Abstract
Purpose of Review
In contrast to well-established relationships between headache and affective disorders, the role of alcohol use in primary headache disorders is less clear. This paper provides a narrative overview of research on alcohol use disorders (AUDs) in primary headache and presents a meta-analysis of the role of alcohol as a trigger (precipitant) of headache.
Recent Findings
The majority of studies on AUDs in headache have failed to find evidence that migraine or tension-type headache (TTH) is associated with increased risk for AUDs or problematic alcohol use. The meta-analysis indicated that 22% (95% CI: 17–29%) of individuals with primary headache endorsed alcohol as a trigger. No differences were found between individuals with migraine (with or without aura) or TTH. Odds of endorsing red wine as a trigger were over 3 times greater than odds of endorsing beer.
Summary
An absence of increased risk for AUDs among those with primary headache may be attributable to alcohol’s role in precipitating headache attacks for some susceptible individuals. Roughly one fifth of headache sufferers believe alcohol precipitates at least some of their attacks. Considerable study heterogeneity limits fine-grained comparisons across studies and suggests needs for more standardized methods for studying alcohol-headache relationships and rigorous experimental designs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary headache disorders of migraine and tension-type headache (TTH) are two of the most prevalent medical conditions globally [1], and migraine remains the sixth leading cause of years lived with disability worldwide [2]. The impact of these conditions is often compounded by co-occurring (comorbid) psychiatric disorders such as major depressive disorder, various anxiety disorders, and bipolar disorder [3,4,5,6,7]. Compared to the abundant literature on the prevalence and impact of mood and anxiety disorders among individuals with migraine or TTH, fewer studies have examined relations between alcohol use disorders (AUDs) and headache. Alcohol use disorders are classified as problematic patterns of alcohol use that result in clinically significant impairment [8]. Alcohol use is of interest also because alcohol may serve as a precipitant (i.e., trigger) of headache attacks for some individuals. The purpose of this paper is to review extant literature on the role of alcohol use in the primary headache disorders of migraine and TTH. The paper first provides a narrative overview of research on AUDs among headache suffers then presents results of a meta-analytic review on the role of alcohol as a potential headache trigger.
Alcohol Use Disorders in Primary Headache
Compared to other substance use disorders, AUDs are relatively common among adults (8.5% annual prevalence), and prevalence peaks between ages 18–29 (16.2% annual prevalence; [8]). Unlike migraine, AUDs affect men more than women. Previously differentiated into separate diagnoses, alcohol abuse and alcohol dependence, the current fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) classification of AUDs recognizes that, like other substance use disorders, maladaptive alcohol use occurs on a continuum of severity (i.e., mild, moderate, severe) and no longer distinguishes two distinct entities [8]. However, most of the studies on AUDs among individuals with headache were conducted prior to this change in diagnostic nomenclature.
The largest epidemiologic studies adhering to diagnostic criteria for AUDs used samples from the USA [6, 7, 9, 10], Canada [5], and Singapore [11]. In the earliest study, Breslau and colleagues [9] examined 1007 members of a health maintenance organization between the ages of 21 and 30. Compared to those without migraine, individuals with migraine with aura (MA) were at greater risk for lifetime alcohol abuse or dependence (20.6 vs 30.5%, odds ratio [OR] = 2.1). Those who had migraine without aura were not at statistically increased risk (24.6%; OR = 1.6). The second U.S. study of 1343 adults found no association between alcohol or drug use disorders at baseline and incident migraine over a decade later (OR = 1.05; [10]). A third large-scale U.S. study of 5692 adults also found no significant relationship between 12-month alcohol abuse or dependence and migraine (4.2% prevalence among migraineurs vs 3.0% for those without headache; OR = 1.4) or TTH (3.4% prevalence among TTH; OR = 1.1; [6]). The most recent U.S. study of 5064 adults similarly found no significant relationship between alcohol abuse (either lifetime or 12-month prevalence) and episodic migraine or chronic daily headache [7].
The Canadian study of 36,984 individuals aged 15+ found no differences in 12-month prevalence of alcohol dependence between individuals with and without migraine (2.3 vs 2.6%; [5]). Finally, Subramaniam et al. [11] assessed lifetime AUDs (i.e., abuse or dependence) among adults with various chronic pain conditions (i.e., migraine, arthritis, back pain) in Singapore. Compared to those without migraine, migraineurs had greater risk for a lifetime AUD (7.8%; OR = 2.1).
