Abstract
Purpose of Review
Enhanced recovery pathways are a multimodal, multidisciplinary approach to patient care that aims to reduce the surgical stress response and maintain organ function resulting in faster recovery and improved outcomes.
Recent Findings
A PubMed literature search was performed for articles that included the terms of metabolic surgical stress response considerations to improve postoperative recovery. The surgical stress response occurs due to direct and indirect injuries during surgery. Direct surgical injury can result from the dissection, retraction, resection, and/or manipulation of tissues, while indirect injury is secondary to events including hypotension, blood loss, and microvascular changes. Greater degrees of tissue injury will lead to higher levels of inflammatory mediator and cytokine release, which ultimately drives immunologic, metabolic, and hormonal processes in the body resulting in the stress response. These processes lead to altered glucose metabolism, protein catabolism, and hormonal dysregulation among other things, all which can impede recovery and increase morbidity. Fluid therapy has a direct effect on intravascular volume and cardiac output with a resultant effect on oxygen and nutrient delivery, so a balance must be maintained without excessively loading the patient with water and salt. All in all, attenuation of the surgical stress response and maintaining organ and thus whole-body homeostasis through enhanced recovery protocols can speed recovery and reduce complications.
Summary
The present investigation summarizes the clinical application of enhanced recovery pathways, and we will highlight the key elements that characterize the metabolic surgical stress response and improved postoperative recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhanced recovery after surgery (ERAS) protocols was developed to reduce complications and improve outcomes after surgery. This is accomplished through a multimodal, multidisciplinary approach to patient care that reduces the physiologic stress response to surgery and maintains organ function resulting in faster recovery [1••, 2••]. Teams consisting of surgeons, anaesthetists, surgical staff, and ERAS coordinators are needed to implement and execute these standardized evidence-based interventions [1••, 3, 4]. ERAS protocols include 22 key elements which on their own may not provide a clinically significant impact, but together work synergistically to enhance recovery [2••]. In this article, we will discuss preoperative optimization, the surgical stress response, metabolic function, fluid management, effects on cognition, and their underlying mechanisms and implications in ERAS patients. See Table 1.
Preoperative Optimization
Preoperative optimization in ERAS protocols aims to reduce patient anxiety, complications, metabolic derangements, infections rates, medication adherence, and postoperative nausea and vomiting among other things [1••, 5]. Starting with preadmission screening, the patients are assessed for excessive alcohol intake as well as smoking history. Patients who stop smoking 3–4 weeks prior to their surgery were found to have a similar incidence of postoperative complications compared with nonsmokers [6]. Chronic diseases should be optimized before surgery to reduce complications.
Once the patient has arrived at the hospital, the patient and their family will be given information that has been tailored to their type of surgery. The involvement of family as well as the patient improves compliance within ERAS protocols and can additionally reduce anxiety [7]. ERAS patients routinely receive preoperative carbohydrate treatment and prophylaxis against nausea and vomiting, infection, and thromboembolism. Preoperative carbohydrate loading with drinks 2 h before surgery has shown to reduce the catabolic effects of surgery, while having positive metabolic effects [8,9,10,11].
Direct Effects of Surgery
Direct effects of surgery are primarily related to cellular injury. There is direct surgical injury and indirect injury. Direct surgical injury occurs through surgical access, organ mobilization, excision, and dissection. Greater degrees of tissue injury lead to higher levels of inflammatory mediator and cytokine release which drive immunologic, metabolic, and hormonal processes in the body known as the surgical stress response [12].
Although the development of minimally invasive, laparoscopic, and robotic surgery has greatly decreased direct tissue injury, open approaches are still necessary for certain types of pathology or procedures. Open abdominal surgical procedures have been intensively studied within ERAS protocols. Much of the direct tissue injury in these surgeries occurs through abdominal wall trauma, which may be reduced through changing incision orientation or minimizing the size of the incision [13]. Some studies have shown that transverse incisions have the potential to reduce postoperative pain but others have shown unclear evidence [14, 15]. Laparoscopic procedures minimize surgical incision sites and allow for less abdominal wall trauma leading to less direct tissue injury [13].
