Abstract
Purpose of Review
The adult skeleton contains stem cells involved in growth, homeostasis, and healing. Mesenchymal or skeletal stem cells are proposed to provide precursors to osteoblasts, chondrocytes, marrow adipocytes, and stromal cells. We review the evidence for existence and functionality of different skeletal stem cell pools, and the tools available for identifying or targeting these populations in mouse and human tissues.
Recent Findings
Lineage tracing and single cell-based techniques in mouse models indicate that multiple pools of stem cells exist in postnatal bone. These include growth plate stem cells, stem and progenitor cells in the diaphysis, reticular cells that only form bone in response to injury, and injury-responsive periosteal stem cells. New staining protocols have also been described for prospective isolation of human skeletal stem cells.
Summary
Several populations of postnatal skeletal stem and progenitor cells have been identified in mice, and we have an increasing array of tools to target these cells. Most Cre models lack a high degree of specificity to define single populations. Human studies are less advanced and require further efforts to refine methods for identifying stem and progenitor cells in adult bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skeleton is a dynamic system that undergoes postnatal growth, remodeling, and regeneration following injury. The processes of osteoblast and chondrocyte differentiation are well defined, and markers are available to selectively identify and genetically target cells at various stages of differentiation. Recent research efforts have focused on identifying the stem and progenitor populations from which these cells derive. Early studies used plastic adherence and the ability to form fibroblastic colonies (CFU-F) following low-density culture to identify mesenchymal stem cells (MSC) that were capable of differentiation into osteogenic, chondrogenic, and adipogenic lineages on a clonal basis [1]. Many studies focus on bone marrow or bone tissue as the source of MSCs; however, cells with similar in vitro characteristics have been isolated from numerous tissues, including fat and muscle [2]. The MSC markers routinely characterized in culture (CD73, CD90, and CD105) do not specifically identify stem cells, and a more nuanced approach to identify stem cells in vivo is required [3, 4].
The focus of recent studies has shifted to identifying skeletal stem cells (SSCs), and various skeletal progenitor populations in vivo. SSC populations should show evidence of self-renewal and the ability to differentiate into lineages including osteoblasts, chondrocytes, adipocytes, and marrow stromal cells that support hematopoiesis. Many studies assume that SSCs form a hierarchy with one stem cell population, similar to what is characterized in the hematopoietic system. However, in reality, there are few situations in vivo where a cell can differentiate into any of these four lineages. Embryonic lineage tracing studies indicate that SSCs in long bones are established locally from the limb bud mesenchyme (identified by Prx1 expression), and from cells expressing chondrogenic markers such as Sox9 and type 2 collagen that invade from the perichondrium [5,6,7]. However, postnatally, it appears that skeletal stem and progenitor pools are established in different bone compartments. There are various lines of evidence indicating that skeletal stem and progenitor cells involved in both growth and healing are derived locally and do not spread between bones [5, 8, 9].
In this review, we will summarize the technical approaches to identifying and isolating different skeletal stem and progenitor populations and recommend approaches for targeting or quantifying different populations. We focus primarily on the limbs. The majority of the studies covered were performed in mice, but some human data is included.
Defining SSCs by Reporters and Lineage Tracing
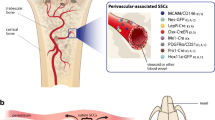
Lineage tracing experiments use transgenic mice and typically involve a cell-specific inducible Cre combined with a suitable fluorescence reporter that is activated by Cre activity (frequently tdTomato) [10]. Depending on the timing of reporter induction, lineage tracing experiments can be used to identify skeletal stem and progenitor cells in vivo during embryonic development, in postnatal and adult bones, and in response to injury. Real-time fluorescent reporters (often GFP) are also useful for identification and isolation of cell subsets. They can be combined with Cre-mediated lineage tracing to validate that similar populations are identified, or track differentiation of stem cells. A summary of Cre models that may be useful for postnatal lineage tracing or gene targeting is presented in Table 1 and Fig. 1.
Markers for different skeletal stem and progenitor populations. a Markers for cell populations in different compartments of the long bone are indicated. Most are based on inducible Cre expression. Stem/progenitors in the growth plate and trabecular region are mainly active during growth. The bone marrow-resident populations are mainly reticular cells that probably do not become osteoblasts during normal bone turnover. The fibrous layer of the periosteum does not appear to contain stem or progenitor cells. b Summary of injury-activated progenitors labeled by Cre lines in a long bone fracture setting. Intra-marrow bone formation is assumed to originate from marrow-resident cells. The approximate % contribution to osteoblasts (OB) and in some cases chondrocytes (CH) is shown. Most data represent labeling in the few weeks before fracture except for the non-inducible models (LepR, Ctsk), and the markers where the timing of tamoxifen (Tam.) is indicated
The majority of lineage markers target one or more defined populations. An interesting exception is Hoxa11, which targets skeletal stem and progenitor cells in all tissue compartments developmentally and throughout adulthood, but only in specific bones. Hox genes are essential for developmental patterning, and some expression is retained throughout life. The expression of Hoxa11-GFP is restricted to the zeugopod limbs (radius/ulna and tibia/fibula) and to undifferentiated mesenchymal cells that are capable of skeletal lineage differentiation in the periosteum, bone marrow, and in the growth plate [9, 32]. The majority of these cells are PDGFRα+/CD51+/LepR+. Lineage tracing of Hoxa11-CreER cells from P3 indicates they contribute to growth plate chondrocytes, trabecular and cortical osteoblasts and osteocytes, and a subset of bone marrow adipocytes [9•]. Hoxa11+ cells identified in adulthood also contribute to osteoblasts and osteocytes, although the timing and extent of this process have not been documented to date [23]. Hoxa11-CreER looks to be a useful tool to target genetic changes to the majority of different types of SSCs, specifically in zeugopod bones, at any stage of life.