Though the findings are not entirely consistent, the majority of high-quality epidemiological studies have failed to find evidence suggesting migraine confers statistically increased risk for AUDs among the general population. Differences in findings between the aforementioned studies may be a result of varying adherence to AUD and migraine diagnostic criteria. Two of the earlier studies [9, 10] used AUD diagnostic criteria prior to publication of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [12], which more strictly defined alcohol dependence than prior editions. Of the three more recent studies, only the Saunders et al. [6] study assessed migraine via adherence to most of the migraine criteria set forth by the International Classification of Headache Disorders (ICHD-3 beta) [13]. To the extent that utilization of structured interviews comporting with established diagnostic criteria is indicative of study quality, existing evidence suggests lack of a clear relationship between AUDs and migraine. Though it is possible that migraine portends increased risk to develop AUDs many years later, epidemiological studies using multiple time points have not found evidence of a delayed risk effect [10, 14], and peak prevalence of AUDs is generally earlier than that of migraine [8, 15].
Other studies have evaluated relations between alcohol use and headache without attempting to formally establish AUD diagnoses, in which scores on validated measures of alcohol use were compared between different headache groups. Among a sample of outpatients at a headache clinic in Brazil, migraineurs were less likely than those with TTH to obtain a score on the Alcohol Use Disorders Identification Test (AUDIT; [16]) indicative of problematic alcohol use (PAU; 5.2 vs 16.1%; [17]). Two other studies compared rates of PAU among individuals with migraine, with non-migrainous headache, and without headache. In the first, 38,508 Norwegians were administered the CAGE questionnaire [18] to assess alcohol overuse [19]. Rates of alcohol overuse (1 or more positive CAGE endorsement) did not differ significantly between any of the three groups; increased frequency of alcohol consumption was associated with reduced odds of both types of headache. In the second study, Brazilian medical students without headache endorsed higher rates of PAU on the AUDIT than students with migraine or non-migrainous headache (20.0 vs 4.0% and 8.6, respectively; [20]). Though differences in study samples and validated measures limit comparisons, these studies do not suggest that individuals with migraine are at greater risk for PAU than individuals without headache or individuals with TTH. In this regard, their findings are similar to those from the aforementioned AUD studies.
Alcohol as a Headache Trigger
The preponderance of cross-sectional evidence suggesting that migraine does not confer increased risk for AUDs may be attributable to its role as a trigger of individual headache attacks, such that headache sufferers may abstain from alcohol to avoid precipitating headache (or be counseled to do so by their treating clinician). Although alcohol is not endorsed as a headache trigger as commonly as stress, hormones (in women), or missing meals or sleep [21], the role of diet in headache has been a source of considerable attention [22••]. Experimental research on precipitants of individual headache attacks is beset with difficulties in confirming causal relationships [23•, 24], and additional challenges present when studying alcohol specifically [25]. These include differentiating headache that is induced rather quickly after ingesting alcohol from a more delayed headache that often occurs 5–12 h later (the latter of which is more common) and teasing out the underlying biochemical mechanisms in various alcoholic drinks. Though few studies have specifically addressed these unique challenges, the overwhelming majority of research on alcohol as a headache trigger is cross-sectional in nature. Multiple narrative reviews of this literature have been published within the last decade [22••, 25, 26•, 27], but a quantitative synthesis of existing studies is lacking. Accordingly, we conducted a meta-analytic review of such studies to quantify the effects of alcohol as a headache trigger given differences in methodologies, samples, and endorsement rates across prior studies. In this meta-analytic review, we hypothesized that alcohol consumption would be perceived as a trigger for both migraine and TTH, and we endeavored to assess variables that might moderate its role as a trigger (e.g., headache diagnosis, type of alcohol).
Methods
The meta-analysis adhered to PRISMA reporting guidelines [28]. Institutional review board approval was not necessary as this was a quantitative review of previously published data.
Search Strategy
The primary author conducted all search and eligibility review processes. On May 6, 2015, a multi-database (i.e., Academic Search Premiere, PsycARTICLES, Psychology and Behavioral Sciences Collection, PsycINFO, MEDLINE) search was conducted using the keyword terms “migraine OR headache” AND “trigger OR precipitant” AND “alcohol.” All abstracts were reviewed for eligibility, bibliographies of retained articles were searched to identify additional articles fulfilling eligibility criteria, and full-texts of candidate articles were reviewed.