Indirect injury occurs through a number of methods including blood loss, alterations in perfusion, microvascular changes, and from anesthetic techniques. Since oxygen delivery is determined by hemoglobin concentration, cardiac output, and oxygen saturation, a decrease in hemoglobin will directly affect oxygen delivery. Decreased oxygen delivery can lead to organ dysfunction and the development of systemic inflammatory response syndrome and sepsis [16]. Although blood loss is inevitable in many surgeries such as hepatic resections, maintaining a low central venous pressure (CVP) can minimize blood flow to the liver in an attempt to decrease surgical blood loss and thus maintain oxygen delivery. Studies have shown that lower CVPs do not decrease inflammation but can decrease the need for blood transfusion [17]. The institution of laparoscopic approaches in colorectal surgery has led to an overall decreased blood loss and reduction in adhesion development [18, 19].
Indirect injury can also occur due to perfusion changes at the microvascular level. Direct surgical manipulation, retraction, ligation of vessels, or removal of tissue or organ components can affect tissue perfusion. When tissues do not receive adequate perfusion, a cascade of events occur which leads to cellular dysfunction. Patient positioning in surgery can also pose a threat to tissue perfusion. Prolonged positions, whether head up or down or tilted positions can cause intravascular fluid shifts and potentially affect tissue perfusion. Prolonged insufflation or high-pressure insufflation of the abdomen for laparoscopic procedures not only causes changes in blood flow and respiratory physiology, but it also triggers a sympathetic response. Pneumoperitoneum causes a rise in afterload that can result in decreased stroke volume and cardiac output, further decreasing oxygen delivery.
The Surgical Stress Response
Direct or indirect injuries lead to cytokine and inflammatory mediator release. In response to cellular injury, neutrophils and macrophages produce proinflammatory cytokines including tumor necrosis factor alpha (TNF-α), and several interleukins (IL-1, IL-6, IL-8) [20]. These cytokines induce the liver to increase synthesis of acute phase proteins such as C-reactive protein (CRP), albumin, ferritin, transferrin, and fibrinogen. Levels of these acute phase reactants, particularly IL-6 and CRP, correlate with the magnitude of the stress response and the development of the systemic inflammatory response [21]. Several studies have demonstrated that laparoscopic surgery, including laparoscopic cholecystectomy and hysterectomy, are associated with lower levels of IL-6 and CRP compared with open procedures [22, 23]. Patients with higher levels of proinflammatory markers are more likely to have postoperative complications [24].
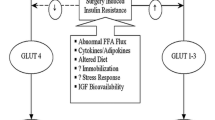
Along with the upregulation of proinflammatory cytokines and acute phase proteins in response to surgical stress, there is activation of the hypothalamic-pituitary-adrenal axis that leads to an elevation in counter-regulatory hormones including cortisol, growth hormone, glucagon, and catecholamines. One of the main surgical concerns regarding the hormonal stress response is the development of insulin resistance through the combination of catecholamine release and impaired immune function [25••]. Catabolic hormones and inflammatory mediators also lead to salt and water retention [13]. In response to surgical injury, mitochondria activity is suppressed with an overall reduction of ATP levels. The increased inflammatory response following surgery leads to increased production of reactive oxygen species (ROS). ROS damage lipids, proteins, and even DNA can lead to impaired vascular permeability. In combination with the release of corticotropin-releasing hormone from the hypothalamus, impaired vascular permeability can eventually result in delayed gastrointestinal function and impaired anastomoses in colorectal procedures [26].
Cell disruption from direct surgical manipulation releases a number of intracellular mediators, including potassium, prostanoids, bradykinin, nerve growth factors, cytokines, and chemokines, which can then cause peripheral sensitization of nociceptors. In addition to these proinflammatory mediators, substance P, and calcitonin gene-related peptide also sensitize nociceptors in adjacent tissues that were not directly injured by surgical trauma. Ultimately, this leads to central sensitization through NMDA receptors in the dorsal horn of the spinal cord and can lead to the development of hyperalgesia, allodynia, and possible chronic postsurgical pain [13].