Growth-Related Stem Cells
During postnatal growth, the growth plate retains an organized structure with the expansion of resting zone cells into proliferative chondrocytes, which ultimately undergo hypertrophy. There is a large body of evidence indicating that hypertrophic chondrocytes from the growth plate can go on to form osteoblasts (reviewed elsewhere [33, 34]). Resting zone chondrocytes in the upper growth plate can be targeted by Pthrp-CreER, and to a lesser extent, Grem1-CreER. These cells continue to contribute locally to osteoblasts and stromal cells, but probably not adipocytes, for at least a year after neonatal labeling [14, 15, 26, 35]. There is also evidence that skeletal stem and progenitor populations reside in the trabecular region. Gli1-CreER targets stem cells in several postnatal settings, including mesenchymal and epithelial stem cells in murine incisors, and SSCs in calvarial sutures [16, 17, 36, 37]. In long bones, Gli1 identifies a population of “metaphyseal mesenchymal progenitors” [18••]. These cells are present at one-month of age and contribute to the majority of trabecular osteoblasts present 1 month later, with a continued contribution to a subset of osteoblasts, stromal cells, and marrow adipocytes in the metaphysis for at least 9 months. Importantly, Gli1 expression is also present in the resting zone of the growth plate. The Gli1+ cell populations in the metaphysis are present during the rapid growth period, but not in older animals. αSMA-CreER, initially developed as a perivascular marker, also identifies cells involved in trabecular osteoblast formation during postnatal growth [27]. αSMA does not target growth plate cells, but long-term tracing studies suggest that these cells are progenitors that give rise to a wave of osteoblasts and osteocytes, rather than self-renewing stem cells [28]. αSMA-lineage cells do not contribute to trabecular osteoblasts in mice over two-months of age. CTGF-GFP also labels cells resident in the trabecular region in growing animals [38]. These CTGF-GFP cells demonstrate MSC characteristics ex vivo, but the in vivo fate of these cells has not been confirmed [38]. Since Gli1-CreER also targets growth plate stem cells, and the other markers do not show evidence of self-renewal in vivo, it is unclear whether there is a metaphyseal stem cell population that contributes to trabecular bone formation, or if contribution continues to come from the growth plate.
Stem Cells Involved in Adult Bone Turnover
Growth-related stem and progenitor cells, particularly those present during adolescent growth, make minimal contribution to cortical osteoblasts. There is little turnover in rodent cortical bone, so osteocytes are likely to have very long lifespans. However, osteoblast turnover continues during postnatal life, with osteoblasts estimated to have a lifespan of a few weeks [21, 26]. Several studies indicate that one source of osteoblasts during adulthood is mature cells within the osteoblast lineage that express late osteoblast markers such as Dmp1 and osteocalcin [26, 39, 40]. Tracing of cells identified by Dmp1-CreER at 2 months of age indicates initial labeling of the vast majority of osteoblasts, a dramatic drop in labeled bone surface cells after 3 weeks, then retention of similar numbers of labeled osteoblasts up to at least 6 months later [26]. These osteoblasts are probably derived from bone lining cells that can reactivate and transcortical channel-resident osteoprogenitors [26, 41].
Leptin receptor (LepR) is widely used as an MSC marker that identifies cells that appear postnatally. LepR+ cells increasingly contribute to osteoblasts as mice age, increasing from < 10% of osteoblasts at two-months of age to around 80% at 14 months of age [7]. Notably, all the LepR studies use constitutively active LepR-Cre, which makes interpretation of the timing of LepR expression impossible. LepR-Cre also identifies a network of reticular stromal cells in the central bone marrow; however, there is accumulating evidence suggesting these cells are not osteoprogenitors contributing to normal bone homeostasis. Further, these cells may inhibit bone formation, as cell ablation mediated by both LepR-Cre and Adipoq-Cre results in rapid formation of trabecular bone in the diaphysis [7, 42]. Notably, 30–60% of bone lining cells and transcortical osteoprogenitors identified by Dmp1-CreER also express LepR [26, 41]. Stromal cells in the bone marrow labeled by Adipoq-Cre, which mostly co-express LepR and Cxcl12, and Cxcl12-CreER labeled cells, never contribute to osteoblasts under homeostatic conditions [24, 42]. Ebf3-CreER labels Cxcl12-abundant reticular (CAR) cells and cells that contribute to osteoblasts during adulthood, similar to LepR-Cre [25•]. However, the timing of this process is unclear. Ebf3 is co-expressed with Adipoq, LepR, and Cxcl12, but presumably targets a broader population that includes most cells capable of CFU-F formation in the bone marrow [25, 42]. Mx1-Cre also targets adult osteoprogenitors that contribute to over 70% of endosteal osteoblasts beginning 20 days after labeling in adult animals [21]. The Mx1 promoter is inducible by stimuli including double-stranded synthetic RNA (poly I: poly C, pIpC) and is often used as a ubiquitous inducible system [43]. Most studies utilizing Mx1-Cre involve irradiation and bone marrow transplantation to eliminate the hematopoietic lineage that is also targeted by Mx1, and pIpC induction is performed over a 20 day period; therefore it is not possible to evaluate the cells labeled initially. These limitations make Mx1-Cre largely unsuitable for targeting genetic changes to SSCs as irradiation has well-established adverse effects on bone homeostasis and healing [44]. It is also challenging to identify these cells in other expression-based studies as Mx1 is not expressed under basal conditions. Overall, the evidence suggests that cells in close proximity to the bone surface contribute to ongoing osteoblastogenesis during adult homeostasis. Some of these cells are committed osteoblast-lineage cells, others, which express Ebf3 and probably LepR, may be more primitive but have not yet been clearly separated from CAR cells using lineage tracing. In summary, Cxcl12-CreER provides a useful model for targeting CAR cells in the central marrow. Ebf3-CreER may provide a method to target LepR+ cells with temporal control, but both these models target a range of stromal populations which make it challenging to interpret results of genetic changes induced by these Cre models.
