Abstract
Purpose of Review
The purpose of this review is to provide an overview of the cluneal nerves, present a summary of pain syndromes secondary to clunealgia, and evaluate current literature for diagnostic and treatment modalities.
Recent Findings
Multiple trials and studies have reported success with numerous modalities ranging from nerve blocks, neuroablation, and even peripheral neuromodulation with varying degrees of clinical benefit.
Summary
Cluneal nerve entrapment or chronic impingement can cause buttock pain or referred pain to nearby areas including the lower back, pelvic area, or even the lower extremities. Clunealgias and associated pain syndromes can often be challenging to diagnose and differentiate. An appreciation of the pathophysiology of clunealgias can assist with patient selection for interventional pain strategies targeted towards the cluneal nerves, including nerve blocks, neuroablation, and peripheral neuromodulation. More research is needed to better delineate the efficacy of these procedures for clunealgias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The cluneal nerves are a group of pure sensory nerves that provide direct cutaneous innervation to the buttocks [1,2,3]. In recent years, clunealgias—pain syndromes secondary to cluneal nerve pathology—have been implicated as the cause of chronic pain that both arises directly from the buttocks and is referred from the lower back, pelvic area, or even lower extremity regions [4,5,6]. However, many of these pain syndromes are often unspecified in etiology given the vast preponderance of other anatomical causes often associated with these conditions, occasionally concomitantly. Thus, it is suggested that true cluneal nerve involvement may be overlooked in certain populations and presentations. Despite there being a sparsity of high-level evidence clearly delineating the pathophysiology of clunealgia, the cluneal nerves have been targeted via multiple interventional pain procedures with varying degrees of clinical benefit [7,8,9,10,11]. A clear appreciation for the pathophysiology, symptomatology, and diagnostics of clunealgias is imperative for appropriate selection of patients and interventional strategies.
Cluneal Nerve Anatomy
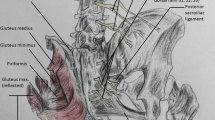
The cluneal nerve complex includes the superior cluneal nerves (SCN), the middle cluneal nerves (MCN), and the inferior cluneal nerves (ICN) (Fig. 1) [1,2,3].
The SCNs are posterior cutaneous branches from the dorsal rami of lower thoracic and upper lumbar spinal nerve roots, usually from the T11-L3 levels [1,2,3]. The SCN travels from superior to inferior and crosses the posterior superior iliac spine (PSIS) while traveling to the quadratus lumborum. The L1–L3 nerves join, pass lateral to the multifidus muscle, and then travel through the erector spinae muscles. The SCN passes through the psoas major and paraspinal muscles and, finally, pierces the inferior latissimus dorsi to provide sensory innervation to the posterior iliac crest and the skin overlying the supero-medial portions of the gluteus maximus. The terminal SCN branches are the medial, middle, and lateral branches across the PSIS. Note that the middle branch of the SCN is a wholly different branch of nerves from the MCN and the distinction must be made. Of clinical significance, the SCN passes through an osteo-fibrous tunnel comprised of the thoracolumbar fascia and iliac crest. This site has been implicated as a cause for LBP (lower back pain) in patients.
The MCNs are sensory nerves derived from the dorsal rami of the upper sacral nerves, including S1, S2, and S3 [1•]. Alternatively, the MCN has been described as originating from the posterior sacrococcygeal plexus (or posterior sacral nerve plexus) that is formed by the S1, S2, and S3 dorsal rami [3]. These nerves exit the sacral foramina and travel inferolaterally under the PSIS and cross the long posterior sacroiliac ligament (LPSL). Whether the MCN is superficial or travels underneath the LPSL is an area of debate, as different anatomic studies have reported variable nerve routes [1•]. The MCNs pierce the gluteus maximus while traveling towards the dorsal sacrum. The MCN innervates the skin overlying the intermediate medial gluteus maximus. Some cadaveric studies have illustrated anastomoses between the MCN and SCN in the subcutaneous tissue of the buttocks [5].
The ICNs are sensory branches of the posterior femoral cutaneous nerve (PFCN), which arises from the S1, S2, and S3 nerves of the sacral plexus [1, 2]. The ICN travels from inferior to superior and wraps around the caudal edge of the gluteus maximus to innervate the skin overlying the inferior aspect of the gluteus maximus muscle and the posterior aspects of the perineum. The ICN also provides cutaneous sensory innervation to the region lateral to the anus (but not the anus itself), as well as the region lateral to either the labium majora or scrotum [2, 12].