Eligibility Criteria
Inclusion criteria were chosen to maximize sensitivity and ensure capture of all relevant data. (1) Articles written in English (2) that utilized human participants (3) with a diagnosis of migraine or TTH that (4) used retrospective recall, prospective diary data, or experimental manipulation were included in the analyses. Exclusion criteria were editorials, review articles, case studies, treatment studies, and articles that focused on pathophysiology, employed static variables, or utilized animals.
Data Collection
Data extracted from retained articles were entered into an Excel database, including: (1) publication metadata (authors, year of publication, journal); (2) sample demographics (sample size, population drawn from, mean age, age range, % female); (3) headache characteristics (diagnostic criteria used, diagnoses, intensity, frequency [days/month or attacks/month]); (4) method of trigger assessment (open-ended query vs provided list of triggers; experimental manipulation; diary); and (5) type of alcohol endorsed as trigger (using verbatim trigger terminology).
Statistical Analyses
We used random effects models to address heterogeneity within and between studies, measured with I2 indices and τ2 indices. Statistical significance was set at p < .05. The primary random effects model quantified the weighted proportion of participants across all studies who endorsed alcohol as a trigger with estimated 95% confidence intervals (95% CIs). Separate random effects models were used to estimate the proportion of individuals with each headache diagnosis who endorsed any form of alcohol as a trigger and to estimate the proportion of individuals with any type of headache who endorsed specific types of alcohol as triggers. Post-hoc meta-regressions were also used to assess other potential moderator variables and sources of heterogeneity (e.g., proportion of females in sample, year of publication). We used R software to run all statistical analyses.
Results
Study Search and Selection
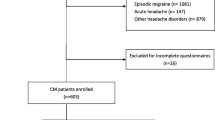
Figure 1 depicts the PRISMA flow diagram from the initial literature search to the retained articles. The initial multi-database search yielded 1929 candidate articles. After the titles and abstracts of these candidate articles were reviewed, 42 articles met inclusion criteria. The bibliography review of the included articles yielded two additional articles appropriate for the study. This process resulted in 44 articles retained for analyses. Table 1 presents summary data for each of these analyzed studies.
Alcohol as a Trigger for All Headache Diagnoses
All 44 articles retained provided sufficient data for calculating the proportion of participants endorsing alcohol as a perceived headache trigger. These studies were published between 1984 and 2014. Results of the random effects model are presented in Fig. 2. Out of 12,763 participants across 44 studies, 22% (95% CI .17–.29) endorsed any form of alcohol as a trigger. Substantial heterogeneity was observed across studies, τ2 = 0.95; I2 = 97.5% (95% CI 97.1–97.8%), which prompted meta-regressions to identify possible sources of heterogeneity. Gender mildly impacted the proportion of trigger endorsement, such that for every 1% increase in proportion of women in the sample, the odds of endorsing alcohol as a trigger increased by 2% (OR = 1.02; 95% CI 1.01–1.05; p = .04. Year of publication (p = .09) did not significantly impact proportion of trigger endorsement.
Alcohol as a Trigger for Specific Headache Diagnoses
Migraine Vs TTH
Forty-two articles provided sufficient data on participants endorsing alcohol as a trigger specifically for either migraine or TTH. Of those 42, all reported data for migraine (11,304 participants), but only 12 reported triggering effects for TTH (1506 participants). Tension-type headache sufferers endorsed alcohol as a trigger slightly less often than migraineurs (14 vs 23%, respectively), but this difference was not statistically significant (OR = 0.59; 95% CI 0.29–1.21; p = .15). Substantial heterogeneity was observed across studies, τ2 = 0.96; I2 = 96.8% (95% CI 96.4–97.3%). The proportion of females in the sample did not significantly impact trigger endorsement as a function of diagnosis (p = .09). Year of publication mildly impacted endorsement such that for each additional (more recent) year of publication, the odds of endorsement decreased by 4% (OR = 0.96; 95% CI .93 to .99; p = .05).