Periosteal Stem Cells
The periosteum is recognized as a critical source of injury-responsive stem cells. The periosteum contains an inner cambium layer and an outer fibrous layer (Fig. 1). Stem and progenitor cells seem to reside in the cambium layer. Most studies indicate that the periosteum is highly enriched for SSCs and osteoprogenitors compared to the endosteum or bone marrow compartment [5, 28, 45]. During fracture healing, when the periosteum is present, the majority of cells that contribute to osteoblasts and chondrocytes within the callus are derived from the periosteum [46]. Graft transplantation studies indicate the presence of rare stem cells that can self-renew and participate in multiple rounds of fracture healing; however, further characterization of these cells to identify markers has not been reported to date [5•].
In recent years, numerous markers for periosteal stem cells have been proposed. Single-cell gene expression studies indicate that there is heterogeneity in periosteal populations, but it is still unclear how the different populations that contribute to fracture healing overlap [45••]. Cathepsin K-driven Cre (Ctsk-Cre) targets the majority of periosteal mesenchymal cells, and a subset of skeletal cells in the fracture callus [45••]. Dual-labeled Mx1-Cre and αSMA-GFP cells are also enriched for ex vivo stem cell characteristics [22•]. Following periosteal injury, dual-labeled Mx1-Cre and αSMA-GFP cells contributed to 80% of callus osteoblasts [22•]. LepR-Cre also targets about half of the osteoblasts and chondrocytes in fracture calluses, but periosteum-resident LepR+ populations have not been otherwise characterized [7, 19]. Several inducible Cre models target a subset of periosteal progenitors contributing to both osteoblasts and chondrocytes in the callus. These include Sox9-CreER, Prx1-CreER, Gli1-CreER, and αSMA-CreER [11, 18, 27, 28, 30, 44, 47]. Postnatal Prx1 expression appears to be mainly restricted to the cambium layer of the periosteum, and the contribution of these cells to the fracture callus appears to be moderate [30, 31, 47]. Sox9+ periosteal resident cells also contribute to a subset of osteoblasts and chondrocytes during long bone fracture and rib injury healing (around 20% in the rib) [11, 12]. Gli1+ cells present at one-month of age contribute about half of osteoblasts and chondrocytes in a later fracture callus, and cells capable of callus contribution are also present in older animals [18, 44]. In addition to cambium cells, Gli1 targets cells in the fibrous layer, and cells that contribute to fibrosis in a radiation-induced non-union model [44]. These fibrotic cells appear to be separate from the cells involved in successful healing. αSMA-CreER targets resident periosteal cells that contribute to 30–40% of osteoblasts, and 27% of chondrocytes in a fracture callus. Some of these cells retain longer-term potential to contribute to fracture, with about 20% of fracture osteoblasts coming from cells that were labeled using αSMA-CreER 90 days earlier [28]. Both Sox9-CreER (in the rib) and αSMA-CreER identify a large proportion of callus osteoblasts (> 80%) when tamoxifen is delivered around the time of injury, suggesting activation of these genes in the early stages of the lineage response to injury [12, 28, 48, 49]. Therefore, these models are useful for targeting genetic changes to a large portion of local progenitor cells during healing without affecting bone development. Finally, Col2.3-CreER+ cells labeled just before fracture contributed to around 10% of osteoblasts but no chondrocytes in the fracture callus, indicating that more committed osteolineage cells in the periosteum can also contribute in an injury setting.
Other Injury-Responsive Cells
There is still debate about the contribution of bone marrow cells to fracture calluses, but they contribute to new bone formation in response to physical injury to the marrow or full-thickness cortical injuries where mechanical integrity of the bone is retained [46]. Cxcl12-CreER derived cells, which comprise a subset of the cells labeled by Cxcl12-GFP, make up 40% of new osteocytes following a cortical injury, and healing is impaired if these cells are ablated [24••]. A separate committed osteolineage population identified by Osx-CreER contributes 12% of new osteocytes following injury [24••]. Thus, like the periosteum, there is diversity in bone marrow cell populations that can contribute to bone formation during healing including cells that would otherwise never enter the osteogenic lineage.
Defining SSCs Using Cell Surface Markers in Murine Models
In the hematopoietic field, different populations are primarily identified by cell surface marker combinations, and these methods have been applied to the skeletal field with some success. After a digestion or dissociation process to isolate cells from the matrix is performed, cell surface marker stains enable identification and isolation of live cells using flow cytometry. These methods do not require transgenic lines making them more widely applicable, and similar markers can potentially be used in different species. The use of marker combinations also allows detailed dissection of specific populations once rigorous validation has been performed. In the bone setting, we do not have established cell surface markers for excluding mature cells such as osteoblasts. However, it is well-accepted that the mesenchymal cell fraction can be identified by excluding cells of the hematopoietic lineage (CD45+), and endothelial lineage (usually CD31+). The frequency of non-hematopoietic cells in adult mouse bone marrow is usually around 0.5% [51]. Markers that have been investigated for prospective isolation of SSCs are summarized in Table 2.
