Abstract
Purpose of Review
This systematic review aims to inform the current state of evidence about the efficacy and effectiveness of medical cannabis use for the treatment of LBP, specifically on pain levels and overall opioid use for LBP. Searches were conducted in MEDLINE (PubMed), Embase, and CINAHL. The search was limited to the past 10 years (2011-2021). Study inclusion was determined by the critical appraisal process using the Joanna Briggs Institute framework. Only English language articles were included. Participant demographics included all adult individuals with LBP who were prescribed medical cannabis for LBP and may be concurrently using opioids for their LBP. Study quality and the risk of bias were both evaluated. A narrative synthesis approach was used.
Recent Findings
A total of twelve studies were included in the synthesis: one randomized controlled trial (RCT), six observational studies (one prospective, four retrospective, and one cross-over), and five case studies. All study results, except for the RCT, indicated a decrease in LBP levels or opioid use over time after medical cannabis use. The RCT reported no statistically significant difference in LBP between cannabis and placebo groups.
Summary
Low back pain (LBP) affects 568 million people worldwide. In the United States, LBP treatment represents more than half of regular opioid users. With the opioid epidemic, alternative methods, particularly medical cannabis, is now increasingly sought by practicing physicians and patients. Due to its infancy, there is minimal high-quality evidence to support medical cannabis use as a first line treatment for LBP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Low back pain (LBP) is one of the most common health complaints and a significant burden at both the individual and population levels [1] with a lifetime prevalence of 49–84% [2, 3]. The World Health Organization (WHO) [4] reports, among musculoskeletal disorders, LBP causes the highest burden with an average annual prevalence of 568 million people across the world reporting LBP every year [4]. LBP is a significant cause of absenteeism in the workplace and is associated with reduced productivity and higher costs of health care spending [3]. In 2016, approximately $34 billion was spent on back pain in the USA alone [5].
Initial recommended approaches of LBP management are non-pharmacological, including spinal manipulative therapy [6•], rest, regular physical exercise, and a healthy lifestyle [7]. However, for more persistent and chronic LBP (for those who do not respond to initial non-pharmacologic treatment), non-steroidal anti-inflammatory drugs (NSAIDs) and opioids are the most frequently recommended drug for LBP relief. Over 50% of opioid users report LBP as one of their primary concerns [8, 9], with opioid prescribing being highest among patients that are over 65 years of age [10]. A recent systematic review of opioids on LBP found that opioid use only provided modest short-term pain relief, with long-term efficacy reported as unknown [11].
Despite insufficient evidence to support opioid use for LBP, there has been an increase in long-term opioid therapy for managing chronic LBP pain [12]. Opioid-related mortalities in the USA have significantly increased, from 56,064 in 2020 to 75,673 in 2021. With this rising burden of the opioid epidemic [13] and increasing rates of opioid drug prescriptions, alternative methods to treat LBP are now increasingly sought by physicians and patients [14,15,16]. One alternative is the use of medical cannabis to manage LBP. Systematic and literature reviews [17,18,19,20] alongside primary studies [21,22,23,24,25], where adult patients reported a decrease in their overall pain levels or a decrease in their opioid medication use over time.
Although, LBP is a leading cause of disability [26] and remains one of the most frequent reasons for medical cannabis prescription [27, 28], the efficacy/effectiveness of medical cannabis for LBP management is unknown. Thus, the aim of this systematic review is to assess the global evidence available on the association between medical cannabis prescription and pain specific to LBP—whether its use can reduce pain levels or reduce pain medication (e.g., opioids) for LBP. To do this, we will define and measure the available evidence on the association between medical cannabis use and LBP in adults (and management of LBP) by measuring LBP outcomes via (1) overall LBP levels, and (2) changes in opioid medication use.
Methods
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist was used (Supplementary Table 1) [29]. The PICO model (population, intervention, comparison, and outcomes) was used to guide our research question [30]: to determine the efficacy/effectiveness of medical cannabis use for the treatment and management of LBP.
Inclusion Criteria
Types of Participants
This review included studies with adults prescribed medical cannabis for treatment of LBP and who may also be receiving opioids for LBP. When available, comparison/control groups included patients who were diagnosed with LBP but were not using medical cannabis or other medications (e.g., opioids) to treat their LBP. This review considered all studies that involved human subjects of legal age who were prescribed medical cannabis by a health care provider/physician to treat or manage their LBP, who may also be prescribed opioids for LBP. As legal age differs between countries, age ranges were from 18 to 21 + years of age.
Types of Intervention
This systematic review focused on the use of medical cannabis (self-medicating using medical cannabis) or medical cannabis therapy prescribed by a practicing physician. The term, “prescription” can be interchangeable with “certification” or “authorization,” depending on the jurisdiction. All forms, doses, frequencies, and types of medical cannabis cultivar were included (oils, sprays, vaporizers, edibles). Interventions of interest included those related to the efficacy or effectiveness of medical cannabis prescription/authorization for LBP including self-reported outcomes, patient-reported outcomes, screening systems, assessment strategies, intervention programs, clinical interventions, and follow-up assessment strategies. Studies that involved interventions using medical cannabis for more than one pain condition that included LBP were also included. An example of this was for the treatment of orthopedic pain or neuropathic pain, with LBP as one subset of pain type. Studies that involved treatment of health conditions that resulted in LBP were also included. An example of this was fibromyalgia, where LBP is a very common medical complaint. Studies that distinctly categorized medical cannabis as part of a larger cannabis study (with both recreational and medical cannabis) were also included.