Migraine with Aura Vs Without Aura
Eleven articles provided sufficient data on participants endorsing alcohol as trigger for migraine with aura (MA; 984 participants) or migraine without aura (MwA; 1077 participants). Of these 11 articles, five articles provided data on both headache diagnoses, four provided data on MA exclusively, and two provided data on MwA exclusively. Individuals with MA (11%) and with MwA (10%) equally endorsed alcohol as a headache trigger (OR = 0.93; 95% CI .32–2.76; p = .90). Substantial heterogeneity was observed across studies, τ2 = 0.87; I2 = 91.1% (95% CI 87.1–93.8%). The proportion of females in the sample did not significantly impact trigger endorsement (p = .13), nor did year of publication (p = .11). The results of the separate random effects models for each headache diagnosis are compiled in Fig. 3.
Alcohol as a Trigger by Type
Twelve articles provided sufficient data on the triggering effects of consuming specific types of alcohol. Of these 12 studies, ten provided data on red wine (3166 participants); seven provided data on beer (1882 participants), white wine (2996 participants), or spirits (1543 participants); and three provided data on sparkling wine (901 participants). The results of the separate random effects models for each alcohol type are compiled in Fig. 4. Red wine (28%) was endorsed most frequently, followed by spirits (14%), white wine (12%), and beer or sparkling wine (10%). Overall differences in endorsement rates were not significant for each type, though endorsement rates for red wine compared to beer approached statistical significance (OR = 3.67; 95% CI: .92–14.67; p = .06). Again, substantial heterogeneity was observed across studies, τ2 = 1.58; I2 = 98.3% (95% CI 98–98.5%). Gender affected endorsement rates, such that for every 1% increase in proportion of females in the samples, the odds of endorsement increased by 9% for any type of alcohol (OR = 1.09; 95% CI 1.04–1.16; p < .001). Year of publication was associated with a decrease in endorsement such that for each additional (more recent) year of publication, the odds of endorsement decreased by 9% (OR = .91; 95% CI .85–.98; p = .01).
Consumption Amount/Frequency and Method of Assessment
There was not a sufficient number of studies to quantify either the effects of consumption amount/frequency or method of assessment (retrospective vs non-retrospective study) on trigger endorsement. Studies have not provided comparative estimates of alcohol’s trigger potency as a function of the amount of alcohol consumed, and only two of the 44 retained articles quantified alcohol’s role as a trigger using a non-retrospective design (one experimental manipulation and one prospective diary study). Re-running the primary random effects model without these two non-retrospective articles changed the overall endorsement rate from 22.2 to 21.0%.
Conclusions
The meta-analysis revealed substantial heterogeneity among studies exploring alcohol as a trigger of migraine and TTH, highlighting considerable methodological differences between studies and suggesting caution when interpreting results. With this caveat in mind, the general pattern of results indicates that: (1) a non-trivial proportion of individuals perceive alcohol as a trigger for migraine and TTH; (2) the precipitating effects do not differ by headache diagnoses; (3) red wine appears to be a more potent trigger than other alcohol types. Roughly one out of five headache sufferers endorse some precipitating effect of alcohol. This overall proportion is consistent with results from a larger, separate meta-analysis of all perceived headache triggers, the latter of which did not use a search strategy specific to alcohol, include non-retrospective studies, or compare types of alcohol [72]. Though this rate of endorsement is considerably less than those for more well-established headache triggers typically reported by 40–80% of headache samples (i.e., stress, hormones in women, sleep, environmental factors [19, 21, 37, 68]), among the dietary triggers alcohol is one of the most commonly endorsed [22••]. The observed endorsement rate may be an underestimate given the amount of heterogeneity found between studies.
The analyses run to assess moderator variables indicated that female gender and year of publication exerted some influence on some of the results, but these variables accounted for little of the observed heterogeneity. The small gender effect may be attributable to an interaction between alcohol and hormonal variables among women [30], and declining endorsement rates with more recent publications may indicate that the clinical advice to avoid headache triggers has become increasingly disseminated to migraineurs [25]. The separate random effects models by diagnosis found small but non-significant differences in rates of endorsement as a function of having migraine vs TTH, clarifying prior mixed findings and providing some indirect support for the notion that these headache disorders may exist on a continuum of severity rather than as discrete pathophysiological entities [73], despite their phenotypic differences. These results imply a common mechanistic effect for ethanol among these primary headache disorders. Though the primary mechanism has not been definitively confirmed, ethanol prompts release of endothelial nitric oxide and calcitonin gene-related peptide at sensory nerves, resulting in trigeminal inflammation and meningeal vasodilation [25, 27].