Sca1-Based Stains
Sca1 (stem cell antigen) is traditionally used as a marker for murine hematopoietic stem cells (HSCs). Sca1+ cells are localized to perivascular regions within the epiphyseal bone marrow, and near the endosteal surface [50, 52]. Sca1+/PDGFRα+ (PαS) bone-associated cells from adult mice are significantly enriched for CFU-F formation and show robust multi-lineage differentiation in vitro and in vivo [7, 53]. The addition of CD24 to the PαS stain (PαS+/CD24+ cells) further enriches for CFU-F and multi-lineage differentiation [54••]. Different combinations of these three markers can also define osteochondroprogenitors (PDGFRα+/Sca1−) or marrow adipocyte progenitors (PDGFRα+/Sca1+/CD24−). Sca1 is also used in combination with CD51 to identify skeletal stem cells, while Sca1−/CD51+ cells have been defined as committed osteoprogenitors or osteoblasts [55,56,57]. Thorough validation of this marker combination has not been published. We have demonstrated that Sca1+/CD51+ cells in the periosteum show efficient CFU-F formation and multi-lineage differentiation in vitro [28]. Sca1−/CD51+ cells contain some mature osteoblasts, in addition to progenitors capable of CFU-F formation, but are committed to the osteoblast lineage [28]. In contrast, a study aimed at identifying periosteal stem cells suggested that these cells were Sca1− [45••]. Hu et al. describe a hierarchy of bone-associated stromal cells where Sca1+/CD146−/CD166− cells represent the multipotent progenitor, and CD146+ and CD166+ populations represent downstream more committed progenitors [50]. The use of Sca1 as a marker for SSCs also has limitations. Expression levels of Sca1 are not consistent in different mouse strains [58]. C57BL/6 mice have strong Sca1 expression, whereas BALB/c and DAB1 strains can have little or no non-hematopoietic Sca1 expression. Despite this, Sca1 is one of the best characterized markers to assist with identification of skeletal stem and progenitor cells, especially in the adult setting.
PDGFRα-Based Stains
PDGFRα is an early mesodermal marker, and its expression overlaps with most Nestin-GFP+ stromal cells, LepR+ cells, and Cxcl12+ cells [7, 59, 60]. In addition to the combination with Sca1 [53], PDGFRα has also been combined with CD51 to define stem/progenitor populations which are enriched for CFU-F ability and tri-lineage differentiation in vivo and in vitro [32, 59]. Park et al. found that the PDGFRα+/CD105+ fraction of bone-associated cells were enriched for CFU-F and used this combination to enrich for periosteal stem cells [21, 22]. In contrast, our studies show that CD105 is one of the few markers that are more prevalent in endosteum than periosteum, and these cells in the periosteum generally perform poorly in CFU-F assays [28]. Flow analysis in our lab identified a large degree of overlap between PαS, Sca1+/CD51+ and PDGFRα+/CD51+ populations in endosteum and periosteum cells. In contrast, CD105 expression is mostly restricted to separate cells: only 17% of endosteal PαS cells are CD105+. In our experience, PDGFRα expression is weak, which means it is important to use a bright fluorophore for flow cytometry. Notably, some studies reported that PDGFRα is absent or very rare in bone marrow [61, 62]. In contrast, using a GFP reporter, Ambrosi et al. reported broad expression of PDGFRα, including the absence of a Sca1+ PDGFRα− population [54••]. This uncertainty highlights the reproducibility challenges surrounding the use of PDGFRα as a marker.
CD51-Based Stains
CD51, or alphaV integrin, is expressed throughout the osteoblast lineage and is abundant in the growth plate [26, 35]. All cells capable of CFU-F formation in endosteum and periosteum are CD51+, as are fetal or neonatal bone cells capable of ossicle formation in vivo [28, 35, 51, 63]. Chan and colleagues have defined a hierarchy of skeletal stem and progenitor cells using cells isolated from neonatal bones, with a focus on cell populations in the growth plate [35, 63, 64]. Differentiation and self-renewal potential was evaluated by transplantation into the kidney capsule and evaluation of the cell and tissue types formed. They identified mouse skeletal stem cells (mSSC) as CD45−/Ter119−/Tie2−/CD51+/CD90−/6C3−/CD105−/CD200+, capable of producing ossicles containing bone, cartilage, and marrow infiltration. These cells give rise to various progenitors, including multipotent bone, cartilage, and stromal progenitors (BCSP; CD51+/CD90−/6C3−/CD105+), as well as five other progenitor populations committed to forming primarily bone, cartilage, or stromal cells. These stains have been applied to various settings, indicating that SSC and BCSP populations expand dramatically in response to fracture, a response that is impaired by irradiation [35, 65]. Similar populations have been identified in the periosteum, also primarily in neonates, where periosteal cells were defined by expression of a Ctsk-Cre driven reporter. They defined periosteal stem cells as CD45−/Ter119−/CD31−/CTSK-GFP+/CD90−/6C3−/CD105−/CD200+, and identified two progenitor populations with varying expression of CD200 and CD105 [45••]. These populations form bone-only ossicles in vivo, except following fracture where they form bone and cartilage.