Metabolic Function
Glucose Homeostatic Function
Major surgery is associated with a significant insult to metabolically active tissues. Intraabdominal and cardiac surgery is particularly burdensome, with intraoperative blood glucose levels in the non-diabetic patient exceeding 10 mmol/L which may remain elevated days after surgery. Increased serum glucose concentrations and peripheral insulin resistance result in persistently elevated blood glucose levels [27]. Metabolic derangements can be further exacerbated with the use of steroids, dextrose-containing solutions, dextrose-rich blood products, or intravenous nutrition [27].
The neurological, cardiovascular, and hematological/immunologic systems are particularly sensitive to alterations in blood glucose. Organ dysfunction, adverse wound outcomes, cardiovascular events, and mortality are all more common in those with hyperglycemia [28,29,30]. Animal models have suggested that fluctuating levels of serum glucose is associated with the poorest outcomes, but long term poor diabetic control, as measured by HbA1c, is also positively correlated with morbidity [31, 32].
Protein Catabolism
Healthy patients can lose 40–80 g of nitrogen after elective abdominal surgery [33]. This loss is equivalent to a loss of approximately 2 kg of skeletal muscle [33]. This is a profound loss for the healthy patient and potentially catastrophic to the malnourished patient. Patients who are already in a catabolic state, such as those with burns or sepsis, experience daily losses of up to 800 g of muscle mass [33]. Protein loss in type 2 diabetic patients after colorectal cancer surgery has been shown to be 50% greater than in non-diabetics [34]. There is no definitive evidence to suggest that older people have a larger protein-wasting deficit.
Proteins are the body’s functional units, and diminishment of stores is associated with delayed mobility, poorer respiratory function, infection from a reduced immunological function, and prolonged recovery [35].
Hormonal Dysregulation
Surgery increases levels of inducible positive glucostat hormones (glucagon, cortisol, and catecholamines) and cytokines that have nuclear effects on metabolism (IL-6, TNF-α) [36]. These hormones and cytokines reduce the effect of insulin, either by inhibiting its secretion or its downstream cytosolic effects. Skeletal muscle seems to be the point of peak effect for insulin resistance and also reflects the largest capacity uptake organ for glucose [35]. Insulin resistance is most pronounced on postoperative day 1 and continues for approximately 3 weeks after surgery. This seems dependent on the nature of the surgery, with open abdominal and cardiac surgery having the greatest impact [36, 37].
Reduced insulin sensitivity is associated with longer hospital stays and is irrespective of a patient’s preoperative diabetic state. Risk of serious morbidity and mortality was doubled with an increased insulin resistance of only 20%.
Preoperative Assessment and Intervention
Quantification of catabolism can be achieved by assessment via anthropomorphic measurements or by the biochemical assessment of synthetic function—however these assessments are neither sensitive nor specific for catabolism [38]. In practice, no good measurement metric exists, and gestalt assessments are the norm.
Avoidance of preoperative fasting may mitigate the stress response to surgery [39]. There must be a balance struck between avoidance of depletion of hepatic glycogen stores from starvation and the anesthetic risks aspiration. Nutritionally replete patients are shown to have less postoperative insulin resistance, decreased protein wasting, and decreased hospital length of stay [40,41,42]. To avoid starvation, administration of carbohydrate rich drinks is a technique employed in many ERAS protocols.
An unexpectedly large percentage of the cohort of patients awaiting surgery have impaired glucose metabolism implying that surgical pathology itself may also predispose to hormonal dysfunction [43].
