Abstract
Purpose of Review
Schizophrenia (SZ) is a debilitating mental illness; existing treatments are partially effective and associated with significant side effect burden, largely due to our limited understanding of disease mechanisms and the trajectory of disease progression. Accumulating evidence suggests that metabolic changes associated with glucose metabolism, mitochondrial dysfunction, and redox imbalance play an important role in the pathophysiology of schizophrenia. However, the molecular mechanisms associated with these abnormalities in the brains of schizophrenia patients and the ways in which they change over time remain unclear. This paper aims to review the current literature on molecular mechanisms and in vivo magnetic resonance spectroscopy (MRS) studies of impaired energy metabolism in patients at clinical high risk for psychosis, with first-episode SZ, and with chronic SZ. Our review covers research related to high-energy phosphate metabolism, lactate, intracellular pH, redox ratio, and the antioxidant glutathione.
Recent Findings
Both first-episode and chronic SZ patients display a significant reduction in creatine kinase reaction activity and redox (NAD + /NADH) ratio in the prefrontal cortex. Chronic, but not first-episode, SZ patients also show a trend toward increased lactate levels and decreased pH value. These findings suggest a progressive shift from oxidative phosphorylation to glycolysis for energy production over the course of SZ, which is associated with redox imbalance and mitochondrial dysfunction.
Summary
Accumulating evidence indicates that aberrant brain energy metabolism associated with mitochondrial dysfunction and redox imbalance plays a critical role in SZ and will be a promising target for future treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia (SZ) is a severe mental illness that places a substantial burden on healthcare systems and can have devastating effects on patients and their caretakers. Still, we know very little about the underlying molecular mechanisms and trajectory of the disease. There is evidence for progressive changes in the illness throughout adolescence and adulthood, but variations in research methods and phenotypic definitions of SZ have hampered research progress. Although the positive symptoms of SZ tend to remain stable or improve over time, cognitive deficits and negative symptoms can become more impairing [1, 2]. Additionally, disease identification and treatment during critical phases of the early disease course—clinical high-risk (CHR) and first-episode (FE) stages—can impact long-term functional outcomes [3]. Thus, there is an urgent need to understand the disease progression mechanisms to provide alternative treatment strategies and tools for earlier intervention.
Despite enormous efforts, SZ pathophysiology is still unclear, and the functional outcome of SZ patients remains poorly managed [4, 5]. Prominent contributors to SZ pathogenesis include impaired neuronal circuitry development, dysfunctional neurotransmission, excessive neuroinflammation, oxidative stress, and metabolic deficits [6, 7]. Treatments that act on the brain’s neurotransmission system, primarily by blocking dopamine receptors, can effectively treat positive symptoms of SZ but often fail to improve negative and cognitive symptoms [8,9,10]. Therefore, researchers have searched for alternative mechanisms to target to alter the course of this disease. A growing body of evidence suggests an “immuno-oxidative” pathway, including the disturbance of glutamatergic neurotransmission with N-methyl-D-aspartate receptor (NMDAR) hypofunction, redox dysregulation, oxidative stress, and neuroinflammation, forming a “central hub” of SZ pathology [6, 7]. The interaction among these processes leads to an excitatory-inhibitory imbalance within local neuronal circuits and impaired connectivity between distant brain regions, ultimately harming information processing and prompting SZ symptom formation [11•, 12]. Accumulating evidence indicates that aberrant brain energy metabolism and mitochondrial dysfunction play a critical role in this “immuno-oxidative” pathway and have become increasingly common in the discussion of SZ pathology [13]. This is not surprising as our brain is the human body’s most metabolically active organ, consuming roughly 20% of the body’s energy at rest despite comprising only 2% of the body’s mass [14]. One of the most fundamental processes supporting the brain’s high energy demand is adenosine triphosphate (ATP) synthesis. Most de novo ATP synthesis is carried out by oxidative phosphorylation (OxPhos) in the mitochondria using a collection of enzyme complexes and electron-shuttling proteins [15]. Reduced electron carriers (e.g., NADH and FADH2) generated from glycolysis and the tricarboxylic acid (TCA) cycle release electrons that aid ATP synthesis [15]. Neurons rely on mitochondria to generate the large amounts of ATP needed for basic cellular homeostasis as well as complex information processing. Glutamatergic neurotransmission, a process that is suspected to be impaired in SZ [16], utilizes most of the brain’s ATP [17]. Furthermore, mitochondrial respiration is the main producer of reactive oxygen species (ROS) [18], known to cause oxidative stress when not properly regulated. Multiple biomarkers of oxidative stress are increased in SZ patients [19], suggesting difficulty in maintaining ROS homeostasis.
Evidence for abnormal energy metabolism in SZ is largely based on animal models, post-mortem brains, and genetic studies. Dysregulated expression of various energy production genes has been found in cortical regions of post-mortem SZ brains [15]. These genes are related to the mitochondrial genome, nucleus mitochondria-crosstalk, and OxPhos [15, 20]. Additionally, the number and size of mitochondria in the anterior cingulate cortex (ACC), prefrontal cortex, hippocampus, caudate, and putamen of post-mortem SZ brains are lower than in healthy control brains [15]. Analysis of the mitochondrial proteome of SZ-derived induced pluripotent stem cells (iPSCs) revealed significant differential regulation of proteins related to calcium transport, mitochondrial location, the TCA cycle, and cell redox homeostasis compared to healthy control iPSCs [21]. Researchers have also found that metabolism in neural stem cells and cerebral organoids derived from SZ patients were heavily reliant on glycolysis rather than oxygen metabolism [21, 22], showed deficits in oxygen consumption and ATP production [23], and had increased ROS levels [21]. Altogether, this suggests abnormal energy metabolism in the SZ brain with a possible shift from the TCA cycle and oxidative phosphorylation to glycolysis and an increase in oxidative stress.