Types of Outcome Measure
According to WHO [31], LBP is defined as any acute, subacute, or chronic pain that resides in the lower back, sacral, or lumbar region, including LBP that results from a strain, ache, trauma, fracture, or other health condition that may cause LBP. For our study, the primary outcomes of interest were (1) changes in the level of LBP score and (2) changes in current opioid use for LBP after medical cannabis use.
Types of Studies
We reviewed all original studies published in peer-reviewed journals that quantitatively examined the association between medical cannabis use and LBP outcomes. LBP outcomes were defined by the level of LBP or number of opioids prescribed/taken for LBP. We only included English language studies or studies translated to English from their original language. We included studies published in the past 10 years (2011–2021) to ensure the evidence was current and aligned with recent cannabis legalization and decriminalization around the world. We did not limit our studies by geographical region.
Exclusion Criteria
We excluded all studies that did not explicitly identify the use of medical cannabis. We excluded studies that measured recreational cannabis or unspecified cannabis. All animal studies were excluded. Any primary studies that were not related to LBP and medical cannabis were excluded. We excluded systematic reviews, literature reviews, clinical reviews, scoping reviews, expert opinion pieces, blogs, and editorials. For systematic reviews, both CL and ED independently reviewed for primary source articles that our review process did not capture.
Information Sources
A subject expert librarian, MB, selected the search terms for LBP. Term selection was broad and focused on capturing every areas of low back and all possible sources of LBP. The primary author, CL, then reviewed these terms with MB.
Databases
CL and MB selected three primary databases: MEDLINE (PubMed), Embase, and CINAHL. The Embase Drug library was selected to capture all generic and standard drug names of medical cannabis that are currently available.
Search Strategy
The search strategy was designed to access both published and unpublished materials. A pilot search was conducted by MB on April 14, 2021, to identify key MeSH terms/words for “medical cannabis” and “low back pain.” MB reviewed the key terms and confirmed that no additional terms would capture more results relevant to the review. MB conducted a search across the three databases on April 16, 2021. To identify and update any new literature since the original search in April 2021, MB repeated the search on October 22, 2021 (Appendix 1).
Screening
First (CL) and second (ED) reviewers independently screened the title and abstract for each study. During the preliminary screening process, we included grey literature from conference proceedings, meeting abstracts, and dissertation regarding medical cannabis use and LBP to capture potential new evidence that we may have missed. Screening was conducted via Rayyan to ensure consistency in inclusion/exclusion of articles. The protocol for title/abstract screening involved reading the title of the citation first and answering a series of eligibility questions (Appendix 1). If the questions could not be answered by the title, CL and ED independently reviewed the citation’s abstract. If the abstract still could not fully answer the questions, the citation was included for the next step in the full-text screening process.
Study Selection
In the full-text screening process, CL and ED used a set of questions (Appendix 2) to determine whether the study was eligible for synthesis. To ensure that all relevant articles were captured, CL and ED independently reviewed all of the references within the included articles, in which they found no additional articles to add. Once CL and ED reviewed all the articles independently, any discrepancies were discussed until consensus was reached.
Data Collection Process
Data extraction on the included studies was completed independently by CL on Microsoft Excel. CL independently extracted data from the studies. ED independently reviewed the data extracted and discussed with CL.
Data Items
Data items included were the first author, year, country, study aim, study period, study design, setting, age of the participants, sample size and characteristics, treatment, comparator group (if available), ethics approval (yes/no/no), unit of measurement for outcomes, statistical methods, number of participants missing, intervention result, control result, and overall study results.
Critical Appraisal
The critical appraisal process was conducted through the Joanna Briggs Institute (JBI) critical appraisal checklist [32]. The JBI offers various checklists appropriate to case control/cohort studies, case studies, and randomized controlled trials (RCTs). CL and ED independently screened and individually appraised the studies using the JBI checklist. After the critical appraisal, the included papers were grouped into study type: (i) RCTs; (ii) observational studies, and (iii) case reports. Second, the risk of bias and quality were assessed for each individual study as well as across all studies.
Risk of Bias
For each study, the risk of bias was evaluated using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) assessment tool under the Cochrane Risk of Bias tool [33]. Overall, CL and ED independently determined the quality of evidence and risk of bias across all included studies using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines (Supplementary Tables 1 and 2 [34]. Every article was assessed individually under GRADE criteria receiving a very low, low, moderate, or high for each of the five GRADE domains. The five GRADE domains consist of (1) Risk of Bias (already determined by ROBINS-I), (2) Imprecision, (3) Inconsistency, (4) Indirectness, and (5) Publication bias. An overall pooled estimate was determined and presented via a GRADE summary of findings table that presented the overall quality and credibility across all studies (Supplementary Table 2).