Anesthetic Technique
Choice of anesthetic drugs and technique play a role in avoiding metabolic dysfunction. Neuraxial, propofol, and opioid heavy anesthetics tend to minimize glucose dysregulation versus inhalational techniques [44]. The intraoperative use of beta blockade to prevent hepatic glycogenolysis has been posited but not widely utilized [45]. Neuraxial anesthesia segmentally inhibits ascending nociceptive pathways and abolishes or attenuates the descending surgical stress response from the locus coeruleus. This phenomenon improves insulin sensitivity and decreases protein catabolism. By way of decreasing insulin resistance, these techniques reduce the amount of energy required to maintain a neutral or positive nitrogen balance [35]. This then prevents the requirement of further proinflammatory or insulin resistant antecedent treatments such as IV nutrition. The use of an epidural has been shown to decrease protein loss by 100 g/day [46]. In this same study, the infusion of small amounts of dextrose and amino acids actually pushed the patient into an anabolic state [46]. Opioid-rich techniques blunt responses within theater, but postoperative catabolism appears to be unaffected.
Maintenance of Glycemic Control and Nutrition
Maintaining glycemic control is associated with better patient outcomes as both lax and rigid controls of normoglycemia have been associated with morbidity and death [47, 48]. Normoglycemia appears to be a target very difficult to maintain, even with continuous insulin infusion, so relative consensus is that capillary glucose levels should be less than 10 mmol/L.
Patients undergoing major surgery are in a state of starvation postoperatively. Without nutritional supplementation, tissue loss occurs. Nutritional supplementation must be tailored such that it is adequate to prevent catabolism but also not so excessive as to cause impaired glucose metabolism, liver dysfunction, or respiratory failure [49]. Most ERAS protocols suggest early intake of oral diet. This may be difficult in abdominal surgery, but previous work has suggested this is safe for anastomoses and is associated with reduced incidence of ileus [50].
Fluid Balance
Fluid therapy has a direct effect on intravascular volume and cardiac output with a resultant effect on oxygen and nutrient delivery to the tissues. The microcirculation is highly sensitive to the modality and volume of fluid administered.
The physiological stress of surgery results in high levels of catecholamines, inflammatory cytokines, aldosterone, ADH, and other hormones that instigate renal salt and water retention. In the surgical abdomen, gut motility is reduced, permeability increased, and alimentary microvascular rheological changes instigate hypercoagulability [12]. Salt and water overload places bowel anastomoses at risk and is also associated with ileus [12]. Increases in body weight greater than 2.5 kg during hospital admission are associated with longer hospital stays and complications [51].
The anesthesiologist must balance maintenance of intravascular volume and tissue perfusion without excessively loading the patient with water and salt. Adjunct monitoring devices may help guide intervention but have not been shown to improve outcomes [52]. ERAS protocols suggest aiming for normovolemia throughout the perioperative period. Due to anesthetic technique and vasopelegia, relative hypotension during the course of the operation is common. Postoperatively oral intake is encouraged, and IV fluids are ceased early to prevent volume overload. Despite these directives, no clear guidance on fluid administration is available, but consensus suggests erring on the side of restrictive practices.
Maintenance of Gastrointestinal Function
The gut, with its intricate enteric nervous system and secretory and absorptive tissues, is sensitive to inflammatory changes from surgical stress. Sympathetic stimulation of the enteric nervous system results in acute intestinal paralysis [53]. Hours after handling and tissue injury, activation of the immune system, and production of reactive oxygen species cause direct cell damage and chemical inhibition of the normal muscular function [53]. This inflammatory state causes reduced bowel motility, increased vascular permeability, and bowel wall edema resulting in ileus. This phenomenon is further exacerbated by large volume of fluid administration and opioid receptor agonism. Those in pathological low serum oncotic pressure states such as hypalbuminemia are further prone to ileus due to readily translocated fluid out of vascular spaces. Ileus and bowel wall edema are associated with longer hospital stays, delayed return of normal gut function, nausea and vomiting, aspiration, and anastomosis failure [26].
