single synovial sample and the smallest and most inflammatory crystals can be easily missed [6]. Observation of the linear calcification in cartilage known as chondrocalcinosis on x-ray is often used to confirm the diagnosis of CPPD. However, it is neither highly sensitive nor specific for this diagnosis [7]. Because issues of sensitivity and specificity plague both commonly used diagnostic tests in CPPD, new, more accurate testing modalities are clearly needed. In this review, we critically assess the recent literature on imaging in CPPD with the hope that newer techniques will contribute to our ability to reliably diagnose CPPD and eventually to improve our management of this common disease.
Conventional Radiography
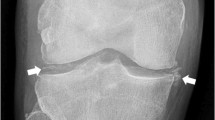
Chondrocalcinosis is a radiographic finding that correlates with the deposition of calcium-containing crystals in articular cartilage. It appears as punctate and linear densities in hyaline and/or fibrocartilage (Fig. 1). Typical locations include the triangular cartilage of the wrist, the fibrocartilage of the pubic symphysis, and the meniscus of the knee. While fibrocartilage may be preferentially involved, similar deposits in the mid-zone of articular cartilage are not-uncommon and follow the contour of the articular surface. Used in accordance with the Ryan-McCarty diagnostic criteria, the presence of chondrocalcinosis along with the observation of positively birefringent crystals in synovial fluid establishes a definite diagnosis of CPPD [5].
Original work demonstrating the pattern of chondrocalcinosis in CPPD patients resulted in radiologic screening recommendations that include x-rays of the hands, pelvis, and knees [8]. Abishek et al. recently investigated the pattern of chondrocalcinosis in an established cross-sectional cohort of 3170 individuals [9•]. The cohort included participants in the Genetics of Osteoarthritis and Lifestyle (GOAL) study of whom 1/3 had clinically severe hip OA, 1/3 had clinically severe knee OA, and 1/3 had no OA. Chondrocalcinosis was assessed on films of the knees (semiflexed and patellar sunrise view), supine pelvis, and AP hands. The mean age of the participants was 66 years old and 48.5 % were women. The prevalence of chondrocalcinosis in this cohort was 13.7 %. As previously demonstrated, the knee was the most common site of chondrocalcinosis. However, between 33 and 45.9 % of participants had evidence of chondrocalcinosis at a location other than the knee and no knee involvement. The wrist was the most commonly involved joint in this subgroup followed by the hips and symphysis pubis. The high prevalence of hip chondrocalcinosis may have been influenced by the selection of patients with known hip OA. Knee chondrocalcinosis was more common in the lateral than the medial compartments. The age of the patient did not correlate with the location of the chondrocalcinosis in hyaline compared to fibrocartilage. The generalizability of these observations is limited by the high frequency of hip OA in this cohort and the potential for reduced detection of chondrocalcinosis with severe OA. The semiflexed positioning of the knee radiographs may also reduce the ability to detect chondrocalcinosis. Nonetheless, this relatively large cohort study reinforces the validity of the current radiographic screening recommendations and the distribution and polyarticular nature of this disease.
Several recent studies support the utility of commonly performed imaging studies to detect chondrocalcinosis at uncommon sites. The frequent use of PA and lateral chest x-rays in clinical practice renders them particularly attractive as a screening tool for detecting CPPD in thoracic joints. Parperis et al. recently examined the prevalence of chondrocalcinosis of the acromioclavicular joint in 1920 consecutive outpatient chest radiographs performed on patients over the age of 50 [10•]. Using magnified digital picture archiving and communication system (PACS) imaging, they demonstrated that 19 (1.1 %) had acromioclavicular joint chondrocalcinosis. These findings suggest that careful re-examinations of chest radiographs using PACS imaging could support a diagnosis of CPPD.