While these populations have been validated in neonatal bones, their identity in adult bones is less clear. BCSPs isolated from adult bones were only capable of forming a bony ossicle lacking a marrow cavity using the same kidney capsule transplant model [50]. It appears that freshly isolated bone-associated cells from adult animals are unable to form ossicles containing multiple lineages when transplanted outside the bone environment. Finally, we have demonstrated that in the endosteum of adult mice, a third of the SSC and BCSP populations expressed mature osteoblast marker Col2.3GFP [28]. On this basis, we do not believe that these stains are a robust method to identify pure stem and progenitor populations in adult bones.
Other Progenitor Markers
CD90 is often stated to identify committed osteogenic cells, although most of the evidence for this appears to come from neonatal systems [63]. In the periosteum, CD90+ cells are enriched for CFU-F and show multi-lineage differentiation capacity in vitro [28]. Single-cell RNAseq analysis also identified CD90, along with Sca1, as a marker of early mesenchymal progenitors [42••]. Nestin-GFP identifies cells in the bone marrow that show SSC potential ex vivo, and the ability to support hematopoiesis [66]. Nestin-GFP cells are also abundant in the periosteum and show similar characteristics [67]. Studies using Nestin-Cre and Nes-CreER do not confirm the contribution of these cells to bone formation in vivo, although they also appear to target different cell populations [68, 69]. The use of Nestin as a marker of MSCs remains controversial.
To summarize, a combination of cell surface markers is required to isolate refined populations of skeletal stem and progenitor cells. In the adult setting, PDGFRα and Sca1 are useful for positive selection and effectively exclude mature osteoblasts. CD51 is also useful for positive selection but must be combined with additional markers which exclude mature lineages. There is scope for further validation and refinement of existing methods to ensure that they are appropriate for the developmental stage and tissue compartment in question.
Identifying SSCs in Human Tissue
It is not always possible to directly translate findings in mice into human systems. The majority of studies on human MSCs have involved in vitro isolation and characterization. Methods for enriching MSCs in culture have been covered elsewhere [70, 71]. Prospective isolation of human skeletal stem cells is limited to staining and ex vivo evaluation. Some of the markers and stains that have been characterized are detailed below.
CD146
Sacchetti et al. reported that the non-hematopoietic CD146+ population in bone marrow included all cells capable of CFU-F formation, and could establish ossicles with a hematopoietic microenvironment in vivo after expansion [72]. This population localizes around sinusoidal blood vessels and expresses HSC niche factors ANG-1 and CXCL12 [2, 72]. Meanwhile, pericytes (CD45−/CD34−/CD146+), which are associated with microvessels, were proposed as a uniform source of MSCs throughout the body, and have been characterized ex vivo from tissues including pancreas, placenta, adipose tissue, brain, and dental pulp [2, 73,74,75]. However, later studies confirmed that while CD146+ cells from different tissues can undergo multi-lineage differentiation in vitro, their in vivo differentiation potential was more restricted, and only bone marrow-derived cells formed ossicles containing marrow [76, 77]. However, CD146 alone is not enough for human SSC isolation, as within CD271+ cells, both CD146+ and CD146− cells show similar CFU-F formation and differentiation in vitro [78].
Growth Plate Stem Cells
Chan et al. identified potential cell surface marker combinations based on comparing gene expression of cells in different zones of the fetal growth plate with mSSC populations [35, 79]. These studies suggested that human SSCs were present in the pre-hypertrophic zone of the growth plate. hSSCs were defined in the stromal fraction (CD45−/CD235a−/Tie2−/CD31−) of fetal bone cells as PDPN+/CD146−/CD73+/CD164+. These cells formed ossicles containing bone, cartilage, and marrow. PDPN+/CD146+/CD73+/CD164+ cells (hBCSPs) were also multipotent. The hSSC population persists in adult bone marrow, retaining osteochondrogenic potential in vitro, and contributing to ossicle formation upon transplantation [79••]. Cells that formed bone robustly were isolated from the surface of trabecular bone rather than marrow alone. hSSC populations are enriched in response to injury. Unlike most of the MSC populations characterized in other studies, hSSCs do not form adipocytes in vitro or in vivo. While impressive, the majority of characterization in this study was performed using fetal samples. Therefore, clearer characterization of populations present in adult tissue is still of interest.
PDGFRα/CD51
PDGFRα+/CD51+ cells are multipotent, have significantly enriched CFU-F formation, and have high expression of HSC niche genes [59]. The frequency of human PDGFRα+/CD51+ cells decreases with age, constituting 6% in fetal but only ~ 0.21% of the stromal population in adult bone marrow. In fetal tissue, PDGFRα+/CD51+ make up a subset of the CD146+ population (15.8%) and enrich this population for colony formation as well as expression of HSC supporting factors and ability to support recruitment of hematopoietic cells.
Periosteum
CD45−/CD235a−/CD31−/CD90−/CD200+/CD105− recognizes human periosteal stem cells, using an equivalent cell surface marker profile to that characterized in mice [45••]. This population constitutes around 5% of non-hematopoietic cells within the femoral periosteal samples evaluated. This population displays multi-lineage potential in vitro and undergoes intramembranous bone formation in vivo.
Conclusion
In summary, mouse studies have enabled the identification of several different populations of postnatal SSCs and progenitors that play different roles in relation to growth, bone turnover and homeostasis, and injury response. Most markers lack specificity for a single cell population. However, we are beginning to understand the diversity and functionality of populations present in the bone marrow space, and to some degree, in the periosteum. Human studies are less advanced, and further efforts to refine methods for identifying different cell populations that appear to be capable of involvement in healing in adults, and ultimately how these may change in aging and relevant disease states are of great interest.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. https://doi.org/10.1038/nm.3028.