Laparoscopic surgical approach is superior to open procedure, and this benefit is further augmented by an ERAS care plan [54]. The use of epidural analgesia minimizes opioid administration and mitigates the release of catecholamines from surgical incision and bowel handling [55]. Epidural has also shown to reduce ileus duration in those undergoing bowel surgery [56]. Avoidance of nasogastric tubes reduce irritation of the alimentary tract and it does not appear to be of any benefit to routine placement [57]. The gut has a very complicated microcirculatory system with approximately 9 l of intestinal fluid turnover in normal states [58]. This complex mechanism is highly sensitive to volume status. Early enteral feeding is a staple of ERAS protocols, and the evidence clearly shows that it reduces ileus, hospital length of stay, anastomotic leak, and effects of malnutrition such as infection [59].
Pharmacological interventions such as peripherally acting mu-receptor antagonists like alvimopan have been trialled with good effect on both returns of gut function and hospital length of stay [60]. Intravenous lidocaine has been posited to decrease levels of inflammatory cytokines and therefore enhance gut recovery [61]. When trialled against control lidocaine infusion at clinically appropriate doses, it was superior for pain scores and gut function [62]. Postoperative pharmacological interventions such as coffee, prokinetic agents, chewing gum, laxatives, nicotine patches, non-steroidal anti-inflammatory drugs, magnesium sulphate, and a traditional Japanese herbal supplement (Daikenchuto) have all been trialled with various efficacy.
Effect of Surgery on Cognition
One of the topics that is garnering more attention and research is how surgery effects cognition [63]. Alterations in cognition postoperatively are associated with poor patient outcomes and can result in longer hospitalizations [6]. Derangements in mental status after surgery can be classified as either postoperative delirium (POD) or postoperative cognitive decline (POCD) [13]. POD is characterized as inattention, disorganized thinking, and altered level of consciousness [64]. POCD is more chronic in nature and described by deficits in coordination, attention, visuospatial recognition, concentration, executive function, verbal memory, and psychomotor speed [13]. Specific patient populations have been identified that are at greater risk to develop postoperative alterations in cognition. These include patients of advanced age, lower education, and those with preexisting conditions such as vascular dementia, attention deficit disorder, and metabolic syndrome [13].
While the exact mechanisms are unknown, ERAS protocols attempt to limit and avoid triggers that may propagate altered cognition in the postoperative period. The surgical stress response results are thought to contribute to neurologic sequelae of altered cognition [65]. Decreasing inflammation via minimally invasive surgical techniques, avoidance of long-acting benzodiazepines, careful titration of intravenous fluids, and multimodal analgesic strategies are all ways to potentially help reduce the onset of cognitive perturbations [66].
Summary
ERAS protocols have proven to be beneficial for patients and healthcare entities by increasing patient satisfaction and reducing healthcare costs. Goals of ERAS protocols are to expedite patient recovery, decrease the chance for adverse events throughout the entire perioperative period, and to save hospitals money by optimizing care and decreasing length of stay. Implementing interventions such as early mobilization, early nutrition, multimodal analgesia, and careful titration of fluid resuscitation are now cornerstones of perioperative care. ERAS protocols will continue to play a pivotal role in the future as we continue to better understand the physiologic response to surgery and as both surgical and anesthetic techniques continue to improve.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
•• Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery. JAMA Surg. 2017;152:292–8. https://doi.org/10.1001/jamasurg.2016.4952 .Excellent review of literature related to enhanced recovery after surgery.
•• Scott MJ, Urman RD. Concepts in physiology and pathophysiology of enhanced recovery after surgery. Int Anesthesiol Clin. 2017;55:38–50. https://doi.org/10.1097/AIA.0000000000000166 Excellent review of concepts in physiology and pathophysiology related to enhanced recovery after surgery.
Horres CR, Adam MA, Sun Z, Thacker JK, Moon RE, Miller TE, et al. Enhanced recovery protocols for colorectal surgery and postoperative renal function: a retrospective review. Perioper Med (London, England). 2017;6:13. https://doi.org/10.1186/s13741-017-0069-0.
Steinthorsdottir KJ, Kehlet H, Aasvang EK. Surgical stress response and the potential role of preoperative glucocorticoids on post-anesthesia care unit recovery. Minerva Anestesiol. 2017;83:1324–31. https://doi.org/10.23736/S0375-9393.17.11878-X.
Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129–35. https://doi.org/10.1016/j.jclinane.2016.07.007.
Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, et al. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–6.
Nygren J, Thacker J, Carli F, Fearon KCH, Norderval S, Lobo DN, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2013;37:285–305. https://doi.org/10.1007/s00268-012-1787-6.
Hausel J, Nygren J, Lagerkranser M, Hellström PM, Hammarqvist F, Almström C, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93:1344–50. https://doi.org/10.1097/00132586-200304000-00042.
Henriksen MG, Hessov I, Dela F, Hansen HV, Haraldsted V, Rodt SA. Effects of preoperative oral carbohydrates and peptides on postoperative endocrine response, mobilization, nutrition and muscle function in abdominal surgery. Acta Anaesthesiol Scand. 2003;47:191–9.
Noblett SE, Watson DS, Huong H, Davison B, Hainsworth PJ, Horgan AF. Pre-operative oral carbohydrate loading in colorectal surgery: a randomized controlled trial. Color Dis. 2006;8:563–9. https://doi.org/10.1111/j.1463-1318.2006.00965.x.
Jankowski CJ. Preparing the patient for enhanced recovery after surgery. Int Anesthesiol Clin. 2017;55:12–20. https://doi.org/10.1097/AIA.0000000000000157.
Scott MJ, Miller TE. Pathophysiology of major surgery and the role of enhanced recovery pathways and the anesthesiologist to improve outcomes. Anesthesiol Clin. 2015;33:79–91. https://doi.org/10.1016/j.anclin.2014.11.006.
Feldheiser A, Aziz O, Baldini G, Cox BPBW, Fearon KCH, Feldman LS, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60:289–334. https://doi.org/10.1111/aas.12651.
Rosenberg TPGJ, Rosenberg J. Vertical compared with transverse incisions in abdominal surgery. Eur J Surg. 2001;167:260–7. https://doi.org/10.1080/110241501300091408.
Seiler CM, Deckert A, Diener MK, Knaebel H-P, Weigand MA, Victor N, et al. Midline versus transverse incision in major abdominal surgery. Ann Surg. 2009;249:913–20. https://doi.org/10.1097/SLA.0b013e3181a77c92.
Stephan RN, Kupper TS, Geha AS, Baue AE, Chaudry IH. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg. 1987;122:62–8.
Page AJ, Ejaz A, Spolverato G, Zavadsky T, Grant MC, Galante DJ, et al. Enhanced recovery after surgery protocols for open hepatectomy—physiology, immunomodulation, and implementation. J Gastrointest Surg. 2015;19:387–99. https://doi.org/10.1007/s11605-014-2712-0.
Dowson HM, Bong JJ, Lovell DP, Worthington TR, Karanjia ND, Rockall TA. Reduced adhesion formation following laparoscopicversus open colorectal surgery. Br J Surg. 2008;95:909–14. https://doi.org/10.1002/bjs.6211.
Schwenk W, Haase O, Neudecker JJ, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 2005;CD003145. https://doi.org/10.1002/14651858.CD003145.pub2.
Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79:757–60.
Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157:362–80. https://doi.org/10.1016/j.surg.2014.09.009.
Kim TK, Yoon JR. Comparison of the neuroendocrine and inflammatory responses after laparoscopic and abdominal hysterectomy. Korean J Anesthesiol. 2010;59:265–9. https://doi.org/10.4097/kjae.2010.59.4.265.
Jakeways MS, Chadwick SJ, Carli F. A prospective comparison of laparoscopic versus open cholecystectomy. Ann R Coll Surg Engl. 1993;75:142.
Podgoreanu MV, Michelotti GA, Sato Y, Smith MP, Lin S, Morris RW, et al. Differential cardiac gene expression during cardiopulmonary bypass: ischemia-independent upregulation of proinflammatory genes. J Thorac Cardiovasc Surg. 2005;130:330–9. https://doi.org/10.1016/j.jtcvs.2004.11.052.