Other radiographic findings have been well described in CPPD and are used as clues to differentiate CPPD from OA, particularly in the absence of chondrocalcinosis. The distribution of joint involvement in CPPD is different from OA and more frequently involves glenohumeral joints, wrists and second and third metacarpophalangeal joints. Other radiographic findings associated with CPPD include large subchondral cysts or geodes and variable osteophyte formation. Severe joint destruction is also often noted. Tendon calcifications are also important clues to the presence of CPPD. These appear as thin linear bands along the tendons’ length. In older studies by Yang et al. [11], tendon calcification around the knee was never present without adjacent chondrocalcinosis and involved the gastrocnemius tendon in 28 % and the quadriceps in 8.4 % of x-rays. Perreira et al. reviewed the prevalence and pattern of tendon calcification in patients with knee chondrocalcinosis and found involvement of Achilles, gastrocnemius, and /or quadriceps tendons in 21–25 % of radiographs [12]. Less commonly observed were involvement of the triceps tendon near the elbow, the rotator cuff, and the long head of the triceps at the shoulder. Recently, Dirim et al. found a high rate of cruciate ligament calcification in knees affected by CPPD, but the sample size was too small to estimate prevalence [13].
CT Scanning
CT scanning has high sensitivity and specificity for detection of calcific deposits in cartilage and soft tissue. This was recently confirmed in a small study by Misra et al. showing that CPPD affects multiple tissues in the knee [14]. Unfortunately, it is not commonly used to image painful joints and requires relatively high radiation doses compared to other imaging techniques. CT scanning improves the visibility of joints typically obscured by soft tissues and facilitates views of small or fibrocartilagenous joints.
Shirazian et al. recently studied 209 triage and emergency room head and cervical spine CT scans performed after trauma to determine the prevalence of chondrocalcinosis in the sternoclavicular joint [15]. All ages were included. They found that 36 (22 women and 14 men) had chondrocalcinosis of the sternoclavicular joint defined by linear, curvilinear, or discrete mottled foci of high attenuating material. Bilateral involvement was seen in 27/36 patients. Sternoclavicular chondrocalcinosis was associated with age, OA of the sternoclavicular joint, and the presence of atlantoaxial chondrocalcinosis. A similar retrospective analysis of 513 cervical spine CT scans in an adult trauma population determined the prevalence of atlantoaxial chondrocalcinosis [16•]. Chang et al. found a prevalence of chondrocalcinosis in the atlantoaxial area of 12.5 % overall. This rose to 34 % in patients over age 60, and 49 % in patients over the age of 80. Interestingly, a similar study done 18 years prior to this one noted only 8.8 % prevalence of chondrocalcinosis in patients over age 60, suggesting that advances in CT technology may improve its sensitivity. Chang et al. also found an increased prevalence of retro-odontoid thickening in patients with atlantoaxial chondrocalcinosis. This finding supports the inclusion of CPP deposition in the differential diagnosis of retro-odontoid space expansion. A recent case collection of 18 cases of crowned dens syndrome confirmed the utility of the CT scan to detect atlantoaxial chondrocalcinosis and also identified co-incident calcification of the cruciform ligament [17].
High resolution CT scanning was recently used to carefully characterize the pattern of CPP crystal deposition in joints ex vivo. Touraine et al. performed high resolution CT scanning in cadaveric knee joints of 68 patients with a mean age of 84 years [18]. In the absence of known clinical history, 34 % had calcification of the menisci and 21 % had calcification of the hyaline cartilage. The presence of calcification in one compartment was highly associated with involvement of another, and there was a good correlation between calcification of the hyaline articular cartilage and meniscal calcification. These detailed studies also highlighted the high prevalence of tibiofibular joint involvement in CPPD. Calcifications of the hyaline cartilage, but not fibrocartilage, correlated with CT-detected cartilage damage. A larger study of 608 cadaver knees from Japan employed histopathologic techniques to evaluate cartilage damage [19] and confirmed crystal identity using FTIR spectroscopy. They demonstrated a clear correlation between the presence of CPP crystals and the depth of cartilage degeneration at the femoro-tibial joint, supporting the notion that crystal deposition is associated with cartilage loss.