Although these studies have provided a strong foundation to study energy metabolism in SZ, in vivo measures of energy metabolism in the brains of humans with SZ are necessary to confirm these theories and to develop novel treatments. Serum and cerebrospinal fluid (CSF) studies provide an estimate of energy metabolism in the central nervous system but cannot quantify the exact levels of metabolites in the brain. 18FDG-PET has been used to directly observe the hypometabolism of glucose in the frontal lobe of SZ patients compared to controls, confirming that energy metabolism is generally disrupted in the frontal lobes [24]; however, this method is invasive and expensive. Measures of insulin action, such as the oral glucose tolerance test, have also detected impaired glucose metabolism and insulin sensitivity in FE psychosis [25], but this method does not directly measure metabolism in the brain. In contrast, magnetic resonance spectroscopy (MRS) is a specialized, noninvasive neuroimaging technique that allows in vivo measurement of numerous energy production metabolites. For instance, 1H-MRS enables us to examine glutathione (GSH), lactate, and glutamate/glutamine levels. Additionally, 31P-MRS provides a means of quantifying the two main pools of high-energy phosphates, ATP and phosphocreatine (PCr), as well as inorganic phosphate (Pi) [26]. Using 31P-MRS, we also can calculate the NAD + /NADH ratio (redox pair, the oxidizing and reducing forms of nicotinamide dinucleotide), intracellular pH value, and the dynamic reaction rates of creatine kinase (CK) and ATPase [27•]. Furthermore, MRS provides a noninvasive tool for tracking the evolution of energy metabolism from the CHR stage to chronic SZ. Thus, this paper will review the recent literature regarding energy metabolism in the brains of human SZ patients, focusing on findings from specific measures obtained via 1H- and 31P-MRS research.
Brain Lactate and Intracellular pH Indicate a Shift to Glycolysis
Researchers have theorized that there may be a shift in the brain from OxPhos to glycolysis for energy production in SZ. For instance, pyruvate dehydrogenase, the enzyme that converts pyruvate to Acetyl-CoA before entering the TCA cycle, is downregulated in the brains of SZ patients reported from the post-mortem studies [28, 29]. Additionally, a 2020 meta-analysis of post-mortem SZ studies found increased lactate in the brains of schizophrenia patients compared to controls [30]. Lactate and parenchymal pH, which can be measured in vivo using 1H- and 31P-MRS, respectively, may elucidate this potential biochemical shift in SZ patients in vivo. Therefore, we can infer the primary mode of energy production by measuring these two indicators.
Lactate
An increase in glycolysis would result in the buildup of lactic acid (lactate), making lactate a biomarker of interest for impaired OxPhos. Four studies have examined lactate levels in chronic or FE SZ patients. Notably, lactate is elevated in the ACC and centrum semiovale regions in chronic SZ patients compared to controls, measured at 7 Tesla (T) [31, 32]. Furthermore, elevated lactate level in chronic SZ was associated with reduced cognitive functioning and functional capacity in one study [31] and negative symptoms in another [32]. In contrast, there were no observed differences in lactate levels between FE and HC groups at 7 T in the ACC, dorsolateral prefrontal cortex, thalamus, centrum semiovale, and orbitofrontal region [33]. Additionally, a longitudinal study found no change in lactate in FE patients over 4 years in the same five brain regions, indicating that energy production may not change significantly over the early course of SZ [34]. To determine whether abnormal lactate levels are present during the prodromal phase of SZ, another study examined differences in lactate between CHR and HC groups at 3 T [35]. Indeed, there was no difference in lactate between CHR and HC groups [35]. Overall, these findings point to normal lactate levels during prodromal and early-phase SZ that becomes elevated in chronic SZ—possibly as a long-term consequence of impaired mitochondrial function.
Intracellular pH
A decrease in intracellular pH, although not solely regulated by lactate concentration, may also point to decreased ETC activity and acidification of tissue [36]. 31P-MRS can be used to examine intracellular pH based on the chemical shift difference between PCr and Pi. Two recent papers have reviewed existing 31P-MRS studies of pH and found no significant results [30, 37]. However, many of the reviewed studies were published in the 1990s and used scanners with low field strengths (1.5 or 2 T) or had insignificant power to detect differences in brain pH [30]. Only one of these studies was conducted at 4 T, which found a significant decrease in pH in chronic SZ patients compared to HCs [27•]. In contrast, an analysis of three 31P-MRS studies of pH at 1.5 T and one at 4 T in FE and the first-degree relatives of psychosis patients found no significant difference in intracellular pH compared to the control group [38], indicating that decreased pH may be a long-term consequence of persistent bioenergetic abnormalities in SZ rather than a risk factor. Of note, there have been no studies of pH in CHR participants, and no study to date has looked at brain pH and lactate simultaneously. Using 1H-MRS to examine lactate and 31P-MRS to examine pH at the same experimental session could indicate whether the body has shifted away from OxPhos toward glycolysis in an individual. Overall, there is a trend toward a progressive decrease in pH from FE to chronic SZ, which is supported by studies with stronger magnetic field strengths and sufficiently powered samples, but further research is needed to confirm this observation.
