Abstract
Purpose of Review
We reviewed recent progress on the role of sclerostin (SOST) and its effects on the immune system in order to summarize the current state of knowledge in osteoimmunology, in regard to hematopoiesis, lymphopoiesis, and inflammation.
Recent Findings
Changes in sclerostin levels affect distinct niches within the bone marrow that support hematopoietic stem cells and B cell development. Sclerostin’s regulation of adipogenesis could also be important for immune cell maintenance with age. Surprisingly, B cell development in the bone marrow is influenced by Sost produced by mesenchymal stem cells and osteoblasts, but not by osteocytes. Additionally, extramedullary hematopoiesis in the spleen and increased pro-inflammatory cytokine levels in the bone marrow are observed in global Sost−/− mice.
Summary
In addition to changes in bone marrow density, sclerostin depletion affects B lymphopoiesis and myelopoiesis, as well as other changes within the bone marrow cavity that could affect hematopoiesis. It is therefore important to monitor for hematopoietic changes in patients receiving sclerostin-depleting therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sclerostin (SOST) is an important regulator of bone homeostasis and is secreted by several cell types, in particular osteocytes (mature mineralized bone cells (OCYs)). This expression inhibits the bone formation through blocking canonical Wnt signaling in osteoblasts (OBs) and preventing OB maturation into OCYs (1,2,3,4). In mice and humans, inhibition of SOST through gene-targeting or monoclonal antibodies results in enhanced Wnt signaling in OBs and increased bone mass (5, 6). A human monoclonal antibody against sclerostin, Romosozumab, was developed by Amgen and UCB Pharma to treat osteoporosis. This is relevant for hematopoietic development and maturation, as canonical Wnt signaling between adult hematopoietic stem and progenitor cells (HSPC) and OBs is as an important regulator of hematopoietic stem cel (HSC) maintenance, self-renewal, and differentiation in the bone marrow microenvironment (7,8,9).
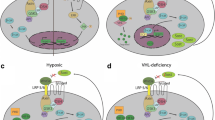
Graphical illustration of the changes in the bone that are caused by loss of sclerostin, and their effects on cells of the immune system. Left: under wild type homeostatic conditions, osteocyte production from mesenchymal stem cells is normal, producing stable levels of sclerostin to control the maturation of osteoblasts to osteocytes. Hematopoietic stem cells and B cell development are supported by niches in the bone marrow cavity. Right: when sclerostin is globally deleted or reduced, osteocyte production is dysregulated, leading to increased bone mass and decreased bone marrow volume and cellularity. Within the sclerostin-deficient bone marrow, we have collected evidence of inflammation, defective B cell development, and possible hematopoietic stem cell migration to the spleen. This figure was created with BioRender.com
Romosozumab binds to SOST to inhibit its activity and leads to increased bone formation in mice and humans (10, 11). Global approval was first awarded to Japan in January of 2019 for the treatment of osteoporosis. The success of Romosozumab is clearly evident with an associated 73% lower risk of new vertebral fractures in postmenopausal women after treatment in Phase III clinical trial “Fracture Study in Postmenopausal Women with osteoporosis” (FRAME, NCT01575834). There is a dramatically positive benefit of treatment with Romosozumab on bone mineral density (BMD), which produces a decreased fracture risk in postmenopausal women. However, this population of patients is also at higher risk for respiratory, urogenital, and gastrointestinal infections and is susceptible to autoimmune disease and mortality, resulting from these challenges to the immune system (12). Hematopoiesis and the bone are elegantly intertwined, and manipulation of one can have drastic effects on the other; it is therefore imperative that the immune system of patients receiving Romosozumab be monitored closely.
The effects of SOST on bone homeostasis, and the close proximity and interaction of the bone with hematopoietic cells in the bone marrow, has led to several investigations of the role that SOST has on hematopoiesis and immune cell development (13–15••). In this review article, we provide an overview of hematopoiesis within the bone marrow niche, a summary of previous and recent findings describing changes in hematopoiesis and immune cell development in studies of mouse models of sclerostin-deficiency, and ideas for future investigations.