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. https://doi.org/10.1016/j.stem.2008.07.003.
Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. 2018;561(7724):455–7. https://doi.org/10.1038/d41586-018-06756-9.
Bragdon BC, Bahney CS. Origin of reparative stem cells in fracture healing. Curr Osteoporos Rep. 2018;16(4):490–503. https://doi.org/10.1007/s11914-018-0458-4.
• de Lageneste Duchamp O, Julien A, Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018;9(1):773. https://doi.org/10.1038/s41467-018-03124-zShowed the high potential of periosteal SSC in skeletal regeneration, which requires the presence of periostin.
Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16(12):1157–67. https://doi.org/10.1038/ncb3067.
Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–68. https://doi.org/10.1016/j.stem.2014.06.008.
Matsushita Y, Ono W, Ono N. Skeletal stem cells for bone development and repair: diversity matters. Curr Osteoporos Rep. 2020;18(3):189–98. https://doi.org/10.1007/s11914-020-00572-9.
• Pineault KM, Song JY, Kozloff KM, Lucas D, Wellik DM. Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nat Commun. 2019;10(1):3168. https://doi.org/10.1038/s41467-019-11100-4Develop Hoxa11-CreER and show that cells labeled in development or early postnatal life continue to contribute to osteoblast formation for many months specifically in the zeugopod limbs, confirming that progenitors involved in growth, turnover, and healing do not travel from other bones.
Matthews BG, Ono N, Kalaizic I. Methods in lineage tracing. Principles of Bone Biology. Academic Press; 2020. p. 1887–98.
He X, Bougioukli S, Ortega B, Arevalo E, Lieberman JR, McMahon AP. Sox9 positive periosteal cells in fracture repair of the adult mammalian long bone. Bone. 2017;103:12–9. https://doi.org/10.1016/j.bone.2017.06.008.
Kuwahara ST, Serowoky MA, Vakhshori V, Tripuraneni N, Hegde NV, Lieberman JR, et al. Sox9+ messenger cells orchestrate large-scale skeletal regeneration in the mammalian rib. eLife. 2019;8:e40715. https://doi.org/10.7554/eLife.40715.
Balani DH, Ono N, Kronenberg HM. Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J Clin Invest. 2017;127(9):3327–38. https://doi.org/10.1172/JCI91699.
•• Mizuhashi K, Ono W, Matsushita Y, Sakagami N, Takahashi A, Saunders TL, et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018;563(7730):254–8. https://doi.org/10.1038/s41586-018-0662-5Established Pthrp-CreER as a marker of a subset of resting growth plate cells and demonstrate their capacity to form chondrocytes that go on to become osteoblasts and stromal cells but not adipocytes in the trabecular area.
Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1–2):269–84.
Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, et al. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. 2016;19(5):628–42. https://doi.org/10.1016/j.stem.2016.08.001.
Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8(5):552–65. https://doi.org/10.1016/j.stem.2011.02.021.
•• Shi Y, He G, Lee WC, McKenzie JA, Silva MJ, Long F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat Commun. 2017;8(1):2043. https://doi.org/10.1038/s41467-017-02171-2Characterization of Gli1-CreER labeled cells in both development and postnatal bone, and demonstration of their functional role in trabecular bone accrual.
Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–9.
Liu YL, Strecker S, Wang LP, Kronenberg MS, Wang W, Rowe DW, et al. Osterix-Cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8(8):e71318. https://doi.org/10.1371/journal.pone.0071318.
Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10(3):259–72. https://doi.org/10.1016/j.stem.2012.02.003.
• Ortinau LC, Wang H, Lei K, Deveza L, Jeong Y, Hara Y, et al. Identification of functionally distinct Mx1+alphaSMA+ periosteal skeletal stem cells. Cell Stem Cell. 2019;25(6):784–96 e5. https://doi.org/10.1016/j.stem.2019.11.003Show that Mx1-Cre targets periosteal stem cells, and that αSMA-GFP enriches for stem-like populations, at least ex vivo. Identify CCL5 as a mediator of migration.
Song JY, Pineault KM, Dones JM, Raines RT, Wellik DM. Hox genes maintain critical roles in the adult skeleton. Proc Natl Acad Sci U S A. 2020;117(13):7296–304. https://doi.org/10.1073/pnas.1920860117.
•• Matsushita Y, Nagata M, Kozloff KM, Welch JD, Mizuhashi K, Tokavanich N, et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat Commun. 2020;11(1):332. https://doi.org/10.1038/s41467-019-14029-wCharacterise functionality of Cxcl12+ central marrow stromal cells using Cxcl12-CreER. Under homeostatic conditions they demonstrate, they do not spread far within the marrow, or form osteoblasts, but become more mobile and osteogenic following injury. Clearly demonstrate heterogeneity of cells involved in injury reponse including those that do not normally contribute to the osteoblast pool.
• Seike M, Omatsu Y, Watanabe H, Kondoh G, Nagasawa T. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev. 2018;32(5–6):359–72. https://doi.org/10.1101/gad.311068.117Establish Ebf3-CreER and examine Ebf3 function in the bone marrow. Lineage tracing data is minimal, and we look forward to more detailed characterization of this model, but functional characterization is striking indicating that Ebf3 is important for inhibiting bone formation in the marrow cavity.
Matic I, Matthews BG, Wang X, Dyment NA, Worthley DL, Rowe DW, et al. Quiescent bone lining cells are a major source of osteoblasts during adulthood. Stem Cells. 2016;34(12):2930–42. https://doi.org/10.1002/stem.2474.