The anatomic locations of the cluneal nerves elucidate how these nerves may contribute to a variety of clinically significant pain syndromes. Table 1 describes many of these symptoms and causes. Buttock and LBP can be caused by entrapment or chronic impingement of the cluneal nerves and are among the most common clinical presentations of cluneal nerve pathology.
Pathophysiology
The pathophysiology of cluneal nerve pain is generally multifactorial. The cluneal nerves are susceptible to entrapment neuropathy phenomena secondary to direct or indirect compression or irritation [2]. The incidence of LBP resulting from SCN entrapment has been estimated to range between 1.6 and 14%. While SCN entrapment can be idiopathic or unspecified, persons with lumbar or pelvic procedures are placed at particular risk. As illustrated in Table 1, there are many discrete causes of cluneal nerve pathology. Nerve damage can occur following spinal fusion procedures, sacroiliac screw placements, decubitus ulcer debridement, or muscle flap surgeries [15]. Posterior iliac bone harvest procedures have been notably implicated for compromising SCN integrity [3, 15]. Traditionally, it was believed that entrapment may be more common in females and older patients [4•]. However, studies have shown that certain young populations, such as soldiers, may be at risk as well [16].
Other less common causes of cluneal nerve injury include trauma, muscle spasms (as seen in the quadratus lumborum causing myofascial compression), lumbar spinal canal stenosis, disc herniation, scoliosis, and vertebral fractures. Parkinson’s disease has also been implicated as abnormal muscle tone, and posturing can result in mechanical compression of the cluneal nerves [4].
Clinical Presentation
The pain symptomatology associated with clunealgia is highly dependent on patterns and degrees of SCN, MCN, or ICN involvement [1,2,3,4,5,6]. In all three types of nerve involvement, pain in the lower back or buttocks and abnormal sensations are typically reported. If motor compromise is reported or appreciated, pure cluneal nerve involvement is unlikely given that they are purely sensory nerves. Among the overall LBP patient population, 20–37% of patients may have a neuropathic pain component [6]. Within this subgroup, SCN and MCN nerve pathologies are underreported and often missed as the cause of neuropathic pain.
In patients with SCN pathology, LBP may be reported with radiation to the superior gluteal regions. Pain may be worse with lumbar movements such as rotation, extension, flexion, standing, walking, or rolling [4•]. Dysesthesia may occasionally be the primary symptom, rather than chronic pain itself [2]. In some cases, SCN injury may mimic the symptoms of sciatica. Studies have explained this anatomically by showing that occasionally, the origin of the SCN may involve the T12-L5 nerve roots [4•]. SCN entrapment itself can produce leg symptoms in as many as 47–84% of patients. Similarly, in patients with MCN pathology, LBP is often the most common presentation. As many as 82% of patients with MCN entrapment may also report symptoms involving the leg. Patients may complain of worsening pain with walking. ICN pathology may present as LBP but can also often present as burning, numbness, or tingling along the inferior-medial aspect of the buttocks, as well as the dorsal/proximal thigh, the lateral anal margin, or the skin of the scrotum in males and labia majora in females [2]. Pain is generally worse when patients are sitting on hard surfaces and may be more frequently seen in bike riders.
The physical examination is an important component of the workup and should be used to rule out other causes of LBP. SCN involvement may present with maximal point tenderness at the height of the (presumed) osteo-fibrous tunnel with reproduction of symptoms after application of pressure [15]. Hyperextension or lateral bending involving the quadratus lumborum muscle may reproduce symptoms. One study suggested that full flexion of the ipsilateral hip and knee joints may provoke SCN nerve symptoms [2]. MCN entrapment may present with trigger point tenderness or reproduction of symptoms when pressure is applied caudally to the PSIS at a slightly lateral point (the edge of the iliac crest) [4]. The ICN may present with hyperesthesia or decreased sensation over the inferior buttocks [2]. Symptoms may be reproducible with palpation over the sciatic notch. No motor deficits should be appreciated on neurologic or musculoskeletal examinations.