While CTs scanning was first introduced in the 1960s, the more recent advent of dual source imaging allowed for scanning at two different energy spectrums and resulted in the development of dual energy CT (DECT). This technique has been particularly useful for imaging calcified tissues. It can subtract bone from angiogram images, detect calcification in atherosclerotic plaques, and because it can distinguish variations in the spectral properties of calcium oxalate compared to urate, it is capable of determining the likely chemical composition of a kidney stone. Incredibly detailed 3-dimensional images of gouty tophi in and around joints have been described [20]. In one recent case study, DECT was used to differentiate articular monosodium urate (MSU) from CPP deposits [21]. In the single patient described, crystal analysis of the affected joint revealed CPP crystals under polarizing light microscopy. The authors suggest that DECT scanning could be useful when conventional radiographs are difficult to interpret or when crystals cannot be examined. Diekhoff et al. used single source DECT to differentiate between urate and CPP crystals in synthetic tissue [22]. Using a model composed of a phantom hand, the investigators placed synthetic MSU and CPP crystals in ultrasound gel and measured imaging parameters using DECT. They were able to detect a lower concentration of CPP crystals than MSU crystals and to clearly differentiate between them using blinded observers. However, the expense, availability, and clinical accuracy of this technology will need to be addressed before it becomes a useful clinical tool.
MRI
Historically, MRI has had little utility in imaging CPPD. Calcifications are not well visualized in articular tissues by MRI. Its use as an imaging modality in conditions associated with soft tissue CPP deposition (tophaceous CPPD) often results in misdiagnosis [23]. Its insensitivity for detecting calcium-containing crystals in cartilage has been recently reinforced by the work of Dirim et al. who showed that examination of SE T1 sequences on a 1.5 T MRI missed 75 % of CPPD deposition in cadaveric knees [13]. While there is some evidence that spoiled gradient 4-T techniques may be superior to others for imaging of CPPD [24], further work on new MR-based techniques is necessary before MRI can be recommended as a useful tool to diagnose CPPD.
Ultrasound
The burgeoning availability and experience with diagnostic ultrasound in clinical rheumatology provides increasing evidence for its utility in diagnosing CPPD. In 2005, Frediani et al. described several pathologic patterns in ultrasound images from patients with crystal-proven CPPD [25]. These included thin hyperechoic bands parallel to the surface of articular cartilage, which likely represent chondrocalcinosis of the hyaline cartilage. A second pattern involved punctate hyperechoic bands in regions of fibrocartilage, which correlate with chondrocalcinosis in the fibrocartilage. The least commonly observed finding was homogeneous hyperechoic nodular or oval deposits in the joint space or bursa likely representing free crystal aggregates. The same group of investigators recently published a study using these ultrasonographic patterns to determine the presence of calcific deposits in 42 well-characterized CPPD patients [26•]. They examined metacarpophalangeal joints, knees, wrists, Achilles tendons, and plantar fascia using both a dichotomous score for the presence of ultrasonographic CPPD as well as a quantitative scoring system to assess the size of the deposits. They found a mean number of 4.7 sites affected (SD ± 1.7, range 2–8) per patient. The knee was most commonly affected and had the highest burden of calcification followed by the wrist, Achilles tendon, plantar fascia, and metacarpophalangeal joints. This high-quality study reinforces the common polyarticular nature of CPPD as well as the frequent involvement of tendons and fascia. The common occurrence of soft tissue CPP deposition is further supported by the recent study of Filippucci et al., who examined the prevalence of ultrasonographic abnormalities in shoulders of 39 gout patients, 43 CPPD patients, and 3 with mixed crystal disease [27]. In this cohort, 30 % had painful shoulders, while 42 % of the shoulders were clinically normal. The remaining shoulder exams revealed some abnormality. Synovial inflammation in the shoulder was more commonly associated with CPPD than gout. Although tendinopathy was a common finding in both gout and CPPD patients, supraspinatous rupture was sevenfold more common in the CPPD group. Despite a lack of age-matched controls, these findings lend support to what we know about the destructive and systemic nature of CPP crystals.