Summary Measures
Principal summary measures included odds ratio (odds of ceasing opioid prescription medication for LBP), percentage point differences in pain level, changes of pain level from 0 to 10 (10 being the highest and worst pain), hours of pain relief, pain questionnaire outcomes, changes in mean pain scores, and changes in opioid medication use (oral morphine equivalence).
Synthesis of Results
The findings are presented in narrative synthesis form because relatively few papers met the inclusion criteria. All data were presented descriptively, with the reporting of mean and standard deviations if continuous data were available, and frequencies or percentages if categorical data were available. A meta-analysis was not possible because there were significant differences in the medical cannabis type of strain, method of consumption, dose, frequencies, populations, and, importantly, comparator groups and outcomes to measure LBP pain across studies. Thus, a quantitative synthesis was not viable due to the heterogeneity between the measures and medical cannabis adult populations across the included studies. The narrative synthesis form included presenting the results in groups by study type: (i) RCTs, (ii) observational studies, and (iii) case reports.
Results
Study Selection
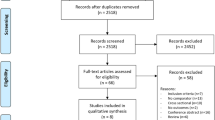
Our search yielded 606 studies, and 526 remained after removing duplicates across the 3 databases. After a title and abstract screening process, 68 studies were included for full article review. After full-text screening, we excluded 56 articles because the studies did not focus on LBP and medical cannabis (n = 26), were scoping or clinical reviews (n = 7), were expert opinions or editorials (n = 7), were abstracts or conference proceedings not pertaining to medical cannabis and LBP (n = 7), were systematic reviews (n = 5), or focused on animal models (n = 4). Once screened, only 12 studies met our inclusion criteria (Fig. 1).
PRISMA flow diagram. From Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71
Study and Participant Characteristics (Table 1)
Sample sizes ranged from 1 to 800 participants. Across all studies, participant mean ages (when reported) ranged from 18 to 87 years. All observational studies included both men and women. For case reports, one case report had only men, three had only women, and one had both. Only one case report and one retrospective cohort study provided information on participant race or ethnicity. Studies were conducted across several countries including the USA (n = 6), Israel (n = 2), Canada (n = 1), Australia (n = 1), Italy (n = 1), and Germany (n = 1).
Study Design
This review included 5 case reports (2 abstracts) [35,36,37,38,39], 4 retrospective cohort studies [40,41,42,43], 1 prospective cohort study [44], 1 observational cross-over study [45] (study where all participants receive the same two or more treatments), and 1 RCT [46]. Only four studies had a comparison or control group: Bebee et al.'s [46] RCT control, Vigil et al. [41] had patients from the same rehabilitation clinic, Yassin et al. [45] had the experimental group serve as their own control, and Takakuwa et al. [40] made comparisons across experimental groups of differing opioid users.
Summary of Interventions
With the exception of the RCT [46], 11 studies reported that cannabis use was highly variable and not standardized with respect to strain, cultivar, method of consumption, frequency of use, percentage of cannabinoid concentrations (cannabidiol [CBD] versus tetrahydrocannabinol [THC]), and the grams of cannabis used per day.
Bebee et al.'s RCT had a defined intervention of 400-mg oral dose of cannabidiol [46]. Ueberall et al. [43] was an intervention with Sativex (an oromucosal THC to CBD ratio spray) as an add-on treatment to concomitant opioid and non-opioid medication for pain. In the Takakuwa et al. [40] retrospective cohort study, participants were granted 1-year approval to either grow a limited amount of medical cannabis or purchase medical cannabis in all forms of consumption and administration of medical cannabis. Vigil et al. [41] study also allowed eligible patients to self-manage the potency, frequency, and type of cannabis product used. In Yassin et al. [45], medical cannabis therapy (MCT) was self-managed and recommended to be 1:4 THC to CBD with THC levels less than 5% with a recommended dose of 20 g/month of MCT for the first 3 months (via smoking or vaporization). After 3 months, the participant was given an option to increase the dose to 30 g/month. Haroutounian et al.’s study [44] had an intervention of prescribed cannabis dose of 20 g/month with the option of smoked, baked cooked, or olive oil extract drops. Mondello et al. [42] was a THC to CBD ratio oleic suspension, but the exact amount of THC or CBD was not monitored.
The case report studies also showed high variability in cannabis prescription, strain, dosage, route of administration, and frequency. The Ko et al. [36] case reports showed a prescription of 1 g per day (9% THC; 13% CBD; via vaporizer; 60 days) for one patient, whereas the other was prescribed 1.5 g per day (5% THC; 8% CBD; via vaporizer; 14 days). The case report by Yeung et al. [39] reported ingesting 10–20 mg CBD orally infused in baked goods (3 times/day; 1 month). For Eskander et al. [35], both case reports were applying CBD cream to the low-back area. Toor et al. [37] reported 2 months of sublingual medical cannabis use. Zarabian et al. [38] had a treatment of adding cannabidiol oil to the current integrative regimen for pain.
Length of Study
The RCT [46] had a follow-up time of 2 h. The retrospective studies [40,41,42,43] ranged from 12-week follow-up time to retrospectively measuring up to 11 years. The cross-over study, Yassin et al. [45], was 6 months of follow-up time. The case reports [35,36,37,38,39] had a follow-up time ranging from 7–8 h to 60 days (one report states longer than 2 months, but the time of final follow-up is unclear).