Diagnosis
In order to diagnose cluneal nerve pathology, clinicians should first perform a thorough history and physical examination paying careful attention to sensory involvement. A comprehensive differential diagnosis should be considered to rule out thoracic and lumbar facet arthropathy, lumbar stenosis, lumbar radiculopathy, discogenic pathology, sacroiliac joint dysfunction, sciatica, obturator neuritis, posterior femoral cutaneous neuritis, myofascial pain syndrome or trigger points, piriformis syndrome, or pelvic pain syndromes [2, 4]. Diagnostic criteria for SCN entrapment and MCN entrapment include lower back pain involving the buttocks that is aggravated by lumbar movements or changes in posture, numbness or radiating pain when the appropriate trigger point is compressed, and symptomatic relief with the use of nerve blocks. In SCN, the trigger point will be over the posterior iliac crest, whereas in MCN, the trigger point is caudal to the PSIS and lateral, approximately at the edge of the iliac crest [4•]. ICN entrapment can often be reproduced by having the patient sit on a hard seat in order to increase nerve compression underneath the ischium [2].
Imaging modalities may play a role in the diagnosis and identification of cluneal nerve pathology. The use of ultrasound imaging in identifying cluneal nerve branches has been described in great detail [17, 18]. Moreover, the use of high-resolution ultrasound has also been described to identify the SCN in patients with lower back pain [15]. Probes of at least 18 MHz were utilized, enabling a resolution of approximately 250 to 500 μm. These findings were then confirmed with diagnostic blocks of the medial branch of the SCN. However, ultrasonic diagnosis and cluneal nerve identification can be limited by obesity, significant muscular atrophy, and practitioner expertise. Nerve blocks—either with fluoroscopic, ultrasound, or anatomical landmark guidance—are considered to be the gold standard for diagnosing cluneal nerve pathology. Ultrasound imaging may be most efficacious given the opportunity for dynamic visualization of anatomical landmarks, but appropriate use can be associated with variability among practitioners. Other imaging modalities, such as computed tomography or magnetic resonance imaging scans play a smaller role. However, they may be useful to rule out alternative causes of LBP or paresthesia in patients [2, 16].
Interventional Management
There currently exists a host of interventional strategies for treating painful conditions secondary to cluneal nerve involvement [7,8,9,10,11,12]. Conservative measures including the use of topical modalities should be optimized first. While pharmacotherapy options are typically first utilized, if reasonable and appropriate, they carry varying adverse effects and may not be tolerated in all persons. The utility of physical therapy can be a reasonable approach but may fail to produce long-standing analgesic benefit [19•].
Given the focal nature of cluneal nerve pain syndromes, localized interventional strategies carry significant promise for analgesic benefit [7,8,9,10,11,12]. However, an appreciation for their clinical considerations and adverse events associated with these procedures is necessary to optimize clinical outcomes. Peripheral nerve blocks have been utilized with varying degrees of benefit for varying duration [7, 17]. Success with neuroablative and neuromodulation modalities has been reported in recent years [8,9,10,11]. Lastly, scenarios of overt cluneal nerve compression or entrapment refractory to conservative or minimally invasive measures may necessitate surgical decompression.
Peripheral Nerve Blocks
Peripheral cluneal nerve blocks with local anesthetics, with or without corticosteroids, can provide rapid analgesic benefits given the immediate effects of local anesthetics [7, 17, 20]. The therapeutic benefit from these nerve blocks may persist across days to weeks. While limited evidence for corticosteroid additions for treating clunealgia exists, there exist data suggesting that corticosteroids can have several detrimental physiologic effects [21]. The incorporation of corticosteroids should be practitioner and patient dependent, with presence of diabetes mellitus with poor glycemic control, poor immunity, and steroid sensitivity considered to be common relative contraindications. While less common, prolotherapy to the cluneal nerves with 12.5–25% dextrose in sterile water has been utilized with varying degrees of benefit for varying duration [22].
Peripheral cluneal nerve blocks can easily be performed at the bedside with anatomical landmarks or ultrasound guidance to directly visualize the nerve [7, 17, 20]. Given the absence of major arteries, nerves, or other vital structures in immediate proximity to the SCN or MCN, ultrasound guidance is not a necessity. However, direct and dynamic cluneal nerve visualization with ultrasound imaging may optimize medication delivery along the nerves and increase the safety profile associated with this procedure [17, 18].