Creatine Kinase and High-Energy Phosphate Metabolism
31P-MRS allows in vivo examination of the two main pools of high-energy phosphates, ATP and PCr, as well as the rates of ATP transportation, synthesis, and consumption [39]. PCr acts as a reservoir for high-energy phosphates in the cytoplasm that can be accessed in response to energy demand, serving as a readily available source to rapidly regenerate ATP. This reversible reaction, catalyzed by creatine kinases (CKs), is critical for maintaining stable ATP levels to support all energy-requiring processes in the brain. Energy homeostasis in the brain supports many essential high-energy processes, including maintenance of ion gradients, synaptic transmission, and neural plasticity [40] as well as white matter integrity [41,42,43].
PCr/ATP Ratio
The importance of the chemical exchange between PCr and ATP has prompted investigators to measure their steady-state ratio (PCr/ATP) using 31P-MRS as a proxy for the metabolic rate of the brain. However, a 2015 31P-MRS review from our group by Yuksel and colleagues found no significant patterns in differences in PCr/ATP between SZ patients and healthy controls (HCs) in the frontal lobes, temporal lobes, and subcortical structures [26]. This held true for both FE and chronic SZ patients. We suggested in that review that small sample sizes, low magnetic field strength, and other methodological discrepancies may have generated these inconsistent findings [26]. Since this literature review, two independent studies with moderate sample sizes from our group have used 4 T 31P-MRS in FE psychosis [44, 45•] and chronic SZ [27•]. One found a significantly lower ratio of PCr/ATP in the FE psychosis patients and their unaffected siblings compared to the HC group [44], while the other FE psychosis study did not [45•]. Of note, a large proportion of the FE psychosis patients in the study with positive findings had bipolar disorder (BD) with psychosis rather than schizophrenia, schizophreniform, or schizoaffective disorder [44]. Furthermore, the study of chronic SZ patients found no difference in PCr/ATP ratio between patient and HC groups [27•].
The 31P-MRS literature has largely focused on high-energy phosphate metabolites at rest. However, the brain’s many complex systems often maintain important physiological parameters within normal limits when faced with challenges. To reveal the malfunction, a system can be perturbed or driven to a state that requires increased utilization of resources [26]. For example, a study by Du and colleagues examined high-energy phosphates in BD patients, where similar bioenergetic abnormalities to SZ are reported [46], during a visual stimulation paradigm. They found no differences between patients and controls at baseline, but there were significant differences in PCr and ATP concentrations during the stimulus presentation [26].
Creatine Kinase Activity
In vivo metabolite level and its ratio quantification with conventional 31P-MRS can provide valuable information about bioenergetic status. However, most in vivo 31P-MRS studies using this conventional approach ignore chemical exchange and partial saturation effects, leading to substantial errors in metabolite quantification. Therefore, the measured PCr/ATP ratio could be affected by the experimental parameters and MR scanners used by individual groups [47]. Another major limitation of measuring the PCr/ATP ratio is that steady-state levels of metabolites may not reflect the dynamics of their metabolism. Indeed, multiple studies have shown that the rate of CK metabolism can significantly shift without any apparent change in PCr or ATP levels [48,49,50]. These limitations can be overcome by phosphorus magnetization transfer spectroscopy, which directly measures the exchange rates of high-energy phosphate metabolites—i.e., CK and ATPase activity [39, 40, 51, 52]. The first study to examine CK reaction rate in vivo in SZ patients found a significant reduction in first-order forward CK rate constant (producing ATP from PCr) at rest in the medial prefrontal cortex (mPFC) of patients with chronic SZ [27•], while the PCr/ATP ratio was not significantly altered. This finding was replicated in another cohort of chronic SZ patients in 2021 [53]. We then wanted to determine if this same deficit exists in FE SZ patients and found that CK reaction rate was significantly reduced in patients compared to control participants [45•]. This finding confirms that metabolic abnormalities are not only present in chronic SZ but also early in the illness course. Decreases in CK reaction rate have also been observed in patients with FE BD with psychotic features—a disorder that is known to have a similar metabolic profile to SZ [46]. Additionally, siblings of patients with psychosis have a decreased CK reaction rate compared to controls, which might suggest that there is a genetic contribution to these metabolic abnormalities [44]. Interestingly, CK flux in the mPFC was significantly associated with functional connectivity between the mPFC with other nodes of the default mode network (DMN) and was associated with stronger anticorrelation between the DMN and the task-positive network (TPN) [53] in healthy controls. However, these relationships were compromised in psychotic patients, where SZ and BD groups showed significantly lower CK flux and decreased anticorrelation between the mPFC and the TPN. Taken together, these findings indicate that lower energy generation rates in SZ might disrupt large-scale neuronal communications, leading to the presentation of psychotic symptoms. Furthermore, interactions between energy metabolism and neural synchrony pathways might be a potential novel treatment target for psychotic disorders.