Assessment of Low-back Pain Outcomes
(A) Low Back Pain Levels
Questionnaires and tools for assessing LBP levels varied across studies. Using a verbal numerical rating scale from 0 to 10 with 10 being the worst, Bebee et al.'s [46] CANBACK trial assessed pain scores in 30-min increments up to 2 h after administration of CBD or placebo. Ueberall et al. [43] used the pain detection questionnaire (PDQ7) and an aggregated nine-factor symptom relief score (ASR-9) to assess pain levels. Specific to LBP measures, these included the pain intensity index (PIX), visual analogue scale (VAS), and Short Form 12 health survey (SF-12). Mondello et al. [42] used the Douleur Neuropathique 4 and Brief Pain Inventory questionnaire. Vigil et al. [41] administered a 1-year post survey to measure pain reduction pre- and post- cannabis program enrollment. Haroutounian et al. [44] used the S-TOPS and Brief Pain Inventory questionnaires. Yassin et al. [45] used the visual analogue scale (VAS), Oswestry disability index (ODI), and Patient’s Global Impression of Change (PGIC) scales. All five case reports [35,36,37,38,39] used a 1–10 pain scale to assess LBP outcomes after medical cannabis use (Table 1).
(B) Opioid Use Outcomes
The RCT [46] did not measure opioid use as an outcome. Takakuwa et al. [40] focused on daily trends, using morphine equivalent (ME) conversions from prescription opioid use to calculate opioid use per day. Opioid reduction was measured by the length of time it took patients to stop opioids. Length of time was the difference between when a patient was initially treated by a cannabis physician and the visit date when a patient was no longer taking opioids. Likewise, Vigil et al. [41] used average prescribed daily dosage of morphine in the last 3 months but measured the time it took for patients to cease opioids and calculated the change in MEs per day (pre- and post-cannabis use) using the GLOBALRPh’s opioid equivalency calculator (medical calculator for clinicians) and 3:1 oral dosage equivalency to measure opioid consumption levels. Ueberall et al. [43] assessed changes in patients’ opioid medication by comparing the pre- and post-12-week period. One of Haroutounian et al. [44]’s secondary outcomes was opioid consumption after 6 months measured in milligrams of daily opioid use. For Yassin et al. [45], opioid drug use was also assessed using the patients’ medical records of pharmacy dispensed medications to determine whether they increased, decreased, or did not change their opioid use during MCT (Table 1).
Two case studies assessed opioid use as an outcome for LBP treatment. In all instances, no standardized tool or measurement was used to evaluate opioid use. Zarabian et al. [38] reported discontinuation of acetaminophen-codeine use and Toor et al. [37] reported the patient had weaned off all opioid use.
Low Back Pain Level Results
After medical cannabis use or prescription, all observational studies reported improvement (with varying degrees) in LBP or pain-related measures. Brief Pain Inventory scores, used in Haroutounian et al. [44] and Mondello et al. [42], improved. VAS scores and PGIC scales, used in Ueberall et al. [43] and Yassin et al. [45], also improved. Otherwise, all other studies used different LBP scales, including the ODI, GLOBALRPh, PDQ7, DN4, and S-TOPS, and all reported improved scores over time. Conversely, the RCT reported minimal improvements in both the cannabis (pain score of 7.1 to 6.2) and placebo group (pain score of 7.4 to 5.8) but no difference in pain score improvement levels (after 2 h) in the cannabis group versus the placebo (absolute difference of − 0.3) [46]. All five case reports [35,36,37,38,39] indicated an improvement in LBP levels in their patients (Table 2).
Opioid Use Results
Specific to complete opioid discontinuation, Haroutounian et al. [44] and Takakuwa et al. [40] reported discontinuation of opioid use in 32 participants—with differing time periods of how long it took to cease opioid use. Ueberall et al. [43] reported a decrease in oral morphine equivalence of −12.0 in the medical cannabis group. Yassin et al. [45] reported a decrease of pharmacy dispensed opioid medications following MCT. Two case studies reported a decrease in opioid use [37, 38] (Table 2).
Harms and Adverse Effects
Harms and adverse effects of medical cannabis use were beyond the scope of this study. However, it is important to note that there was also a wide range of adverse effects reported in 5 of the 12 studies. In the RCT [46], no serious adverse events were reported. Overall, 4 studies reported mild adverse events. In Takakuwa et al. [40], compared to the 75% of patients who decreased opioid use, 17 (20%) medical cannabis patients had an increase in opioid use, and 3 (4.9%) had no change in opioid use. As this was a short-term study, this may have been due a higher initial opioid use in this subset of patients who had severe chronic pain and were prescribed a higher short-term opioid dose to control their LBP. Given the nature of the study design, it is difficult to determine the true underlying cause of the initial increase. Both Mondello et al. [42] and Ueberall et al. [43] reported short-term mild adverse events, in which drowsiness, attention, dry mouth, and headache were the most commonly reported. In Yassin et al. [45], adverse effects including red eyes, increased appetite, and sore throat were considered mild, which did not require changes in MCT. Conversely, the work by Haroutounian et al. [44] was the only study that reported serious adverse effects in two participants (elevated liver transaminases and hospitalization due to a confused state), causing them to discontinue the study. The remaining 7 studies did not discuss or report any adverse harms or effects. There is a separate category of cannabis research that juxtaposes its therapeutic benefit, focusing on recreational cannabis and its association with high risk behaviors [47, 48], cannabis’ potential harms [19, 49], cannabis use disorder [50], and other side effects from long-term use [51]. Even though evidence may exist that may be contrary to our review, medical cannabis use is an emergent therapeutic method, and we cannot necessarily overlook the potential therapeutic benefit that was reported in our included studies.