By comparison to the results for headache diagnoses, a large effect was observed for red wine as compared to other forms of alcohol. Though this effect fell just short of statistical significance, more than twice as many individuals endorsed red wine as a trigger than any other alcohol type. These results are concordant with Littlewood et al.’s [48] classic experimental study, in which migraineurs who believed red wine triggered their attacks consumed either a Spanish red wine or a vodka-lemonade cocktail of equivalent alcohol content. Participants were blinded to alcohol type through consumption from a dark bottle and straw. The large majority (81%) of those who consumed red wine developed a migraine within 3 h, compared to none of those given vodka. Collectively, these findings imply that, at least among some susceptible individuals, components of red wine other beyond ethanol per se (e.g., sulfites, tannins, histamines, phenols) may be responsible for higher incidence of headache following consumption [74••]. Unlike beer or white wine, red wine exerts potent releasing effects on serotonin [75].
To better understand triggering effects of certain wine components, Krymchantowski and Jevoux [76, 77] conducted two studies with migraine patients who perceived their attacks to be triggered by wine, one examining red wine type and the other examining region of production. Tannat and malbec were more potent headache triggers than cabernet sauvignon and merlot, triggering headache in 51.7 and 48.2% of patients, respectively [76]. In the second study, French cabernets triggered headache more than those from South America (60.9 vs 39.1% of patients, respectively [77]). Notably, in both of these open studies participants did not usually experience a headache after consumption. The authors concluded that tannins and phenolic flavonoids, which were most abundant in the wines most likely to induce headache, may be the underlying mechanisms of wine’s triggering effects. Given that neither of these studies were double-blinded or used a non-wine control group, further experimental studies are needed both to clarify the mechanisms underlying alcohol’s effects on headache and individual variables that may moderate these effects.
Collectively, our review indicates that primary headache does not appear to confer increased risk for AUDs and provides support for the commonly held notion that alcohol (and red wine in particular) is perceived as a headache trigger for a sizeable subset of individuals with migraine or TTH. Despite these general findings, numerous unanswered questions and directions for future research remain. As is evident from the large amount of heterogeneity present in existing literature, an effort toward enhancing uniformity and standardization in alcohol trigger studies is warranted. Given that the vast majority of reviewed studies used retrospective self-report designs, our review also highlights a need for rigorous experimental studies that can appropriately satisfy assumptions of causality and identify underlying mechanisms, particularly given that prior double-blind studies of other triggers (e.g., chocolate) have not always verified patient perceptions of their potency [78]. An ideal within- and between-subjects experimental design to establish causality would assign individuals to drink alcohol or a non-alcoholic drink (in double-blinded fashion) on numerous randomly selected days over multiple weeks, allowing sufficient time between exposures to minimize carryover effects and interactions with other triggers [23•].
Future research in this area should also assess the importance of consumption frequency and quantity in attempt to determine the threshold necessary for alcohol to precipitate an attack, as well as moderator variables that make some individuals susceptible only when present simultaneously with alcohol consumption (e.g., high stress, poor sleep, menstruation). Incorporating electronic diaries may be useful in assessing frequency and consumption of alcohol in temporal relation to headache onset. Clinically, we concur with Panconesi and colleagues [27] in their assertion that there is little reason to routinely advise headache patients against modest consumption of alcohol. Such advice might be appropriate among patients for whom a relationship between consumption and headache has been definitively established, but there is little evidence that “elimination diets” are effective or practical [79] and growing evidence suggests that therapeutic exposure to headache triggers holds promise as an adaptive alternative coping strategy to complete avoidance [80, 81•].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;280:2163–96.
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800.
Baskin SM, Lipchik GL, Smitherman TA. Mood and anxiety disorders in chronic headache. Headache. 2006;46:76–87.
Baskin SM, Smitherman T. Migraine and psychiatric disorders: comorbidity, mechanisms, and clinical applications. Neurol Sci. 2009;30(Suppl 1):61–5.