Several recent studies suggest that ultrasound may be more sensitive for the detection of chondrocalcinosis than plain radiography. Gutierrez et al. examined the sensitivity, specificity, and accuracy of ultrasound for chondrocalcinosis in the knee in 74 patients with CPPD and 83 controls with other types of arthritis [28]. CPPD was defined by the presence of synovial fluid CPP crystals detected by polarizing light microscopy in the affected joint. Meniscal chondrocalcinosis was detected by ultrasound in at least one knee in 90 % of the patients with CPPD, compared to 83.7 % with conventional radiography. Detection rates for calcification of hyaline cartilage were 59.5 % by ultrasound and 45.9 % by radiography. Specificity (true negatives/false positives + true negatives) was 100 %, when the gold standard diagnostic test was the detection of synovial fluid CPP crystals. Study limitations include the special expertise of the investigators in this study and the poorly described inclusion criteria. Barskova et al. compared plain radiography, CT scans, and ultrasound in 25 patients with crystal-proven CPPD of the knee [29]. Oddly, only patients less than age 60 were enrolled in the study, and no control group was included. Thirteen had chondrocalcinosis on conventional radiography, 18 had chondrocalcinosis on CT scanning, and 25 had signs of chondrocalcinosis on ultrasound. They conclude that ultrasound detects chondrocalcinosis with greater sensitivity than CT scanning or conventional radiography. Ellabban et al. examined the shoulder, wrist, and knees of 60 patients with knee effusions including a subgroup with synovial fluid aspirates containing CPP crystals [30]. They excluded patients with known chondrocalcinosis, gout, or autoimmune disease. They found that 32/60 had chondrocalcinosis by ultrasound, and all of these patients had CPP crystals in synovial fluid aspirates. Their data demonstrate that ultrasound accurately detected 84.2 % of CPPD cases compared to 13.2 % for conventional radiography. The specificity was 100 % for both imaging techniques, based again on the presence of synovial fluid CPP crystals.
Many of the studies published on ultrasound in CPPD are from groups of investigators with special expertise in the interpretation of ultrasonography findings in CPPD. The generalizability of these findings is called into question by a recent study by Loffler et al. who suggested that in clinical practice, it may be difficult to distinguish between the double contour sign of gout and articular chondrocalcinosis [31•] and warned against overreliance on these subtle findings.
Other Imaging Techniques
Other imaging techniques, including both those in the research phase and those not typically used to image joints may contribute to our diagnostic armamentarium in CPPD. Li et al. recently published a study of diffraction-enhanced synchrotron imaging (DEI) in cadaveric knee joints [32]. This technique is based on radiographic technology that harnesses the x-ray refraction and scatter rejection properties not available with conventional radiography and results in very detailed images of articular cartilage. While these elegant studies were performed at a synchrotron facility, and this technique is not yet feasible in living patients, chondrocalcinosis is beautifully illustrated by this technology. Similar techniques may prove useful diagnostically once adapted for human use.
Conclusions
The advantages and disadvantages of currently available imaging modalities for CPPD are summarized in Table 1. Recent work reinforces the challenging nature of imaging in CPPD. Conventional radiography and CT scanning continue to provide important diagnostic information in this disease. In concert with ultrasound, these techniques highlight the polyarticular and systemic nature of CPPD, the high frequency of tendon and ligament calcification, and the inflammation and tissue destruction associated with CPP crystals. DECT and DEI are currently research tools, but hold promise as future diagnostic tests for CPPD. Improved imaging modalities will significantly increase our ability to accurately diagnose CPPD and contribute to better management of the many patients with this type of arthritis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–703.
Nalbant S, Martinez JAM, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cartilage. 2003;11:50–4.
Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–4.
Gerster JC, Varisco PA, Kern J, Dudler J, So AK. CPPD crystal deposition disease in patients with rheumatoid arthritis. Clin Rheumatol. 2006;25:468–9.
Ryan L, McCarty D. Calcium pyrophosphate crystal deposition disease: pseudogout: articular chondrocalcinosis. in Arthritis and Allied Conditions, 11th Edition (McCarty, D. ed.). Philadelphia: Lea & Febiger; 1989. p. 1711–36.
Gordon C, Swan A, Dieppe P. Detection of crystals in synovial fluids by light microscopy: sensitivity and reliability. Ann Rheum Dis. 1989;48:737–42.