Risk of Bias Within and Across Studies
At the individual level, the risk of bias was at the serious or critical level for all studies except the RCT [46]. The prospective [44], retrospective [40,41,42,43], and cross-over [45] studies had critical risk of bias due to deviations from intended interventions and critical bias in the measurement of outcomes. All case reports [35,36,37,38,39] were assessed to be unknown or at a critical level of risk for bias, as the majority of the domains could not be answered since no information was given about the patient populations’ demographics or characteristics (Supplementary Table 2).
GRADE Study Quality
The RCT received an “excellent” to “good” levels for all domains of GRADE. However, the five case studies received a “poor” level of quality for all domains. The prospective, retrospective, and cross-over studies were similar with quality ranging from “poor” to “good.” Four studies were funded by a cannabis company or stated that at least one of the authors had a disclosure of interest with a cannabis company. In all, the cumulative pooled estimate (Supplementary Table 2) showed “poor” to “fair” levels. This was due to low statistical power due to small sample size, lack of covariates, lack of generalizability outside of the study, all studies being a single-site observational study, and inconsistency of baseline and post-intervention measurements (including follow-up) of both pain and medical cannabis use across studies. Publication bias was the only category to receive a “good” level of quality.
Discussion
This systematic review provides preliminary evidence that medical cannabis use may be associated with reducing pain levels and concurrent opioid medication use among individuals with LBP. However, the results do not provide any evidence of a dose–response gradient or novel findings about the efficacy/effectiveness of medical cannabis for improvement of LBP outcomes. All case reports [35,36,37,38,39] stated a numerical decrease in pain scale level. Retrospective cohort studies [40,41,42,43] reported a reduction in overall opioid use in medical cannabis users. All observational studies concluded that medical cannabis use was associated with some level of LBP relief in a subset of their participants, despite the discrepancies on statistical significance on LBP levels, the differing study types, lengths, and contexts.
Our results are consistent with existing cannabis research, in that there is mixed evidence about medical cannabis’ effectiveness towards decreasing LBP. From a pathophysiology perspective, there are a number of studies that show medical cannabis’ interaction with the CB1 (expressed in both CNS and PNS functions of the brain including a role in appetite, learning, memory, anxiety, addiction, and stroke) and CB2 receptors (expressed mostly in immune cells) [52], which are two cannabinoid receptors of the human endocannabinoid system that have been linked to pain reduction and reduction in inflammation [53]. AEA (N-arachidonoyl ethanolamine) [54] is another primary endocannabinoid in the body that has been observed to act on TRPV1 receptors, which have been implicated by Zou and Kumar [54] to be associated with pain processing. Recent studies and reviews [54,55,56] have shown plausibility that a specific CBD strain may be able to target specific CB1 receptors localized in peripheral issues or selectively target CB2 receptors as they are predominantly expressed outside of the brain. This suggests that future RCTs may consider specific strains of medical cannabis to optimize its effects on low LBP.
The findings from the RCT are important to highlight as it is the first trial, of 5 older RCTs [57,58,59,60,61] that showed no difference between cannabis and placebo groups in pain levels and opioid use. This is the only RCT with a large cohort, whereas the 5 previous RCTs had cohort sizes ranging from 1 (“n of 1” studies) to 63. Other systematic reviews echo these findings and report that current synthetic cannabinoid drugs such as nabilone [53] are still considered a weak recommendation as a third-line therapy for pain. Another clinical trial [62] demonstrated that the FAAH endocannabinoid modulator was ineffective against osteoarthritic pain. Noteworthy in this review, only the RCT [46] and Yassin et al. [45] used a recommended standard dose of medical cannabis (RCT, 400 mg CBD; Yassin, 1:4 THC to CBD with THC levels less than 5%; 20 g per month for the first 3 months via vaporization). The remaining studies treated participants with different formulations of medical cannabis or allowed them to self-manage their dose, frequency, strain, and route of administration. Thus, future studies need to control for percentages of THC and CBD to further understand which specific strain can mitigate side effects while also providing an appropriate level of therapeutic benefit.