Redox State
The balance between reductive and oxidative (redox) reactions in the brain depends on various biological pathways, but primarily on the ETC, which produces ROS during OxPhos [54]. Disruption of the brain’s redox balance can lead to redox imbalance and mitochondrial dysfunction, which can both impair the development of high-energy neural circuitry—i.e., peri-ventricular interneuron (PVI) microcircuits and their perineuronal nets (PNNs). PVI circuitry supports the high-frequency neuronal synchrony responsible for complex human behaviors such as cognitive control and is suggested to be responsible for cognitive deficits in SZ [12]. An antioxidant-deficient rodent model of SZ that displays PVI damage shows changes in redox balance, ATP, PCr, intracellular pH, glutamine and glutamate, and membrane phospholipid metabolites [55, 56]. In the same rodent model with additional peri-pubertal insults, oxidative stress induces upregulation of the microRNA miR-137 dysregulation, leading to decreased COX6A2 (a subunit of cytochrome c oxidase complex IV) in PVIs, mitophagy, and accumulation of damaged mitochondria [56]. Furthermore, increased miR-137 and decreased COAX2 are also peripherally observed in a subgroup of FE psychosis patients and are associated with worse cognitive, positive, and negative symptoms, as well as functional outcomes, indicating that redox state, antioxidant levels, and mitochondrial function are intertwined. Combined with findings of potential oxidative damage in humans with SZ, these preclinical models led researchers to turn their attention to the molecular regulators of redox state.
Glutathione
The primary antioxidant regulator of redox balance in the brain is GSH [57], so researchers have used 1H-MRS to determine if increased oxidative stress is caused by dysfunction in the GSH system. There are more than fifteen published 1H-MRS studies measuring GSH in SZ patients as of 2022, but there is little consensus about GSH in the SZ literature thus far. Two recent meta-analyses of 3 T and 7 T 1H-MRS studies found small but significant decreases of GSH in ACC between SZ and HC groups [58, 59], with no significant effect of age or illness chronicity. Antipsychotic medication dosage and type were rarely reported, although 80–100% of SZ patients were medicated [59]. Therefore, future studies of GSH, especially those in chronic SZ, must record and analyze the impact of medication. Both meta-analyses consisted of sizeable FE and chronic SZ samples, but the authors did not examine differences in GSH between these two groups. Since these two meta-analyses, there has been one 1H-MRS study of ACC GSH in FE SZ patients and three in chronic SZ patients. Contrary to the findings of the meta-analyses, GSH levels were higher in FE SZ patients than controls [60], and all three studies in chronic SZ patients found no difference in ACC GSH [61,62,63]. In response to these conflicting findings, researchers have started to probe GSH levels in CHR populations to identify disease effects without the confounds of disease chronicity and medication status. To date, there are three 1H-MRS studies of GSH in CHR populations. All three found no differences in GSH between CHR and HC groups [35, 64, 65]. However, Jeon and colleagues found a positive association between Social and Occupational Functioning Assessment Scale score and GSH concentration in the CHR but not the HC group [64]. This is in line with a study by the same group that found a significant association between higher GSH levels at baseline and employment/education status at baseline and 1-year follow-up in FE SZ patients [60]. Taken together, these inconsistencies in GSH findings are likely due to the use of widely divergent technical approaches and sample sizes and because GSH levels may vary based on disease chronicity, age, and symptom severity, as well as compensatory pathways.
Redox Ratio
GSH is essential to various complex biological systems, including redox regulation and NMDAR signaling. Therefore, other biological parameters related to redox state may adjust to maintain GSH levels. The redox pair NAD + and NADH are known to regulate energy metabolism in the brain. Most NADH is produced from NAD + during glycolysis in the cytosol and the TCA cycle in mitochondria, where the majority of ATP is generated [66]. NADH is subsequently converted back to NAD + during OxPhos to power the mitochondrial electron transport chain (ETC) [66]. All major metabolic pathways, including glycolysis, the TCA cycle, ETC, fatty acid oxidation, and ketone body metabolism, rely on redox reactions, for which NAD(H) is an important co-substrate. Thus, NAD + and NADH reflect the redox state of the cellular environment, and disruption of their homeostasis likely impacts these metabolic pathways.
There have only been two publications to date examining the redox ratio (NAD + /NADH) in SZ patients. One study examined the redox ratio in chronic and FE SZ patients [67•], while the other looked at FE and BD patients [44]. The consensus of both studies was that both FE and chronic psychosis patients showed a significant decrease in redox ratio. The FE patients had more severe redox abnormalities than chronic patients, suggesting an active process in the early illness [67•]. The decreased redox ratio found in FE and chronic SZ patients is due to an increase in NADH levels with slightly decreased levels of NAD + [44, 67•]. Indeed, NADH can accumulate when it is continuously produced by the TCA cycle and cannot be re-oxidized through OxPhos [66]. Furthermore, the rise in NADH suggests increased reductive stress in the cellular environment, which can cause increased ROS production [68]. In combination with findings of increased lactate and reduced intracellular pH, NADH accumulation and decreased redox ratio indicate a shift away from OxPhos toward glycolysis, resulting in reductive stress. It is difficult to study the progression of SZ before the first episode of psychosis, so rodent models have been used to characterize the disorder’s early development. Although decreased redox ratio in FE and chronic SZ patients points to reductive stress, rodent models tell us that there may be an increased redox ratio accompanied by oxidative stress during adolescence [69, 70]. The same study also observed a reduction in redox ratio during adulthood, consistent with our findings in adult SZ patients [67•]. Compensatory antioxidant responses can maintain oxidative stress within normal physiological ranges for a time [71] but may eventually reach a limit [55, 72, 73]. Then, ROS-producing metabolic processes will decrease, and ROS production may fall below the normal range; this will limit NAD + -dependent respiration and inhibit normal cellular functions of ROS, such as cellular signaling [68, 74, 75].