Grcevic D, Pejda S, Matthews BG, Repic D, Wang LP, Li HT, et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30(2):187–96. https://doi.org/10.1002/stem.780.
Matthews BG, Sbrana FV, Novak S, Funnell JL, Cao Y, Buckels EJ, et al. Heterogeneity of murine periosteum osteochondroprogenitors involved in fracture healing. bioRxiv. 2020:2020.06.24.169003. https://doi.org/10.1101/2020.06.24.169003.
Prater MD, Petit V, Alasdair Russell I, Giraddi RR, Shehata M, Menon S, et al. Mammary stem cells have myoepithelial cell properties. Nat Cell Biol. 2014;16(10):942–50. https://doi.org/10.1038/ncb3025.
Kawanami A, Matsushita T, Chan YY, Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun. 2009;386(3):477–82. https://doi.org/10.1016/j.bbrc.2009.06.059.
Moore ER, Yang Y, Jacobs CR. Primary cilia are necessary for Prx1-expressing cells to contribute to postnatal skeletogenesis. J Cell Sci. 2018;131(16):jcs217828. https://doi.org/10.1242/jcs.217828.
Rux DR, Song JY, Swinehart IT, Pineault KM, Schlientz AJ, Trulik KG, et al. Regionally restricted Hox function in adult bone marrow multipotent mesenchymal stem/stromal cells. Dev Cell. 2016;39(6):653–66. https://doi.org/10.1016/j.devcel.2016.11.008.
Matsushita Y, Ono W, Ono N. Growth plate skeletal stem cells and their transition from cartilage to bone. Bone. 2020;136:115359. https://doi.org/10.1016/j.bone.2020.115359.
Wolff LI, Hartmann C. A second career for chondrocytes—transformation into osteoblasts. Current Osteoporosis Reports. 2019;17(3):129–37. https://doi.org/10.1007/s11914-019-00511-3.
Chan Charles KF, Seo Eun Y, Chen James Y, Lo D, McArdle A, Sinha R, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1–2):285–98. https://doi.org/10.1016/j.cell.2014.12.002.
Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14(2):160–73. https://doi.org/10.1016/j.stem.2013.12.013.
Zhao H, Feng JF, Ho TV, Grimes W, Urata M, Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015;17(4):386. https://doi.org/10.1038/ncb3139.
Wang W, Strecker S, Liu Y, Wang L, Assanah F, Smith S, et al. Connective tissue growth factor reporter mice label a subpopulation of mesenchymal progenitor cells that reside in the trabecular bone region. Bone. 2015;71:76–88. https://doi.org/10.1016/j.bone.2014.10.005.
Kim SW, Lu Y, Williams EA, Lai F, Lee JY, Enishi T, et al. Sclerostin antibody administration converts bone lining cells into active osteoblasts. J Bone Miner Res. 2017;32(5):892–901. https://doi.org/10.1002/jbmr.3038.
Kim SW, Pajevic PD, Selig M, Barry KJ, Yang JY, Shin CS, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27(10):2075–84. https://doi.org/10.1002/jbmr.1665.
Root SH, Wee NKY, Novak S, Rosen CJ, Baron R, Matthews BG, et al. Perivascular osteoprogenitors are associated with transcortical channels of long bones. Stem Cells. 2020;38:769–81. https://doi.org/10.1002/stem.3159.
•• Zhong L, Yao L, Tower RJ, Wei Y, Miao Z, Park J, et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife. 2020;9:e54695. https://doi.org/10.7554/eLife.54695Present single cell RNAseq data from bone marrow and endosteal cells, clearing mapping out different stromal populations and showing expression of most of the previously proposed markers in these populations. Discover cells with adipocyte gene signature that are preadipogenic reticular cells that inhibit bone formation that appear to greatly overlap with populations including LepR-Cre+ cells.
Joseph C, Quach JM, Walkley CR, Lane SW, Lo Celso C, Purton LE. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell. 2013;13:520–33.
Wang L, Tower RJ, Chandra A, Yao L, Tong W, Xiong Z, et al. Periosteal mesenchymal progenitor dysfunction and extraskeletally-derived fibrosis contribute to atrophic fracture nonunion. J Bone Miner Res. 2019;34(3):520–32. https://doi.org/10.1002/jbmr.3626.
•• Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562(7725):133–9. https://doi.org/10.1038/s41586-018-0554-8Identify periosteal stem cells using a combination of lineage markers and cell surface markers.
Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274–82. https://doi.org/10.1359/jbmr.081003.
Wang T, Zhang X, Bikle DD. Osteogenic differentiation of periosteal cells during fracture healing. J Cell Physiol. 2016;232:913–21. https://doi.org/10.1002/jcp.25641.
Kolind M, Bobyn JD, Matthews BG, Mikulec K, Aiken A, Little DG, et al. Lineage tracking of mesenchymal and endothelial progenitors in BMP-induced bone formation. Bone. 2015;81:53–9. https://doi.org/10.1016/j.bone.2015.06.023.
Matthews BG, Grcevic D, Wang L, Hagiwara Y, Roguljic H, Joshi P, et al. Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. J Bone Miner Res. 2014;29(5):1283–94. https://doi.org/10.1002/jbmr.2140.
Hu X, Garcia M, Weng L, Jung X, Murakami JL, Kumar B, et al. Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat Commun. 2016;7:13095. https://doi.org/10.1038/ncomms13095.