From a harm reduction perspective, this systematic review indicates that medical cannabis may potentially play a role in containing the opioid epidemic. Harm reduction approach models focus on principles of decreasing the negative effects of opioid use and small gains that lead towards wellness [63]. This study is one of the first reviews to better understand these risk-reduction approaches and has potential implications for medical cannabis in reducing the opioid use for LBP, a condition that heavily relies on opioids. Chronic opioid use can lead to escalation in dosage, which can lead to addiction and a chronic dependency endpoint of death [64]. Although this review could not quantify the population-level effect and magnitude on opioid reduction from medical cannabis use, we know the opioid epidemic in North America is growing, with devastating outcomes for individuals and their families [65]. Although this research is in its infancy, we may infer that any type of decrease in overall opioid use could be an indicator of a potentially beneficial impact for current opioid-using or opioid-dependent individuals [66]. Specifically, the cross-over study [45] and retrospective cohort study [41] emphasized that pre-enrollment and observation time periods were too short to fully observe the potential therapeutic benefit and that patients may have reduced their opioid consumption over a longer time period (> 12 months). For smaller effects on opioid use, it is unclear how clinically important the improvement was in comparison to those who started with a higher dosage of opioids [15].
In the context of North American guidelines for medical cannabis, our findings align with the clinical recommendations from the US NASEM [67] on the use of medical cannabis for chronic pain. Conversely, our findings also align with Canadian clinicians [68, 69] in their recommendation against medical cannabis (particularly smoked) as a primary avenue of treatment for any type of pain (including LBP).
While this is the first systematic review to assess the relationship between medical cannabis use on LBP, it is not without limitations. First, the studies included were based on small sample sizes (based on single sites), which limits the generalizability of individual findings. Heterogeneity of measurement and methodology notably limits our ability to compare results across studies. Prior use of opioids and other drugs for pain were either assumed or grouped as a general “low” or “high” with no standardized dosage or type. The absence of RCTs and reliance on observational designs also limits our ability to draw conclusions about causal inference. Second, most cohort studies did not capture those with LBP who obtained medical cannabis through unauthorized methods and did not include those who self-medicated rather than seeking a physician authorization. There is a possibility that adults are already managing their pain with medical cannabis without authorization, and thus, the data captured in our study may underestimate the true population of LBP cannabis users. Further, there is uncertainty as to whether the medical cannabis authorized was consumed as prescribed, and if patients elected to use alternative treatments for their pain symptoms/management, including any concomitant use with other non-prescription drugs. Given the wide variability of the type of cannabis products or cannabis cultivars used, we cannot pinpoint one specific strain or dose of medical cannabis that may have attributed to the reduction of opioid use or type of pain. Third, most studies in this review were subject to several forms of bias, resulting in low internal validity. Last, the studies did not include the exact time of onset or baseline assessment of pain symptoms for each participant.
LBP is a serious health condition that afflicts individuals worldwide. With the opioid epidemic and opioid-related mortalities rising, the rationale for the use of medical cannabis as an alternative treatment for LBP is also increasing. Investment in studying alternative avenues of treatment such as medical cannabis is pertinent for containing the crisis. This systematic review indicates that there is minimal high-quality evidence to support medical cannabis as a first line treatment at the population level. However, there may be circumstances where certain patients or small subgroups of patients may benefit from using medical cannabis synergistically with other pain medications to alleviate their LBP. Robust RCTs are needed to investigate the safety and efficacy profile of medical cannabis’s prolonged use. Future researchers can take advantage of this emerging literature and study the efficacy of medical cannabis in reducing the opioid burden among LBP patients.
Availability of Data and Materials
Data extraction supplementary tables can be accessed by request to corresponding author.
Abbreviations
- AEA:
-
N-Arachidonoyl ethanolamine
- ASR-9:
-
Aggregated nine factor symptom relief score
- BPI:
-
Brief Pain Inventory
- CBD:
-
Cannabidiol
- DN4:
-
Douleur Neuropathique 4
- GRADE:
-
Grading of Recommendations, Assessment, Development and Evaluations
- LBP:
-
Low back pain
- ME:
-
Morphine equivalence
- ODI:
-
Oswestry disability index
- PIX:
-
Pain intensity index
- PDQ7:
-
Pain detection questionnaire
- PGIC:
-
Patient’s Global Impression of Change
- PICOS:
-
Population, intervention, comparison and outcomes
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT:
-
Randomized controlled trial
- ROBINS-I:
-
Risk of Bias in Non-randomized Studies of Interventions
- SF-12:
-
Short Form 12 health survey
- S-TOPS:
-
Treatment Outcomes in Pain Survey—short form
- THC:
-
Tetrahydrocannabinol
- VAS:
-
Visual analogue scale
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Angst F, Angst J, Ajdacic-Gross V, Aeschlimann A, Rössler W. Epidemiology of back pain in young and middle-aged adults: a longitudinal population cohort survey from age 27–50 years. Psychosomatics. 2017;58(6):604–13.
Casiano VE, Dydyk AM, Varacallo M. Back Pain. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC. 2021.
Allegri M, Montella S, Salici F, Valente A, Marchesini M, Compagnone C, et al. Mechanisms of low back pain: a guide for diagnosis and therapy. F1000Res. 2016;5.
Organization WH. https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions. Published 2021.
Relieving pain in america: a blueprint for transforming prevention, care, education, and research. Washington (DC). 2011.
• Rubinstein SM, de Zoete A, van Middelkoop M, Assendelft WJJ, de Boer MR, van Tulder MW. Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;364:l689. A systematic review on current modalities for low back pain treatment and management.
Traeger A, Buchbinder R, Harris I, Maher C. Diagnosis and management of low-back pain in primary care. CMAJ. 2017;189(45):E1386–95.
Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380.
Kolber MR, Ton J, Thomas B, Kirkwood J, Moe S, Dugré N, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67(1):e20–30.
Chou R, Wagner J, Ahmed AY, Blazina I, Brodt E, Buckley DI, et al. Treatments for acute pain: a systematic review. Rockville (MD). 2020.
Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(7):958–68.
Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872–82.
Lee SW, Shen J, Kim SJ, Chun SY, Kim P, Riaz J, et al. US trends of opioid-use disorders and associated factors among hospitalized patients with spinal conditions and treatment from 2005 to 2014. Spine. 2020;45(2):124–33.
Nicol AL, Hurley RW, Benzon HT. Alternatives to opioids in the pharmacologic management of chronic pain syndromes: a narrative review of randomized, controlled, and blinded clinical trials. Anesth Analg. 2017;125(5):1682–703.
Lee C, Lin M, Martins KJB, Dyck JRB, Klarenbach S, Richer L, et al. Opioid use in medical cannabis authorization adult patients from 2013 to 2018: Alberta, Canada. BMC Public Health. 2021;21(1):843.
Safakish R, Ko G, Salimpour V, Hendin B, Sohanpal I, Loheswaran G, et al. Medical cannabis for the management of pain and quality of life in chronic pain patients: a prospective observational study. Pain Med. 2020;21(11):3073–86.
Okusanya BO, Asaolu IO, Ehiri JE, Kimaru LJ, Okechukwu A, Rosales C. Medical cannabis for the reduction of opioid dosage in the treatment of non-cancer chronic pain: a systematic review. Systematic Reviews. 2020;9(1).
Montero-Oleas N, Arevalo-Rodriguez I, Nunez-Gonzalez S, Viteri-Garcia A, Simancas-Racines D. Therapeutic use of cannabis and cannabinoids: an evidence mapping and appraisal of systematic reviews. BMC Complement Med Ther. 2020;20(1):12.
Pratt M, Stevens A, Thuku M, Butler C, Skidmore B, Wieland LS, et al. Benefits and harms of medical cannabis: a scoping review of systematic reviews. Syst Rev. 2019;8(1):320.
Khan SP, Pickens TA, Berlau DJ. Perspectives on cannabis as a substitute for opioid analgesics. Pain Manag. 2019;9(2):191–203.
• Lucas P, Boyd S, Milloy MJ, Walsh Z. Cannabis significantly reduces the use of prescription opioids and improves quality of life in authorized patients: results of a large prospective study. Pain Med. 2021;22(3):727–39. A recent study on medical cannabis and opioid use.
Takakuwa KM, Sulak D. A survey on the effect that medical cannabis has on prescription opioid medication usage for the treatment of chronic pain at three medical cannabis practice sites. Cureus. 2020;12(12):e11848.
Shah A, Hayes CJ, Lakkad M, Martin BC. Impact of medical marijuana legalization on opioid use, chronic opioid use, and high-risk opioid use. J Gen Intern Med. 2019;34(8):1419–26.
Barry AR, Chris CE. Treatment of chronic noncancer pain in patients on opioid therapy in primary care: a retrospective cohort study. Can Pharm J (Ott). 2020;153(1):52–8.
Orhurhu V, Urits I, Olusunmade M, Olayinka A, Salisu Orhurhu M, Uwandu C, et al. Cannabis use in hospitalized patients with chronic pain. Adv Ther. 2020;37(8):3571–83.
Ehrlich GE. Low back pain. Bull World Health Organ. 2003;81(9):671–6.
• First L, Douglas W, Habibi B, Singh JR, Sein MT. Cannabis use and low-back pain: a systematic review. Cannabis Cannabinoid Res. 2020;5(4):283–9. A systematic review on general cannabis use and low back pain.
Kim TE, Townsend RK, Branch CL, Romero-Sandoval EA, Hsu W. Cannabinoids in the treatment of back pain. Neurosurgery. 2020;87(2):166–75.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
Training C. Cochrane handbook for systematic reviews of interventions. 2022.
Duthey B. https://www.who.int/medicines/areas/priority_medicines/BP6_24LBP.pdf. Published 2004.
(JBI) JBI. https://jbi.global/critical-appraisal-tools.
Bias CM. https://methods.cochrane.org/bias/risk-bias-non-randomized-studies-interventions. Published 2022.
Practice BB. https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/. Published 2022.
Eskander JP, Spall J, Spall A, Shah RV, Kaye AD. Cannabidiol (CBD) as a treatment of acute and chronic back pain: a case series and literature review. J Opioid Manag. 2020;16(3):215–8.
Ko GD, Bober SL, Mindra S, Moreau JM. Medical cannabis - the Canadian perspective. J Pain Res. 2016;9:735–44.
Toor J, Kars M, Deygoo J, Dowling O, Hameed A. Medical marijuana and the discontinuation of opioids in a patient with neuropathic chronic pain. Reg Anesth Pain Med. 2017;42(6).
Zarabian K, Wannon A, Chin M, Kogan M. The intersection between integrative medicine and neuropathic pain: a case report. Explore (NY). 2021.