Neuroablation
In cases where peripheral cluneal nerve blocks provide meaningful but only temporary relief, neurolysis may be pursued to provide patients with a longer duration of analgesic benefit [23]. Cluneal neurolysis, usually with 5–8% aqueous phenol, can produce longer term analgesic benefit, sometimes up to 6–9 months or longer. However, it is usually performed only after a diagnosis of clunealgia is well established with an anesthetic nerve block. Phenol neurolysis is a relatively safe procedure, but dysesthesias have been reported post-neurolysis, suspected in cases of incomplete neurolysis or nerve regrowth [24]. Similarly, radiofrequency ablation (RFA) can be utilized to thermally ablate implicated cluneal nerves [19•]. Knight et al. have reported a radiofrequency treatment pathway of the SCNs wherein over 90% of patients reported good to excellent benefit across a 12–24 month follow-up period. Vorobeychik et al. are also exploring benefits of ablation to MCN branches supplying the posterior sacroiliac joint complex; the results of this study are forthcoming [25].
Neuromodulation
While there exist many technologies and modalities of neuromodulation, peripheral nerve stimulation (PNS) provides opportunity for the most targeted treatment of focal pain syndromes [8]. Cases of dorsal root ganglion stimulation have been reported to treat cluneal pain syndromes, but it is thought to be a less utilized modality [9, 11]. While high-level evidence supporting PNS for clunealgias does not exist, several cases of successful pain relief post-implantation have been reported [9,10,11].
There exist well-designed studies with high-level data demonstrating considerable benefits of PNS systems in managing peripheral neuropathic pain [8,9,10,11]. PNS implantation is a relatively safe procedure performed in the ambulatory setting wherein an implantable electrode catheter is placed in close proximity to the implicated nerve. This catheter, which is powered and programmed externally using a programmer device, delivers rapid electrical stimuli which produce paresthesia and inhibit the propagation of peripheral pain [26]. This therapy is particularly favorable relative to other implantable neuromodulatory interventions given that the PRN electrode catheter can be easily removed in non-responder patients and responder patients do not require a separate permanent implantation procedure.
Surgical Decompression
Unfortunately, certain patients with clunealgia may fail to achieve meaningful or sustained analgesic benefit with peripheral nerve blocks, neuroablation, and neuromodulation interventions. In such patients, reevaluation of other pain phenomena is imperative to ensure that cluneal nerve involvement is truly the precipitating noxious pathology. If cluneal nerve involvement is thought to be the true etiology, such patients may warrant surgical decompression [12,13,14].
In cases of overt compression or entrapment, as can be assessed with varying imaging modalities, surgical decompression may provide meaningful benefit. Knight et al. reported their experience in patients with SCN pain pathology who failed to respond to RFA [19•]. Those RFA non-responder patients who achieve some pain reduction with nerve root blocks had eventual success with transforaminal endoscopic lumbar decompression and foraminoplasty with excellent or good outcomes.
Conclusion
The cluneal nerves, consisting of the SCN, MCN, and ICN, are a group of cutaneous nerves providing sensory innervation to the buttocks. Cluneal nerve entrapment or impingement can cause buttock pain or referred pain to nearby areas including the lower back, pelvic area, or even lower extremities. A thorough understanding of cluneal nerve anatomy can help provide an appreciation for clunealgias and other associated pain syndromes, which can often be challenging to diagnose and differentiate. Moreover, an appreciation of the pathophysiology and varying presentations can assist with patient selection for varying interventional pain strategies targeted towards the cluneal nerves. Despite a sparsity of high-level evidence clearly delineating the efficacy of these procedures for clunealgias, multiple reports and studies have reported good success with varying degrees of clinical benefit.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Kikuta S, Iwanaga J, Watanabe K, Tubbs RS. Revisiting the middle cluneal nerves: an anatomic study with application to pain syndromes and invasive procedures around the sacrum. World Neurosurg. 2019;127:1228–31 Anatomy and pain syndromes related to MCN.
Dallas-Prunskis T. Inferior cluneal nerve entrapment. Peripheral nerve entrapments. Spinger. Cham. 2016;52:565–70.
Tubbs RS, Levin MR, Loukas M, Potts EA, Cohen-Gadol AA. Anatomy and landmarks for the superior and middle cluneal nerves: application to posterior iliac crest harvest and entrapment syndromes. J Neurosurg Spine. 2010;13(3):356–9.
• Isu T, Kim K, Morimoto D, Iwamoto N. Superior and middle cluneal nerve entrapment as a cause of low back pain. Neurospine. 2018;15(1):25–32. https://doi.org/10.14245/ns.1836024.012Diagnosis of SCN and MCN nerve entrapment.
Konno T, Aota Y, Saito T, Qu N, Hayashi S, Kawata S, et al. Anatomical study of middle cluneal nerve entrapment. J Pain Res. 2017;10:1431–5.