Boulais PE, Mizoguchi T, Zimmerman S, Nakahara F, Vivie J, Mar JC, et al. The majority of CD45– TER119– CD31– bone marrow cell fraction is of hematopoietic origin and contains erythroid and lymphoid progenitors. Immunity. 2018;49:627–39.
Nakamura Y, Arai F, Iwasaki H, Hosokawa K, Kobayashi I, Gomei Y, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116(9):1422–32. https://doi.org/10.1182/blood-2009-08-239194.
Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–96. https://doi.org/10.1084/jem.20091046.
•• Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771–84 e6. https://doi.org/10.1016/j.stem.2017.02.009Thorough characterization of bone marrow populations isolated based on expression of PDGFRα, Sca1, and CD24. Find populations that are multipotent, osteo-chondral, and adipocyte progenitors as well as more committed preadipocytes.
Lundberg P, Allison SJ, Lee NJ, Baldock PA, Brouard N, Rost S, et al. Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J Biol Chem. 2007;282(26):19082–91. https://doi.org/10.1074/jbc.M609629200.
Arthur A, Nguyen TM, Paton S, Zannettino ACW, Gronthos S. Loss of EfnB1 in the osteogenic lineage compromises their capacity to support hematopoietic stem/progenitor cell maintenance. Exp Hematol. 2019;69:43–53. https://doi.org/10.1016/j.exphem.2018.10.004.
Winkler IG, Barbier V, Wadley R, Zannettino ACW, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–85. https://doi.org/10.1182/Blood-2009-07-233437.
Anjos-Afonso F, Bonnet D. Prospective identification and isolation of murine bone marrow derived multipotent mesenchymal progenitor cells. Best Pract Res Clin Haematol. 2011;24(1):13–24. https://doi.org/10.1016/j.beha.2010.11.003.
Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, et al. PDGFR alpha and CD51 mark human Nestin(+) sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–67. https://doi.org/10.1084/jem.20122252.
Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. https://doi.org/10.1038/nature11885.
Abbuehl JP, Tatarova Z, Held W, Huelsken J. Long-term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell transplantation. Cell Stem Cell. 2017;21(2):241–55.e6. https://doi.org/10.1016/j.stem.2017.07.004.
Li H, Ghazanfari R, Zacharaki D, Ditzel N, Isern J, Ekblom M, et al. Low/negative expression of PDGFR-α identifies the candidate primary mesenchymal stromal cells in adult human bone marrow. Stem Cell Reports. 2014;3:965–74.
Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457(7228):490–4. https://doi.org/10.1038/nature07547.
Chan CKF, Lindau P, Jiang W, Chen JY, Zhang LF, Chen C-C, et al. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc Natl Acad Sci. 2013;110(31):12643–8. https://doi.org/10.1073/pnas.1310212110.
Marecic O, Tevlin R, McArdle A, Seo EY, Wearda T, Duldulao C, et al. Identification and characterization of an injury-induced skeletal progenitor. Proc Natl Acad Sci U S A. 2015;112(32):9920–5. https://doi.org/10.1073/pnas.1513066112.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. https://doi.org/10.1038/nature09262.
Tournaire G, Stegen S, Giacomini G, Stockmans I, Moermans K, Carmeliet G, et al. Nestin-GFP transgene labels skeletal progenitors in the periosteum. Bone. 2020;133:115259.
Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. https://doi.org/10.1038/nature10783.
Ono N, Ono W, Mizoguchi T, Nagasawa T, Frenette PS, Kronenberg HM. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell. 2014;29:330–9.
Mabuchi Y, Matsuzaki Y. Prospective isolation of resident adult human mesenchymal stem cell population from multiple organs. Int J Hematol. 2016;103(2):138–44. https://doi.org/10.1007/s12185-015-1921-y.
Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–19. https://doi.org/10.1002/stem.1681.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. https://doi.org/10.1016/j.cell.2007.08.025.
Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–11. https://doi.org/10.1093/humrep/dem265.
Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, et al. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One. 2012;7(4):e35577. https://doi.org/10.1371/journal.pone.0035577.
Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. https://doi.org/10.1359/jbmr.2003.18.4.696.
Applebaum M, Kalcheim C. Mechanisms of myogenic specification and patterning. Results Probl Cell Differ. 2015;56:77–98. https://doi.org/10.1007/978-3-662-44608-9_4.
Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, et al. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports. 2016;6(6):897–913. https://doi.org/10.1016/j.stemcr.2016.05.011.
Tormin A, Li O, Brune JC, Walsh S, Schutz B, Ehinger M, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117(19):5067–77. https://doi.org/10.1182/blood-2010-08-304287.
•• Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, et al. Identification of the human skeletal stem cell. Cell. 2018;175(1):43–56 e21. https://doi.org/10.1016/j.cell.2018.07.029They established novel staining protocols to isolate skeletal stem cells from human tissue. They focused on the pre-hypertrophic zone of the growth plate in fetal tissue as the main source of these cells, which does not completely align with the mouse data, but the study is impressive nonetheless.
Funding
This work has been supported by the Health Research Council of New Zealand Sir Charles Hercus Fellowship, the Auckland Medical Research Foundation, and the American Society for Bone and Mineral Research Rising Star Award to BGM. YC is supported by a University of Auckland Doctoral Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skeletal Biology and Regulation
Rights and permissions
About this article
Cite this article
Cao, Y., Buckels, E.J. & Matthews, B.G. Markers for Identification of Postnatal Skeletal Stem Cells In Vivo. Curr Osteoporos Rep 18, 655–665 (2020). https://doi.org/10.1007/s11914-020-00622-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00622-2