Fisseler-Eckhoff A, Muller KM. Arthroscopy and chondrocalcinosis. Arthroscopy. 1992;8:98–104.
Resnick D, Niwayama G, Goergen TG, Utsinger PD, Shapiro RF, Haselwood DH, et al. Clinical, radiographic and pathologic abnormalities in calcium pyrophosphate dihydrate deposition disease (CPPD): pseudogout. Radiology. 1977;122:1–15.
Abhishek A, Doherty S, Maciewicz R, Muir K, Zhang W, Doherty M. Chondrocalcinosis is common in the absence of knee involvement. Arthritis Res Ther. 2012;14:R205. This large study of over 1000 patients provides information on the pattern of chondrocalcinosis in a population with and without osteoarthritis.
Parperis K, Carrera G, Baynes K, Mautz A, Dubois M, Cerniglia R, et al. The prevalence of chondrocalcinosis (CC) of the acromioclavicular (AC) joint on chest radiographs and correlation with calcium pyrophosphate dihydrate (CPPD) crystal deposition disease. Clin Rheumatol. 2013;32:1383–6. This interesting study uses digital imaging and PACS technology on chest x-rays to detect chondrocalcinosis in acromioclavicular joints.
Yang BY, Sartoris DJ, Resnick D, Clopton P. Calcium pyrophosphate dihydrate crystal deposition disease: frequency of tendon calcification about the knee. J Rheumatol. 1996;23:883–8.
Pereira ER, Brown RR, Resnick D. Prevalence and patterns of tendon calcification in patients with chondrocalcinosis of the knee: radiologic study of 156 patients. Clin Imaging. 1998;22:371–5.
Dirim B, Resnick D, Abreu M, Wangwinyuvirat M, Trudell DJ, Haghighi P. Relationship between the degeneration of the cruciate ligaments and calcium pyrophosphate dihydrate crystal deposition: anatomic, radiologic study with histologic correlation. Clin Imaging. 2013;37:342–7.
Misra D, Guermazi A, Sieren JP, Lynch J, Torner J, Neogi T, et al. CT imaging for evaluation of calcium crystal deposition in the knee: initial experience from the Multicenter Osteoarthritis (MOST) study. Osteoarthr Cartil. 2014. doi:10.1016/j.joca.2014.10.009.
Shirazian H, Chang EY, Wolfson T, Gamst AC, Chung CB, Resnick DL. Prevalence of sternoclavicular joint calcium pyrophosphate dihydrate crystal deposition on computed tomography. Clin Imaging. 2014;38:380–3.
Chang EY, Lim WY, Wolfson T, Gamst AC, Chung CB, Bae WC, et al. Frequency of atlantoaxial calcium pyrophosphate dihydrate deposition at CT. Radiology. 2013;269:519–24. This large study re-examines cervical spine CT scans performed for trauma and correlates the presence of CPPD with widening of the retro-odontoid space.
Godfrin-Valnet M, Godfrin G, Godard J, Prati C, Toussirot E, Michel F, et al. Eighteen cases of crowned dens syndrome: presentation and diagnosis. Neurochirurgie. 2013;59:115–20.
Touraine S, Ea HK, Bousson V, Cohen-Solal M, Laouisset L, Chappard C, et al. Chondrocalcinosis of femoro-tibial and proximal tibio-fibular joints in cadaveric specimens: a high-resolution CT imaging study of the calcification distribution. PLoS One. 2013;8:e54955.
Ryu K, Iriuchishima T, Oshida M, Kato Y, Saito A, Imada M, et al. The prevalence of and factors related to calcium pyrophosphate dihydrate crystal deposition in the knee joint. Osteoarthritis Cartilage. 2014;22:975–9.
Bongartz T, Glazebrook KN, Kavros SJ, Murthy NS, Merry SP, Franz WB, 3rd Michet CJ, Akkara Veetil BM, Davis JM, 3rd Mason TG, 2nd Warrington KJ, Ytterberg SR, Matteson EL, Crowson CS, Leng S, McCollough CH. Dual-energy CT for the diagnosis of gout: an accuracy and diagnostic yield study. Ann Rheum Dis. 2014.