Widespread Impacts of Metabolic Dysfunction and Redox Dysregulation
Energy metabolism and redox state interact with other systems that show dysfunction in SZ, such as the excitatory-inhibitory balance of neural circuitry maintained by glutamatergic neurotransmission. A large body of evidence suggests that glutamatergic neurotransmission and NMDAR hypofunction are involved in SZ pathology—particularly in cognitive dysfunction. NMDAR hypofunction can be induced through both transient GSH deficits and changes in redox balance [12]. This can suppress the PVIs that rely on NMDARs to support properly functioning glutamatergic circuitry and create a hyper-glutamatergic state [76], leading to excitotoxicity and oxidative stress. Additionally, NMDAR hypofunction can cause greater redox imbalance through transcriptional control of antioxidant genes [12], creating a self-propagating cycle of NMDAR hypofunction. There is also evidence for increased neuroinflammation in SZ based on elevated plasma inflammatory biomarkers and altered concentrations of myoinositol in vivo [55]. Importantly, several inflammatory mediators are activated by ROS, and immune cells can induce the secretion of ROS [77]. The details of glutamatergic transmission, NMDAR hypofunction, and neuroinflammation in SZ are out of the scope of this manuscript. However, 1H-MRS studies from our group [76] and others, including a recent meta-analysis [78], consistently show that glutamate (Glu) is elevated in never- or minimally treated FE [79,80,81] but reduced in antipsychotic-medicated FE [33, 82] and chronic SZ patients [78, 81, 83, 84]. A summary of metabolic alterations as SZ progresses and a diagram of underlying molecular mechanisms in chronic SZ are outlined in Figs. 1 and 2.
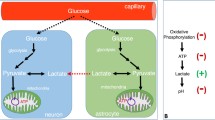
Diagram of proposed metabolic abnormalities in chronic schizophrenia. Multiple factors point to decreased OxPhos and increased glycolysis in SZ patients. Broadly, glucose metabolism in FE and chronic SZ patients is decreased, and there is a trend for increased lactate concentration and decreased pH in the brains of chronic SZ patients. Multiple metabolites involved in ATP synthesis, storage, and consumption differ in SZ patients compared to controls. The redox ratio (NAD + /NADH) is decreased in the mPFC of FE and chronic SZ patients compared to controls, mostly due to an increase in NADH, pointing to a decrease in electron transport chain activity. Additionally, the forward (ATP-producing) reaction rate of creatine kinase is significantly lower in the mPFC of FE and chronic SZ patients compared to controls. These abnormalities likely impair redox homeostasis, increase redox stress, and impact the functioning of high-energy neural circuitry
Progression of metabolite levels from prodromal to chronic sages of SZ. Vertical axis: the percent difference in metabolite level between SZ and control groups. Horizontal axis: the illness phase in chronological order from the prodrome to chronic SZ. A The NAD + NADH ratio is decreased by 48% in FE and 38% in chronic SZ patients [67•] compared to healthy controls. Rodent models indicated that there may be an increased redox ratio during adolescence, illustrated by the star in A. The PCr/ATP ratio seems a slight decrease in FE but did not differ between SZ and HC groups. The forward reaction rate of CK is significantly decreased by 15% in FE SZ [45•], which falls even further to 22% in chronic SZ [27•]. There is no difference in pH between FE and HC groups [44], while there is a small but significant decrease in pH between chronic SZ patients and HC [27•]. B The hypofunction of NMDAR receptors can be induced by excess ROS, redox imbalance, and GSH deficits. This hypofunction likely leads to elevated glutamate (Glu) during the early phase of SZ, followed by a decrease in Glu due to excitotoxicity. C Hypothesis for ROS and GSH levels over the course of SZ. Production of ROS is likely high during the prodrome creating oxidative stress, and production slows during the first-episode and chronic phases due to compensatory mechanisms. GSH level may then increase during the prodrome to compensate for high ROS levels but decreases as the supply of GSH is used up and ROS production slows. Conflicting GSH results reported in the literature may indicate fluctuations in GSH concentrations over time. Note: o---experimental data from our in vivo MRS measurements
Taken together, the conceptual framework driven by the preliminary data summarized above indicates: First, the initial active phase of SZ is characterized by redox dysregulation and excessive glutamatergic neurotransmission. Second, these two processes progressively become downregulated, or “burn out,” as brain energy metabolism shifts from OxPhos to glycolysis in chronic SZ, leading to a subsequent reduction in pH. The findings are consistent with a picture where there is elevated Glu signaling early in the disease associated with reduced ATP availability (indexed by the decreased CK reaction rate and PCr/ATP ratio) and redox imbalance. With disease progression, the brain responds by shifting from OxPhos toward glycolysis, supported by the increased lactate and reduced redox ratio in chronic SZ patients. This compensatory response addresses two critical problems for neuronal function seen in FE SZ patients: attenuating the redox imbalance and returning ATP availability to normal. The proposed shift to glycolysis in chronic SZ is reinforced by the reports of altered electron transport chain activity [15] associated with increased lactate/pyruvate ratios in post-mortem SZ brains [28, 36] and in those at genetic high risk for SZ, such as children with the 22q11.2 deletion syndrome [85, 86].
Finally, the research included in the current review has shed light on these mechanisms in early-phase SZ and the transition to chronic SZ, but most data we and others have collected are limited to mid-life. Furthermore, little progress has been made in understanding metabolic changes in older adults with SZ, even though there is evidence for accelerated aging of metabolic processes in the brains of SZ patients [87,88,89]. Thus, studying the evolution of energy-related metabolites in mid- to late-life in SZ will be essential in the future.