Jette N, Patten S, Williams J, Becker W, Wiebe S. Comorbidity of migraine and psychiatric disorders: a national population-based study. Headache. 2008;48:501–16.
Saunders K, Merikangas K, Low NCP, Von Korff M, Kessler RC. Impact of comorbidity on headache-related disability. Neurology. 2008;70:538–47.
Peterlin BL, Rosso AL, Sheftell FD, Libon DJ, Mossey JM, Merikangas KR. Post-traumatic stress disorder, drug abuse and migraine: new findings from the National Comorbidity Survey Replication (NCS-R). Cephalalgia. 2011;31:235–44.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
Breslau N, Davis GC, Andreski P. Migraine, psychiatric disorders, and suicide attempts: an epidemiologic study of young adults. Psychiatry Res. 1991;37:11–23.
Swartz KL, Pratt LA, Armenian HK, Lee LC, Eaton WW. Mental disorders and the incidence of migraine headaches in a community sample: results from the Baltimore epidemiologic catchment area follow-up study. Arch Gen Psychiatry. 2000;57:945–50.
Subramaniam M, Vaingankar JA, Abdin E, Chong SA. Psychiatric morbidity in pain conditions: results from the Singapore Mental Health Study. Pain Res Manag. 2013;18:185–90.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition, (DSM-IV). Washington, DC: American Psychiatric Association; 1994.
International Headache Society. The international classification of headache disorders, 3rd ed. Beta version. Cephalalgia. 2013;33:645–61.
Merikangas KR, Angst J, Isler H. Migraine and psychopathology: results of the Zurich cohort study of young adults. Arch Gen Psychiatry. 1990;47:849–53.
Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–9.
Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–32.
Domingues RB, Domingues SA, Lacerda CB, Machado TV, Duarte H, Teixeira AL. Alcohol use problems in migraine and tension-type headache. Arq Neuropsiquiatr. 2014;72:24–7.
Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252:1905–7.
Aamodt AH, Stovner LJ, Hagen K, Bathen G, Zwart J. Headache prevalence related to smoking and alcohol use. The Head-HUNT Study. Eur J Neur. 2006;13:1233–8.
Domingues RB, Domingues SA. Headache is associated with lower alcohol consumption among medical students. Arq Neuropsiquiatr. 2011;69:620–3.
Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007a;27:394–402.
•• Martin VT, Vij B. Diet and headache: part 1. Headache. 2016;56:1543–52. This is a rather comprehensive paper on diet and headache that reviews not only alcohol but other foods and ingredients within foods.
• Turner DP, Smitherman TA, Martin VT, Penzien DB, Houle TT. Causality and headache triggers. Headache. 2013;53:628–35. This paper outlines the numerous assumptions that must be met to experimentally confirm that a stimulus is a headache trigger.
Lipton RB, Pavlovic JM, Haut SR, Grosberg BM, Buse DC. Methodological issues in studying trigger factors and premonitory features of migraine. Headache. 2014;54:1661–9.
Panconesi A. Alcohol and migraine: trigger factor, consumption, mechanisms. A review. J Headache Pain. 2008;9:19–27.
• Dueland AN. Headache and alcohol. Headache. 2015;55:1045–9. This is a recent narrative review on the triggering effects of alcohol.
Panconesi A, Bartolozzi ML. Alcohol and migraine: what should we tell patients? Curr Pain Headache Rep. 2011;15:177–84.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Amery WK, Vandenbergh V. What can precipitating factors teach us about the pathogenesis of migraine? Headache. 1987;27:146–50.
Andress-Rothrock D, King W, Rothrock J. An analysis of migraine triggers in a clinic-based population. Headache. 2010a;50:1366–70.
Baldacci F, Vedovello M, Ulivi M, Vergallo A, Poletti M, Borelli P, et al. How aware are migraineurs of their triggers? Headache. 2013;53:834–7.
Bank J, Marton S. Hungarian migraine epidemiology. Headache. 2000;40:164–9.
Blau JN. Water deprivation: a new migraine precipitant. Headache. 2005;45:757–9.
Fishbain DA, Cutler R, Cole B, Rosomoff HL, Rosomoff RS. International Headache Society headache diagnostic patterns in pain facility patients. Clin J Pain. 2001;17:78–93.