Aota Y. Entrapment of middle cluneal nerves as an unknown cause of low back pain. World J Orthop. 2016;7(3):167–70.
• Nielsen TD, Moriggl B, Barckman J, et al. Randomized trial of ultrasound-guided superior cluneal nerve block. Reg Anesth Pain Med. 2019;44:772–80 Ultrasound guided nerve blocks of SCN.
Mehta P. Peripheral nerve stimulation. In: Deer's treatment of pain 2019. Cham: Springer. p. 607–14.
Deer T, Pope J, Benyamin R, Vallejo R, Friedman A, Caraway D, et al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation. 2016;19(1):91–100.
Abd-Elsayed A. Wireless peripheral nerve stimulation for treatment of peripheral neuralgias. Neuromodulation: Technology at the Neural Interface. 2020.
McRoberts WP, Deer TR, Abejon D, Barolat G. Stimulation of the extraspinal peripheral nervous system. In: Deer TR, Pope JE, editors. Atlas of implantable therapies for pain management. New York: Springer Science; 2016. p. 171–83.
Darnis B, Robert R, Labat J, et al. Perineal pain and inferior cluneal nerves: anatomy and surgery. Surg Radiol Anat. 2008;30(3):177–83.
Iwanaga J, Simonds E, Patel M, et al. Anatomic study of superior cluneal nerves: application to low back pain and surgical approaches to lumbar vertebrae. World Neurosurg. 2018;116:766–8.
Maigne JY, Doursounian L. Entrapment neuropathy of the medial superior cluneal nerve: nineteen cases surgically treated, with a minimum of 2 years’ follow-up. Spine. 1997;22(10):1156–9.
Bodner G, Platzgummer H, Meng S, Brugger PC, Gruber GM, Lieba-Samal D. Successful identification and assessment of the superior cluneal nerves with high-resolution sonography. Pain Physician. 2016;19(3):197–202.
Ermis MN, Yildirim D, Durakbasa MO, et al. Medial superior cluneal nerve entrapment neuropathy in military personnel; diagnosis and etiologic factors. J Back Musculoskelet Rehabil. 2011;24(3):137–44.
Nielsen TD, Moriggl B, Barckman J, Jensen JM, Kolsen-Petersen JA, Søballe K, et al. Randomized trial of ultrasound-guided superior cluneal nerve block. Reg Anesth Pain Med. 2019;44:772–80.
Chang KV, Wu WT. Is it possible to exactly visualize the superior cluneal nerve using ultrasound imaging?. Reg Anesth Pain Med. 2019:rapm-2019.
• Knight M, Karangoda I, Ahmed RN, D’Angelo T, Inklebarger J, Abbas B, et al. A radiofrequency treatment pathway for cluneal nerve disorders. EC Orthopaedics. 2020; Radiofrequency ablation for cluneal nerve disorders.
Talu GK, Özyalçin S, Talu U. Superior cluneal nerve entrapment. Reg Anesth Pain Med. 2000;25:6.
Brinks A, Koes BW, Volkers AC, Verhaar JA, Bierma-Zeinstra SM. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11(1):206.
Inklebarger J, Galanis N. The management of cluneal nerve referred pain with prolotherapy. J Prolotherapy. 2018;10:982–91.
Ramamurthy S, Walsh NE, Schoenfeld LS, Hoffman J. Evaluation of neurolytic blocks using phenol and cryogenic block in the management of chronic pain. J Pain Symptom Manag. 1989;4(2):72–5.
Karri J, Mas MF, Francisco GE, Li S. Practice patterns for spasticity management with phenol neurolysis. J Rehabil Med. 2017;49(6):482–8.
Vorobeychik Y Optimal performance of RFA of the nerves supplying the posterior sacroiliac joint complex. https://clinicaltrials.gov/ct2/show/NCT02808962. Updated March 30, 2020. Accessed April 27, 2020.
Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008 Jan 1;5(1):100–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jay Karri, Mani Singh, Vwaire Orhurhu, Mihir Joshi, and Alaa Abd-Elsayed declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuropathic Pain
Rights and permissions
About this article
Cite this article
Karri, J., Singh, M., Orhurhu, V. et al. Pain Syndromes Secondary to Cluneal Nerve Entrapment. Curr Pain Headache Rep 24, 61 (2020). https://doi.org/10.1007/s11916-020-00891-7
Published:
DOI: https://doi.org/10.1007/s11916-020-00891-7