•• Carli F. Physiologic considerations of enhanced recovery after surgery (ERAS) programs: implications of the stress response. Can J Anesth Can Anesth. 2015;62:110–9. https://doi.org/10.1007/s12630-014-0264-0 Excellent review of concepts in stress pathophysiology related to enhanced recovery after surgery.
Chowdhury AH, Lobo DN. Fluids and gastrointestinal function. Curr Opin Clin Nutr Metab Care. 2011;14:469–76. https://doi.org/10.1097/MCO.0b013e328348c084.
Eberhart LH, Graf J, Morin AM, Stief T, Kalder M, Lattermann R, et al. Randomised controlled trial of the effect of oral premedication with dexamethasone on hyperglycaemic response to abdominal hysterectomy. Eur J Anaesthesiol. 2011;28:195–201. https://doi.org/10.1097/EJA.0b013e32834296b9.
Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80:862–6. https://doi.org/10.4065/80.7.862.
Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–8. https://doi.org/10.4065/78.12.1471.
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–82. https://doi.org/10.1210/jcem.87.3.8341.
Deane AM, Horowitz M. Dysglycaemia in the critically ill - significance and management. Diabetes Obes Metab. 2013;15:792–801. https://doi.org/10.1111/dom.12078.
Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95:4338–44. https://doi.org/10.1210/jc.2010-0135.
Kinney JM, Elwyn DH. Protein metabolism and injury. Annu Rev Nutr. 1983;3:433–66. https://doi.org/10.1146/annurev.nu.03.070183.002245.
Schricker T, Gougeon R, Eberhart L, Wykes L, Mazza L, Carvalho G, et al. Type 2 diabetes mellitus and the catabolic response to surgery. Anesthesiology. 2005;102:320–6.
Schricker T, Lattermann R. Perioperative catabolism. Can J Anesth. 2015;62:182–93. https://doi.org/10.1007/s12630-014-0274-y.
Thorell A, Loftenius A, Andersson B, Ljungqvist O. Postoperative insulin resistance and circulating concentrations of stress hormones and cytokines. Clin Nutr. 1996;15:75–9.
Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69–78.
Downs JH, Haffejee A. Nutritional assessment in the critically ill. Curr Opin Clin Nutr Metab Care. 1998;1:275–9.
Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2013;37:259–84. https://doi.org/10.1007/s00268-012-1772-0.
Crowe PJ, Dennison A, Royle GT. The effect of pre-operative glucose loading on postoperative nitrogen metabolism. Br J Surg. 1984;71:635–7.
Ljungqvist O, Thorell A, Gutniak M, Häggmark T, Efendic S. Glucose infusion instead of preoperative fasting reduces postoperative insulin resistance. J Am Coll Surg. 1994;178:329–36.
Yagci G, Can MF, Ozturk E, Dag B, Ozgurtas T, Cosar A, et al. Effects of preoperative carbohydrate loading on glucose metabolism and gastric contents in patients undergoing moderate surgery: a randomized, controlled trial. Nutrition. 2008;24:212–6. https://doi.org/10.1016/j.nut.2007.11.003.
Hatzakorzian R, Bui H, Carvalho G, Pi Shan WL, Sidhu S, Schricker T. Fasting blood glucose levels in patients presenting for elective surgery. Nutrition. 2011;27:298–301. https://doi.org/10.1016/j.nut.2010.02.003.
Horber FF, Krayer S, Miles J, Cryer P, Rehder K, Haymond MW. Isoflurane and whole body leucine, glucose, and fatty acid metabolism in dogs. Anesthesiology. 1990;73:82–92.
Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–9. https://doi.org/10.1056/NEJMoa010342.
Schricker T, Meterissian S, Wykes L, Eberhart L, Lattermann R, Carli F. Postoperative protein sparing with epidural analgesia and hypocaloric dextrose. Ann Surg. 2004;240:916–21.
NICE-SUGAR Study Investigators, Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–18. https://doi.org/10.1056/NEJMoa1204942.
Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67. https://doi.org/10.1056/NEJMoa011300.