The Bone Marrow Niches that Support Hematopoietic Stem Cells and B Cell Development
It is widely accepted that crosstalk between cells of the bone microenvironment and hematopoietic cells affects each other’s behavior (16, 17). HSC self-renewal, quiescence, migration, and differentiation is regulated by the cellular and molecular interactions between the stem cells and its local microenvironment (18, 19). For adult mammalian HSCs, there are two main stem cell niches: the endosteal niche, which is located where the bone and bone marrow (BM) meet, and the perivascular niches, which contains blood vessels that are located more central within the BM cavity. In addition to hematopoietic cells, the BM contains non-hematopoietic cells, loosely termed bone marrow stromal cells (BMSCs), which are critical for HSC maintenance and differentiation. BMSCs are located in close proximity to HSCs and cells of differentiated blood lineages. BMSCs include mesenchymal stem cells (MSCs), osteoblasts (OBs), and endothelial cells (ECs), amongst others. All of these play distinct roles in HSC maintenance and differentiation. HSC behavior and fate are also influenced differently by other cell types, such as adipocytes, endothelial cells, and macrophages in the bone marrow microenvironment (20, 21). In addition, elegant studies combining tissue-specific knockout mice, imaging, and functional assays of hematopoiesis and immune development provided important details about the BM microenvironment that support early B cell progenitors. In particular, within the BM, perivascular stromal cells, osteoprogenitor cells, osteoblasts, and osteocytes are critical B cell “niches,” providing CXCL12, SCF, IL7, and IGF for B cell progenitors (20,21,22,23,24,25). In addition, other factors, such as oxygen levels within the BM are key influencers of hematopoietic stem cells and B cell progenitor survival and maintenance, via preferred energy metabolism pathways. In the BM, B cell progenitors are localized in the relatively hypoxic perisinusoidal regions of the marrow (26, 27). Osteocytes are bone-matrix embedded cells throughout the bone that communicate with hematopoietic, endothelial, and other bone cell types via their canaliculi network. Several studies demonstrate the role of osteocytes in the regulation of B cell development (14, 15•, 28●●, 29, 30). However, the molecular mechanisms that regulate crosstalk between hematopoietic and these different niche cells in the bone are incompletely understood.
Sclerostin and Lymphoid Cells
Studies in global Sost−/− mice (31) revealed Sost’s influence on the regulation of immune cell development. Wnt signaling is enhanced in the absence of Sost, and Wnt signaling has been implicated in the maintenance of hematopoietic stem cells and during B cell development (32). Therefore, it was hypothesized that hematopoiesis would be altered in Sost−/− mice. Cain et al. found that the numbers of hematopoietic progenitors was unaffected in Sost−/− mice. However, B lymphocyte development was severely affected. B cell numbers were diminished, and B lymphocyte apoptosis increased in the Sost−/− bone marrow (14). Reciprocal bone marrow transplantation experiments demonstrated that the impaired B cell development in Sost−/− mice was due to a B cell-extrinsic (i.e., microenvironmental) mechanism. Analysis of the microenvironment revealed that expression of CXCL12, a critical B cell growth factor, was reduced in Sost−/− bone marrow stromal cells. These studies revealed a novel role of SOST on B cell development in the bone marrow (16). As SOST is expressed primarily by osteocytes in the bone, these data suggested that osteocytes were an important regulator of B cell development. However, using conditional Sost knockout mice (SostiCOIN/iCOIN, MGI:5544793), Yee et al. recently demonstrated that osteoblasts and mesenchymal stem cells also express Sost, and that Sost in specific osteolineage cells differentially contribute to B cell development (15●●). Remarkably, B cell development was unaffected by the deletion of Sost in osteocytes (using Dmp1-Cre). Surprisingly, MSC-specific deletion of Sost (using Prx1-Cre) and OB-specific deletion of Sost (using Col1-Cre) reduced total numbers of B cells in the BM. However, MSC-specific Sost influences proper transitions through B cell developmental stages. In contrast, Sost in OBs and OCYs do not appear to regulate B cell precursors and immature B cell subsets (15●●).
The role of SOST on B cell development was further confirmed indirectly by studies of mice in which the von-Hippel-Lindau (Vhl) gene was deleted specifically in osteocytes (28●●). Vhl is a gene in the hypoxia response signaling pathway. In Vhl-knockout mice, hypoxia-inducible factor 1a (Hif1α) is stabilized. Evidence in the literature supports both positive and negative effects of hypoxia and Hif1α on Sost expression. Sost expression is positively regulated by the direct binding of Hif1α to the SOST promoter (33). In contrast, enhanced Hif1α expression in osteocytes decreased sclerostin expression (34), and osteoblast-specific deletion of Vhl results in Hif1α activation with concomitant reduction in Sost-positive osteocytes (35). Osteocyte-specific Vhl-conditional knockout mice (Dmp1-Cre;Vhlfl/fl (herein abbreviated VhlcKO) (36), display decreased Sost expression (28●●). In addition, VhlcKO mice and Sost−/− mice both display high bone mass and impaired B cell developmental phenotypes, but the effects are more severe in the VhlcKO. For example, we have observed no differences in the frequency and number of mature B lymphocytes in the spleens of global Sost−/− mice, whereas peripheral B cells are significantly reduced in the VhlcKO spleens (Chicana and Manilay et al., unpublished results). Functional analysis of B cells in Sost−/− mice support the conclusion that B cell responses to T-dependent antigens may be altered (37), but functional B cell responses to antigens in VhlcKO mice require further investigation. In any case, these findings imply that patients receiving sclerostin-depleting therapies for osteoporosis might suffer from defects in B cell developmental and function. We are unaware if these changes occur in humans, as these data are not reported in recent reports of the Romosozumab clinical trial results (38). Given our observations in Sost-deficient mice, it would be interesting to add analysis of peripheral blood B cells and antibody titers after vaccinations in patients receiving Romosozumab and other sclerostin-depleting drugs.