Potential Therapeutic Interventions
Numerous MRS findings, supported by preclinical research, have pinpointed multiple systems that can be targeted for therapeutic intervention: the brain’s antioxidant defense system, redox state, and mitochondrial function. N-acetylcysteine (NAC), a precursor to GSH, has been extensively studied as an antioxidant therapy in SZ research [55, 90]. In FE psychosis patients, NAC treatment has been shown to increase brain GSH levels [91]. Some studies indicate that NAC could improve neurocognition, reduce NMDAR-related mismatch negativity, and reduce negative symptoms with long-term use [55, 90]. Additionally, NAC has been found to improve peripheral redox status in a subgroup of FE psychosis patients with high serum oxidative stress levels [91]. NAD + supplements and ketogenetic diet may also be able to rebalance the redox ratio and have the potential to boost the TCA cycle and OxPhos in FE and chronic SZ patients. While NAD + precursor molecules like nicotinamide riboside (NR) have not been tested in humans with psychotic disorders, they have shown promise in preclinical and some clinical studies for cognitive dysfunction in other disorders with redox imbalance [87, 92]. Therapies that target mitochondrial dysfunction have also been explored. Mitochondrial-targeted antioxidants like MitoQ can rescue miR-137 and COAX2 dysregulation that leads to PVI impairments in an antioxidant-deficient rodent model of SZ [56]. Since these impairments are also found in a subgroup of FE SZ patients, MitoQ has potential for use as a specialized treatment for mitochondrial impairment in SZ [12]. In summary, molecules such as NAC and MitoQ, as well as NAD + supplements, show potential for therapeutic intervention in SZ by addressing oxidative stress, redox imbalance, and mitochondrial dysfunction. Further research and clinical trials are needed to explore their efficacy, safety, and use in personalized treatment of SZ patients.
Conclusion
Although the literature is still limited, using MRS to study the brains of SZ patients in vivo has provided insight into the molecular mechanisms responsible for metabolic deficits in SZ. This paper reviewed MRS findings related to pH and lactate, the CK/PCr system, GSH, and redox dysregulation. Generally, there is evidence for decreased OxPhos and increased glycolysis, which is primarily present in chronic SZ. First, the rate of CK action is significantly decreased in both FE and chronic SZ. Second, there is a buildup of NADH in both FE and chronic SZ and decreased NAD + /NADH ratio. This could indicate normal pyruvate oxidation and/or TCA cycle functioning without the ability to re-oxidize NADH to NAD + during oxidative phosphorylation. Lastly, there is a trend toward increased lactate and decreased pH in chronic but not FE SZ patients, pointing to a shift toward glycolysis in chronic SZ. These abnormalities have the potential to alter various systems throughout the brains of SZ patients and may serve as useful targets for therapeutic treatments in the future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. 2023.
Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–34.
Goff DC, Li C, Thorpe L. Does early intervention improve the long-term course of schizophrenia? Am J Psychiatry. 2020;177(4):288–90.
Lally J, et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. Br J Psychiatry. 2017;211(6):350–8.
AlAqeel B, Margolese HC. Remission in schizophrenia: critical and systematic review. Harv Rev Psychiatry. 2013;20(6):281–97.
Cuenod M, et al. Caught in vicious circles: a perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Mol Psychiatry. 2022;27(4):1886–97.
Steullet P, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res. 2016;176(1):41–51.
Demjaha A, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981–9.
Kaar SJ, et al. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704.
McCutcheon RA, Marques TR, Howes OD. Schizophrenia—an overview. JAMA Psychiat. 2020;77(2):201–10.
• Perkins DO, Jeffries CD, Do KQ. Potential roles of redox dysregulation in the development of schizophrenia. Biol Psychiatry. 2020. This paper provides a summary of the evidence supporting redox dysregulation as a pathological mechanism driving the development of psychosis.
Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17(2):125–34.
Henkel ND, et al. Schizophrenia: a disorder of broken brain bioenergetics. Mol Psychiatry. 2022;27(5):2393–404.
Balasubramanian V. Brain power. Proc Natl Acad Sci. 2021;118(32):e2107022118.
Roberts RC. Mitochondrial dysfunction in schizophrenia: with a focus on postmortem studies. Mitochondrion. 2021;56:91–101.
Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2020;205:107426.
Hyder F, et al. Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J Cereb Blood Flow Metab. 2013;33(3):339–47.
Kowalczyk P, et al. Mitochondrial oxidative stress-a causative factor and therapeutic target in many diseases. Int J Mol Sci. 2021;22(24).
Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiat. 2013;74(6):400–9.
Hjelm BE, et al. Evidence of mitochondrial dysfunction within the complex genetic etiology of schizophrenia. Complex Psychiatry. 2015;1(4):201–19.
Zuccoli GS, et al. Mitochondrial, cell cycle control and neuritogenesis alterations in an iPSC-based neurodevelopmental model for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023:1–16.
da Silveira Paulsen B, et al. Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transplant. 2012;21(7):1547–59.
Kathuria A, et al. Disease-specific differences in gene expression, mitochondrial function and mitochondria-endoplasmic reticulum interactions in iPSC-derived cerebral organoids and cortical neurons in schizophrenia and bipolar disorder. Discover Mental Health. 2023;3(1):8.
Townsend L, et al. Brain glucose metabolism in schizophrenia: a systematic review and meta-analysis of 18FDG-PET studies in schizophrenia. Psychol Med. 2022:1–18.
Chouinard VA, et al. Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol Psychiatry. 2019;24(10):1513–22.