Fraga MDB, Pinho RS, Andreoni S, Vitalle MSDS, Fisberg M, Peres MFP, et al. Trigger factors mainly from the environmental type are reported by adolescents with migraine. Arq Neuropsiquiatr. 2013;71:290–3.
Fragoso YD, Carvalho R, Ferrero F, Lourenço DM, Paulino ER. Crying as a precipitating factor for migraine and tension-type headache. Sao Paulo Med J. 2003;121:31–3.
Fukui PT, Conçalves TRT, Strabelli CG, Lucchino NMF, Matos FC, Moreiera dos Santos JP, et al. Trigger factors in migraine patients. Arq Neuropsiquiatr. 2008a;66:494–9.
Galinović I, Vuković V, Trošelj M, Antić S, Demarin V. Migraine and tension-type headache in medical students: a questionnaire study. Coll Antropol. 2009;33:169–73.
Haimanot RT, Seraw B, Forsgren L, Ekbom K, Ekstedt J. Migraine, chronic tension-type headache, and cluster headache in an Ethiopian rural community. Cephalalgia. 1995;15:482–8.
Hauge AW, Kirchmann M, Olesen J. Characterization of consistent triggers of migraine with aura. Cephalalgia. 2010a;31:416–38.
Hauge AW, Kirchmann M, Olesen J. Trigger factors in migraine with aura. Cephalalgia. 2010b;30:346–53.
Hung CI, Liu CY, Wang SJ. Precipitating or aggravating factors for headache in patients with major depressive disorder. J Psychosom Res. 2008;64:231–5.
Ierusalimschy R, Filho PFM. Precipitating factors of migraine attacks in patients with migraine without aura. Arq Neuropsiquiatr. 2002;60(3-A):609–13.
Karli N, Zarifoglu M, Calisir N, Akgoz S. Comparison of pre-headache phases and trigger factors of migraine and episodic tension-type headache: do they share similar clinical pathophysiology? Cephalalgia. 2005;25:444–51.
Karli N, Akgoz S, Zarifoglu M, Akis N, Erer S. Clinical characteristics of tension-type headache and migraine in adolescents: a student-based study. Headache. 2006;46:399–412.
Kelman L. Migraine changes with age: IMPACT on migraine classification. Headache. 2006;46:1161–71.
Lipton RB, Newman LC, Cohen JS, Solomon S. Aspartame as a dietary trigger of headache. Headache. 1989;29:90–2.
Littlewood JT, Glover V, Davies PTG, Gibb C, Sandler M, Clifford RF. Red wine as a cause of migraine. Lancet. 1988a;331:558–9.
Matuja WB. Headache: pattern and features as experienced in a neurology clinic in Tanzania. East Afr Med J. 1991;68:935–43.
Mollaoğlu M. Trigger factors in migraine patients. J Health Psychol. 2012;18:984–94.
Mounstephen AH, Harrison RK. A study of migraine and its effects in a working population. Occup Med. 1995;45:311–7.
Panconesi A, Franchini M, Bartolozzi ML, Mugnai S, Guidi L. Alcoholic drinks as triggers in primary headache. Pain Med. 2013;14:1254–9.
Peatfield RC, Glover V, Littlewood JT, Sandler M, Rose F. The prevalence of diet-induced migraine. Cephalalgia. 1984;4:179–83.
Peatfield RC. Relationship between food, wine, and beer—precipitated migrainous headaches. Headache. 1995;35:355–7.
Phanthumchinda K, Sithi-Amorn C. Prevalence and clinical features of migraine: a community survey in Bangkok, Thailand. Headache. 1989;29:594–7.
Rains JC, Penzien DB. Precipitants of episodic migraine: behavioral, environmental, hormonal, and dietary factors. Headache. 1996;36:274–5.
Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53:65–72.
Russell MB, Rasmussen BK, Fenger K, Olesen J. Migraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996;16:239–45.
Savi L, Rainero I, Valfre W, Gentile S, Lo GR, Pinessi L. Food and headache attacks. A comparison of patients with migraine and tension-type headache. Panminerva Med. 2002;44:27–31.
Scharff L, Turk DC, Marcus DA. Triggers of headache episodes and coping responses of headache diagnostic groups. Headache. 1995;35:397–403.
Schürks M, Buring JE, Kurth T. Migraine features, associated symptoms and triggers: a principal component analysis in the Women’s Health Study. Cephalalgia. 2011;31:861–9.