Kim HR, Lee JH, Kim NR, Lee SH. Detection of calcium pyrophosphate dihydrate crystal deposition disease by dual-energy computed tomography. Korean J Intern Med. 2014;29:404–5.
Diekhoff T, Kiefer T, Stroux A, Pilhofer I, Juran R, Mews J, Blobel J, Tsuyuki M, Ackermann B, Hamm B, Hermann KG. Detection and Characterization of Crystal Suspensions Using Single-Source Dual-Energy Computed Tomography: A Phantom Model of Crystal Arthropathies. Invest Radiol. 2014.
Srinivasan V, Kesler H, Johnson M, Dorfman H, Walter K. Tophaceous pseudogout of the thoracic spine. Acta Neurochir (Wien). 2012;154:747–50. discussion 750.
Suan JC, Chhem RK, Gati JS, Norley CJ, Holdsworth DW. 4 T MRI of chondrocalcinosis in combination with three-dimensional CT, radiography, and arthroscopy: a report of three cases. Skeletal Radiol. 2005;34:714–21.
Frediani B, Filippou G, Falsetti P, Lorenzini S, Baldi F, Acciai C, et al. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: ultrasonographic criteria proposed. Ann Rheum Dis. 2005;64:638–40.
Filippou G, Filippucci E, Tardella M, Bertoldi I, Di Carlo M, Adinolfi A, et al. Extent and distribution of CPP deposits in patients affected by calcium pyrophosphate dihydrate deposition disease: an ultrasonographic study. Ann Rheum Dis. 2013;72:1836–9. This study supports the polyarticular nature of CPPD and reinforces the utility of ultrasound for the diagnosis of CPPD.
Filippucci E, Delle Sedie A, Riente L, Di Geso L, Carli L, Ceccarelli F, et al. Ultrasound imaging for the rheumatologist. XLVII Ultrasound of the shoulder in patients with gout and calcium pyrophosphate deposition disease. Clin Exp Rheumatol. 2013;31:659–64.
Gutierrez M, Di Geso L, Salaffi F, Carotti M, Girolimetti R, De Angelis R, et al. Ultrasound detection of cartilage calcification at knee level in calcium pyrophosphate deposition disease. Arthritis Care Res (Hoboken). 2014;66:69–73.
Barskova VG, Kudaeva FM, Bozhieva LA, Smirnov AV, Volkov AV, Nasonov EL. Comparison of three imaging techniques in diagnosis of chondrocalcinosis of the knees in calcium pyrophosphate deposition disease. Rheumatology (Oxford). 2013;52:1090–4.
Ellabban AS, Kamel SR, Omar HA, El-Sherif AM, Abdel-Magied RA. Ultrasonographic diagnosis of articular chondrocalcinosis. Rheumatol Int. 2012;32:3863–8.
Loffler C, Sattler H, Peters L, Loffler U, Uppenkamp M, Bergner R. Distinguishing Gouty Arthritis from calcium pyrophosphate disease and other arthritides. J Rheumatol. 2014. http://www.jrheum.org/content/early/2014/11/11/jrheum.140634 This interesting work stresses the difficulty of differentiating between gout and CPPD with ultrasonograhy alone.
Li J, Wilson N, Zelazny A, Meyer J, Zhong Z, Muehleman C. Assessment of diffraction-enhanced synchrotron imaging for cartilage degeneration of the human knee joint. Clin Anat. 2013;26:621–9.
Compliance with Ethics Guidelines
Conflict of Interest
Jennifer Miksanek and Ann K. Rosenthal declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Crystal Arthritis
Supported by a grant from the National VA Research Service (AKR 101BX000812).
Rights and permissions
About this article
Cite this article
Miksanek, J., Rosenthal, A.K. Imaging of Calcium Pyrophosphate Deposition Disease. Curr Rheumatol Rep 17, 20 (2015). https://doi.org/10.1007/s11926-015-0496-1
Published:
DOI: https://doi.org/10.1007/s11926-015-0496-1