Yuksel C, et al. Phosphorus magnetic resonance spectroscopy studies in schizophrenia. J Psychiatr Res. 2015;68:157–66.
• Du F, et al. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiat. 2014;71(1):19–27. This paper uses a novel 31P-MT-MRS approach to examine creatine kinase reaction rate and intracellular pH in vivo in chronic schizophrenia pateints. Creatine kinase rate and intracellular pH were significnatly reduced in schizophrenia pateints compared to controls, indicating bionenergetic abnormalities.
Dean B, et al. Evidence for impaired glucose metabolism in the striatum, obtained postmortem, from some subjects with schizophrenia. Transl Psychiatry. 2016;6(11):e949–e949.
Prabakaran S, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9(7):684–97.
Pruett BS, Meador-Woodruff JH. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: a focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr Res. 2020;223:29–42.
Rowland LM, et al. Elevated brain lactate in schizophrenia: a 7 T magnetic resonance spectroscopy study. Transl Psychiatry. 2016;6(11):e967.
Wijtenburg SA, et al. Metabolite alterations in adults with schizophrenia, first degree relatives, and healthy controls: a multi-region 7T MRS study. Front Psychiatry. 2021;12:656459.
Wang AM, et al. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiat. 2019;76(3):314–23.
Wang M, et al. Longitudinal changes in brain metabolites in healthy controls and patients with first episode psychosis: a 7-Tesla MRS study. Mol Psychiatry. 2023.
Da Silva T, et al. Glutathione, the major redox regulator, in the prefrontal cortex of individuals at clinical high risk for psychosis. Int J Neuropsychopharmacol. 2018;21(4):311–8.
Park H-J, Choi I, Leem K-H. Decreased brain pH and pathophysiology in schizophrenia. Int J Mol Sci. 2021;22(16):8358.
Dogan AE, et al. Brain lactate and pH in schizophrenia and bipolar disorder: a systematic review of findings from magnetic resonance studies. Neuropsychopharmacology. 2018;43(8):1681–90.
Romeo B, et al. Magnetic resonance spectroscopy studies in subjects with high risk for psychosis: A meta-analysis and review. J Psychiatr Res. 2020;125:52–65.
Du F, et al. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57(1):103–14.
Du F, et al. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105(17):6409–14.
Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34(8):1270–82.
Kole K, et al. Parvalbumin basket cell myelination accumulates axonal mitochondria to internodes. Nat Commun. 2022;13(1):7598.
Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11(4):275–83.
Chouinard V-A, et al. Brain bioenergetics and redox state measured by 31P magnetic resonance spectroscopy in unaffected siblings of patients with psychotic disorders. Schizophr Res. 2017;187:11–6.
• Yuksel C, et al. Abnormal brain bioenergetics in first-episode psychosis. Schizophr Bull Open. 2021;2(1). This study used 31P-MT-MRS to reveal a decrease in creatine kinase reaction rates in first-episode schizophrenia, building upon previous research that identified the same abnormality in chronic schizophrenia patients.
Du F, et al. Abnormalities in high-energy phosphate metabolism in first-episode bipolar disorder measured using 31P-magnetic resonance spectroscopy. Biol Psychiat. 2018;84(11):797–802.
Kim SY, et al. Rapid and simultaneous measurement of phosphorus metabolite pool size ratio and reaction kinetics of enzymes in vivo. J Magn Reson Imaging. 2018;47(1):210–21.
Chen W, et al. Increase of creatine kinase activity in the visual cortex of human brain during visual stimulation: a 31P NMR magnetization transfer study. Magn Reson Med. 1997;38(4):551–7.
Kašparová S, et al. A study of creatine kinase reaction in rat brain under chronic pathological conditions—chronic ischemia and ethanol intoxication. Brain Res Bull. 2000;53(4):431–5.
Mlynárik V, et al. Creatine kinase reaction rates in rat brain during chronic ischemia. Magn Reson Mater Phys, Biol Med. 1998;7:162–5.
Du F, et al. In vivo proton MRS to quantify anesthetic effects of pentobarbital on cerebral metabolism and brain activity in rat. Magn Reson Med. 2009;62(6):1385–93.
Chaumeil MM, et al. Multimodal neuroimaging provides a highly consistent picture of energy metabolism, validating 31P MRS for measuring brain ATP synthesis. Proc Natl Acad Sci U S A. 2009;106(10):3988–93.
Song X, et al. Bioenergetics and abnormal functional connectivity in psychotic disorders. Mol Psychiatry. 2021;26(6):2483–92.
Zhao RZ, et al. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 2019;44(1):3–15.
Dwir D, et al. Redox and immune signaling in schizophrenia: new therapeutic potential. Int J Neuropsychopharmacol. 2023;26(5):309–21.
Khadimallah I, et al. Mitochondrial, exosomal miR137-COX6A2 and gamma synchrony as biomarkers of parvalbumin interneurons, psychopathology, and neurocognition in schizophrenia. Mol Psychiatry. 2022;27(2):1192–204.
Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62(6):649–71.
Das TK, et al. Antioxidant defense in schizophrenia and bipolar disorder: a meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:94–102.
Sydnor VJ, Roalf DR. A meta-analysis of ultra-high field glutamate, glutamine, GABA and glutathione 1HMRS in psychosis: implications for studies of psychosis risk. Schizophr Res. 2020;226:61–9.