Sjöstrand C, Savic I, Laudon-Meyer E, Hillert L, Lodin K, Waldenlind E. Migraine and olfactory stimuli. Curr Pain Headache Rep. 2010;14:244–51.
Spierings ELH, Ranke AH, Honkoop PC. Precipitating and aggravating factors of migraine versus tension-type headache. Headache. 2001;41:554–8.
Téllez-Zenteno JF, García-Ramos G, Zermeño-Pöhls F, Velazquez A. Demographic, clinical and comorbidity data in a large sample of 1147 patients with migraine in Mexico City. J Headache Pain. 2005;6:128–34.
Tonini MC, Frediani F. Headache at high school: clinical characteristics and impact. Neurol Sci. 2012;33:185–7.
Ulrich V, Olesen J, Gervil M, Russell MB. Possible risk factors and precipitants for migraine with aura in discordant twin-pairs: a population-based study. Cephalalgia. 2000;20:821–5.
Van den Bergh V, Amery WK, Waelkens J. Trigger factors in migraine: a study conducted by the Belgian Migraine Society. Headache. 1987;27:191–6.
Wang JJ. Triggers of migraine and tension-type headache in China: a clinic-based survey. Eur J Neur. 2013a;20:689–96.
Wöber C, Holzhammer J, Zeitlhofer J, Wessely P, Wöber-Bingöl Ç. Trigger factors of migraine and tension-type headache: experience and knowledge of the patients. J Headache Pain. 2006;7:188–95.
Wöber C, Brannath W, Schmidt K, Kapitan M, Rudel E, Wessely P, et al. Prospective analysis of factors related to migraine attacks: the PAMINA study. Cephalalgia. 2007;27:304–14.
Ying G, Fan W, Li N, Wang J, Li W, Tan G, et al. Clinical characteristics of basilar-type migraine in the neurological Clinic of a University Hospital. Pain Med. 2014;15:1230–5.
Walters Pellegrino AB, Davis-Martin RE, Houle TT, Turner DP, Smitherman TA. Perceived triggers of primary headache disorders: a meta-analysis. Under review.
Turner DP, Smitherman TA, Black AK, Penzien DB, Porter JAH, Lofland KR, et al. Are migraine and tension-type headache diagnostic types or points on a severity continuum?: an exploration of the latent taxometric structure of headache. Pain. 2015;156:1200–7.
•• Krymchantowski AV, Jevoux CC. Wine and headache. Headache. 2014;54:967–75. A thorough review on wine and headache, this paper provides a nice discussion of likely underlying mechanisms.
Jarman J, Glover V, Sandler M. Release of (14C)5-hydroxytryptamine from human platelets by red wine. Life Sci. 1991;48:2297–300.
Krymchantowski AV, Jevoux CC. Red wine-type and triggering of migraine: an open prospective study. Headache. 2012;52:884.
Krymchantowski AV, Jevoux CC. Cabernet sauvignons from France do trigger migraine more often than those from South America: an open prospective study. Cephalalgia. 2013;33(Suppl. 8):90.
Marcus DA, Scharff L, Turk D, Gourly LM. A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia. 1997;17:855–62.
Rothrock JF. The truth about triggers. Headache. 2008;48:499–500.
Martin PR, MacLeod C. Behavioral management of headache triggers: avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483–95.
• Martin PR, Reece J, Callan M, MacLeod C, Kaur A, Gregg K, et al. Behavioral management of the triggers of recurrent headache: a randomized controlled trial. Behav Res Ther. 2014;61:1–11. This novel RCT presents data on the efficacy of progressive exposure to versus avoidance of headache triggers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ashley N. Polk and Rachel E. Davis-Martin declare that they have no conflict of interest.
Todd A. Smitherman has received personal fees from Alder Biopharmaceuticals outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Psychological and Behavioral Aspects of Headache and Pain
Rights and permissions
About this article
Cite this article
Davis-Martin, R.E., Polk, A.N. & Smitherman, T.A. Alcohol Use as a Comorbidity and Precipitant of Primary Headache: Review and Meta-analysis. Curr Pain Headache Rep 21, 42 (2017). https://doi.org/10.1007/s11916-017-0642-8
Published:
DOI: https://doi.org/10.1007/s11916-017-0642-8