Askanazi J, Rosenbaum SH, Hyman AI, Silverberg PA, Milic-Emili J, Kinney JM. Respiratory changes induced by the large glucose loads of total parenteral nutrition. JAMA. 1980;243:1444–7.
Boelens PG, Heesakkers FFBM, Luyer MDP, van Barneveld KWY, de Hingh IHJT, Nieuwenhuijzen GAP, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery. Ann Surg. 2014;259:649–55. https://doi.org/10.1097/SLA.0000000000000288.
Levy BF, Scott MJP, Fawcett WJ, Rockall TA. 23-hour-stay laparoscopic colectomy. Dis Colon Rectum. 2009;52:1239–43. https://doi.org/10.1007/DCR.0b013e3181a0b32d.
Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt SÅ, Hansen B, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth. 2012;109:191–9. https://doi.org/10.1093/bja/aes163.
Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004;16:54–60. https://doi.org/10.1111/j.1743-3150.2004.00558.x.
Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61:1181–7. https://doi.org/10.1111/j.1742-1241.2007.01415.x.
Holte K, Kehlet H. Epidural anaesthesia and analgesia - effects on surgical stress responses and implications for postoperative nutrition. Clin Nutr. 2002;21:199–206.
Marret E, Remy C, Bonnet F. Postoperative pain forum group. Meta-analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Surg. 2007;94:665–73. https://doi.org/10.1002/bjs.5825.
Nelson R, Tse B, Edwards S. Systematic review of prophylactic nasogastric decompression after abdominal operations. Br J Surg. 2005;92:673–80. https://doi.org/10.1002/bjs.5090.
Michell AR. Diuresis and diarrhea: is the gut a misunderstood nephron? Perspect Biol Med. 2000;43:399–405.
Zhuang C-L, Ye X-Z, Zhang C-J, Dong Q-T, Chen B-C, Yu Z. Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of randomized clinical trials. Dig Surg. 2013;30:225–32. https://doi.org/10.1159/000353136.
Tan EK, Cornish J, Darzi AW, Tekkis PP. Meta-analysis: alvimopan vs. placebo in the treatment of post-operative ileus. Aliment Pharmacol Ther. 2006;0:061017010431001–??? https://doi.org/10.1111/j.1365-2036.2006.03150.x.
McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery. Drugs. 2010;70:1149–63. https://doi.org/10.2165/10898560-000000000-00000.
Sun Y, Li T, Wang N, Yun Y, Gan TJ. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery. Dis Colon Rectum. 2012;55:1183–94. https://doi.org/10.1097/DCR.0b013e318259bcd8.
Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–9. https://doi.org/10.1056/NEJMoa1112923.
Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96:747–53. https://doi.org/10.1093/bja/ael094.
Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, et al. Depletion of bone marrow–derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–36. https://doi.org/10.1097/ALN.0b013e3182834d94.
Rasmussen LS, Steentoft A, Rasmussen H, Kristensen PA, Moller JT. Benzodiazepines and postoperative cognitive dysfunction in the elderly. ISPOCD group. International study of postoperative cognitive dysfunction. Br J Anaesth. 1999;83:585–9.
Disclaimer
The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript and are involved in institutional protocols and policies for enhanced recovery pathways.
Corresponding author
Ethics declarations
Conflict of Interest
Erik M. Helander, Michael P. Webb, Bethany Menard, Amit Prabhakar, John Helmstetter, Elyse M. Cornett, and Viet H. Nguyen declare no conflict of interest. Alan Kaye is on the speaker bureau for the Merck and Depomed, Inc. Richard D. Urman received research funding from the Medtronic.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Other Pain
Rights and permissions
About this article
Cite this article
Helander, E.M., Webb, M.P., Menard, B. et al. Metabolic and the Surgical Stress Response Considerations to Improve Postoperative Recovery. Curr Pain Headache Rep 23, 33 (2019). https://doi.org/10.1007/s11916-019-0770-4
Published:
DOI: https://doi.org/10.1007/s11916-019-0770-4