Yeung B, Patel P, Nelson A, Dayal R. Cannabidiol in the geriatric population: a novel treatment option for chronic back pain. Pain Physician. 2018;21(3):E294.
Takakuwa KM, Hergenrather JY, Shofer FS, Schears RM. The impact of medical cannabis on intermittent and chronic opioid users with back pain: how cannabis diminished prescription opioid usage. Cannabis Cannabinoid Res. 2020;5(3):263–70.
Vigil JM, Stith SS, Adams IM, Reeve AP. Associations between medical cannabis and prescription opioid use in chronic pain patients: a preliminary cohort study. PLoS ONE. 2017;12(11):e0187795.
Mondello E, Quattrone D, Cardia L, Bova G, Mallamace R, Barbagallo AA, et al. Cannabinoids and spinal cord stimulation for the treatment of failed back surgery syndrome refractory pain. J Pain Res. 2018;11:1761–7.
Ueberall MA, Essner U, Mueller-Schwefe GH. Effectiveness and tolerability of THC:CBD oromucosal spray as add-on measure in patients with severe chronic pain: analysis of 12-week open-label real-world data provided by the German Pain e-Registry. J Pain Res. 2019;12:1577–604.
Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain. 2016;32(12):1036–43.
Yassin M, Oron A, Robinson D. Effect of adding medical cannabis to analgesic treatment in patients with low back pain related to fibromyalgia: an observational cross-over single centre study. Clin Exp Rheumatol. 2019;37 Suppl 116(1):13–20.
Bebee B, Taylor DM, Bourke E, Pollack K, Foster L, Ching M, et al. The CANBACK trial: a randomised, controlled clinical trial of oral cannabidiol for people presenting to the emergency department with acute low back pain. Med J Aust. 2021;214(8):370–5.
Miller NS, Ipeku R, Oberbarnscheidt T. A review of cases of marijuana and violence. Int J Environ Res Public Health. 2020;17(5).
Gunn RL, Skalski L, Metrik J. Expectancy of impairment attenuates marijuana-induced risk taking. Drug Alcohol Depend. 2017;178:39–42.
Memedovich KA, Dowsett LE, Spackman E, Noseworthy T, Clement F. The adverse health effects and harms related to marijuana use: an overview review. CMAJ Open. 2018;6(3):E339–46.
Simpson AK, Magid V. Cannabis use disorder in adolescence. Child Adolesc Psychiatr Clin N Am. 2016;25(3):431–43.
Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–27.
Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79(7):516–25.
McGolrick D, Frey N. Nabilone for chronic pain management: a review of clinical effectiveness and guidelines - an update. Ottawa (ON). 2018.
Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3).
Hossain MZ, Ando H, Unno S, Kitagawa J. Targeting peripherally restricted cannabinoid receptor 1, cannabinoid receptor 2, and endocannabinoid-degrading enzymes for the treatment of neuropathic pain including neuropathic orofacial pain. Int J Mol Sci. 2020;21(4).
Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43(1):52–79.
Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford). 2006;45(1):50–2.
Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112(3):299–306.
Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133(1–3):210–20.
Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 “N of 1” studies. Anaesthesia. 2004;59(5):440–52.
Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17(1):21–9.
Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153(9):1837–46.
International HR. The global state of harm reduction 2020. Aldgate, London, United Kingdom. 2020.
Azadfard M, Huecker MR, Leaming JM. Opioid addiction. StatPearls. Treasure Island (FL). 2021.
Belzak L, Halverson J. The opioid crisis in Canada: a national perspective. Health Promot Chronic Dis Prev Can. 2018;38(6):224–33.
Coussens NP, Sittampalam GS, Jonson SG, Hall MD, Gorby HE, Tamiz AP, et al. The opioid crisis and the future of addiction and pain therapeutics. J Pharmacol Exp Ther. 2019;371(2):396–408.
The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington (DC). 2017.
Allan GM, Finley CR, Ton J, Perry D, Ramji J, Crawford K, et al. Systematic review of systematic reviews for medical cannabinoids: pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64(2):e78–94.
Lee C, Round JM, Klarenbach S, Hanlon JG, Hyshka E, Dyck JRB, et al. Gaps in evidence for the use of medically authorized cannabis: Ontario and Alberta, Canada. Harm Reduct J. 2021;18(1):61.
Funding
This systematic review was funded by Northwestern University. ED was supported by the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) (grant #90ARHF0003, PI: Allen Heinemann). AK was supported by the National Institute on Mental Health (K01 MH121854).
Author information
Authors and Affiliations
Contributions
CL designed the study and MB conducted the search. CL and ED were independent reviewers for the systematic review. All other authors (ED, NJ, AK, DE) revised it critically for important intellectual content and approved the final version to be published. All authors are accountable for the work and integrity of the work.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Ethics approval was not applicable nor needed for this systematic review.
Disclaimer
CL affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and if relevant) have been explained.
Consent for Publication
All authors consent to the publication of this manuscript.
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, C., Danielson, E.C., Beestrum, M. et al. Medical Cannabis and Its Efficacy/Effectiveness for the Treatment of Low-Back Pain: a Systematic Review. Curr Pain Headache Rep 27, 821–835 (2023). https://doi.org/10.1007/s11916-023-01189-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-023-01189-0