MacKinley M, et al. Central oxidative stress and early vocational outcomes in first episode psychosis: a 7-Tesla Magnetic Resonance Spectroscopy study of glutathione. Schizophr Bull. 2022;48(4):921–30.
Coughlin JM, et al. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol Psychiatry. 2021;26(7):3502–11.
Ravanfar P, et al. In vivo 7-Tesla MRI investigation of brain iron and its metabolic correlates in chronic schizophrenia. Schizophrenia. 2022;8(1):86.
Iwata Y, et al. Glutathione levels and glutathione-glutamate correlation in patients with treatment-resistant schizophrenia. Schizophr Bull Open. 2021;2(1):sgab006.
Jeon P, et al. Glutathione as a molecular marker of functional impairment in patients with at-risk mental state: 7-Tesla 1H-MRS Study. Brain Sci. 2021;11(7):941.
Da Silva T, et al. Mitochondrial function in individuals at clinical high risk for psychosis. Sci Rep. 2018;8(1):6216.
Bonora M, et al. ATP synthesis and storage. Purinergic Signal. 2012;8(3):343–57.
• Kim S-Y, et al. Redox dysregulation in schizophrenia revealed by in vivo NAD+/NADH measurement. Schizophr Bull. 2017;43(1):197–204. This study uses MRS to reveal significant redox dysregulation (reduced NAD+/NADH) in both first-episode and chronic schizophrenia pateints.
Xiao W, Loscalzo J. Metabolic responses to reductive stress. Antioxid Redox Signal. 2020;32(18):1330–47.
Skupienski R, Do KQ, Xin L. In vivo (31)P magnetic resonance spectroscopy study of mouse cerebral NAD content and redox state during neurodevelopment. Sci Rep. 2020;10(1):15623.
Skupienski R, et al. Developmental changes in cerebral NAD and neuroenergetics of an antioxidant compromised mouse model of schizophrenia. bioRxiv. 2022.
Lushchak VI, Storey KB. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. Excli J. 2021;20:956–67.
Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311–24.
Aoyama K. Glutathione in the brain. Int J Mol Sci. 2021;22(9).
Sies H, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23(7):499–515.
Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–9.
Kim SY, et al. In vivo brain glycine and glutamate concentrations in patients with first-episode psychosis measured by echo time-averaged proton magnetic resonance spectroscopy at 4T. Biol Psychiatry. 2018;83(6):484–91.
Mladenov M, et al. Oxidative stress, reductive stress and antioxidants in vascular pathogenesis and aging. Antioxidants (Basel). 2023;12(5).
Merritt K, et al. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic esonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiat. 2021;78(6):667–81.
Bustillo JR, et al. (1)H-MRS at 4 Tesla in minimally treated early schizophrenia. Mol Psychiatry. 2009.
Theberge J, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159(11):1944–6.
Merritt K, et al. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiat. 2016;73(7):665–74.
Egerton A, et al. Effects of antipsychotic administration on brain glutamate in schizophrenia: a systematic review of longitudinal (1)H-MRS studies. Front Psychiatry. 2017;8:66.
Brandt AS, et al. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: a (1)H MRS study at 7 Tesla. Schizophr Res. 2016;172(1–3):101–5.
Smucny J, Carter CS, Maddock RJ. Medial prefrontal cortex glutamate is reduced in schizophrenia and moderated by measurement quality: a meta-analysis of proton magnetic resonance spectroscopy studies. Biol Psychiatry. 2021;90(9):643–51.
Napoli E, et al. Mitochondrial citrate transporter-dependent metabolic signature in the 22q11.2 deletion syndrome. J Biol Chem. 2015;290(38):23240–53.
Cleynen I, et al. Genetic contributors to risk of schizophrenia in the presence of a 22q11.2 deletion. Mol Psychiatry. 2021;26(8):4496–510.
Covarrubias AJ, et al. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–41.
Nguyen TT, Eyler LT, Jeste DV. Systemic biomarkers of accelerated aging in schizophrenia: a critical review and future directions. Schizophr Bull. 2017;44(2):398–408.
Okusaga OO. Accelerated aging in schizophrenia patients: the potential role of oxidative stress. Aging Dis. 2014;5(4):256–62.
Smaga I, Frankowska M, Filip M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br J Pharmacol. 2021;178(13):2569–94.
Conus P, et al. N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophr Bull. 2018;44(2):317–27.
Reiten OK, et al. Preclinical and clinical evidence of NAD+ precursors in health, disease, and ageing. Mech Ageing Dev. 2021;199:111567.
Acknowledgements
The authors thank our volunteers and Mr. Elliot Kuan and Ms. Margaret Gardner for their assistance in the experiments and subject recruitment; Drs. Xiaopeng Song, Xi Chen, Cagri Yuksel, Virginie-Anne Chouinard, Kim Do Cuenod, and Bruce Cohen for their thoughtful discussions.
Funding
This research work was supported by National Institutes of Health (NIH) grants: R21MH114020, R01MH114982, P50MH115846, K24MH104449, R01AG066670, and R01MH095809.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Over the past 3 years, Dr. Dost Ongur has received honoraria from Neumora Inc. and Guggenheim LLC for scientific presentations. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. None of the other authors have conflict of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stein, A., Zhu, C., Du, F. et al. Magnetic Resonance Spectroscopy Studies of Brain Energy Metabolism in Schizophrenia: Progression from Prodrome to Chronic Psychosis. Curr Psychiatry Rep 25, 659–669 (2023). https://doi.org/10.1007/s11920-023-01457-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11920-023-01457-1