Sclerostin and Inflammation
It is still not fully understood how the bone marrow microenvironment influences changes in long-term hematopoietic stem cell (LT-HSC) behavior. It is widely accepted that Sost-deficiency increases the bone mass; however, there are limited data as to how this change influences the bone marrow microenvironment. To investigate how the absence of Sost in the bone microenvironment affects hematopoietic differentiation, we performed transplantation of wild-type (WT) hematopoietic stem cells and progenitor cells (HSPCs) into global Sost−/− recipient mice. This demonstrated the Sost−/− bone marrow niche favors myeloid differentiation in WT ➔ Sost−/− chimeras when compared with WT ➔ control chimeras (Donham et al., manuscript in preparation). Inflammation in the BM can be characterized by rapid mobilization and overproduction of myeloid lineages as well as decreased production of lymphoid cells (39). Analysis of Sost−/− mice revealed an increase in TNFα in the BM, as well as upregulation of several other pro-inflammatory cytokines in the BM, perhaps in an attempt to upregulate Sost expression (Donham and Manilay et al. unpublished data). TNFα suppresses HSC activity during an inflammatory response (40) and is produced predominantly by activated macrophages. TNFα’s involvement in decreasing B lymphopoiesis is also documented (41●). Further research is needed to assess if the increased myelopoiesis in Sost−/− mice results in overproduction of activated macrophages. Using an osteoarthritis mouse model, Chang et al. recently demonstrated that Sost expression in the bone is upregulated by TNFα after injury (42●), further linking sclerostin and the processes of inflammation in the bone.
In addition to changes in the bone marrow cytokines and cellular composition, inflammation causes changes in the spleen such as extramedullary hematopoiesis (EMH) (43). Sost−/− mice display splenomegaly, and evidence of EMH, including increases in erythroid and myeloid production in the spleens (Donham et al. unpublished data). To our knowledge, extramedullary hematopoiesis has not been monitored in humans harboring inactivating Sost mutations, despite their having a similar bone marrow cavity occlusion phenotypes to that seen in Sost−/− mice, (44,45,46). Our studies of Sost−/− mice indicate that anti-sclerostin treatment in patients may alter developmental hematopoiesis in the bone marrow and spleen, and this could lead to complications for patients with impaired immune systems, such as the elderly. This is important as the primary recipient population for osteoporotic therapies are patients over the age of 50.
Future Directions
Comparison of Bone Marrow Microenvironments and Roles of Sclerostin in Different Bone Types
The bone marrow plays a key role in HSC maintenance and functionality, and it is found in numerous bones throughout the body, which vary in structure and cellular maintenance. Common sites used to study the bone marrow niche are calvaria, femurs, tibia, and vertebrae. Whether the bone marrow microenvironment from different bone types can lead to differences in BM function is a point of debate. Certainly, the development of marrow adipose tissue (MAT) in the long bones and vertebrae occurs within different time frames, which could affect the composition and frequency of non-hematopoietic and hematopoietic cells within the marrow during development (47). Comparison of bone marrow in calvaria and femurs demonstrated that they contain distinct HSC niches (48). However, analysis of 8 different bone types, suggested that BM harvest from a single bone is representative of the rest of the BM present in a mouse (49). Sclerostin is generally expressed by osteocytes in all bone types, and sclerostin is detected in the serum, so studies to determine if sclerostin production from a particular bone or tissue has endocrine effects would be very interesting. Genetic tools that delete the Sost gene in specific cell types, such as Prx1-Cre, which is activated in the limb bones but not in the vertebrae (15●●), could help to reveal endocrine versus paracrine effects of SOST on bone marrow niches.
Sclerostin, Adipose Tissue, and Immunity
Fairfield et al. first demonstrated a link between sclerostin and the regulation of the bone marrow adipose tissue development (50●●). Treatment of primary BM-MSCs with recombinant SOST increased adipocyte differentiation, and treatment of the 3T3-L1 cell line with osteocyte-conditioned media also increased adipocyte differentiation, showing a direct effect of osteocyte-produced SOST on BM adipogenesis. In addition, Sost−/− mice display reduced BM adipose tissue (BMAT) at 6 weeks of age, and treatment of wild-type mice with sclerostin antibody reduced BM adiposity. These results were confirmed by Li et al., who demonstrated that sclerostin antibody treatment reversed the increase in marrow adiposity observed in in ovariectomized rabbits (51). The reduced BM adipose tissue observed by Fairfield et al. and Li et al. is contradictory to the observation of decreased B cell development in the BM of Sost−/− mice (14), since increased BM adiposity of the bone marrow is correlated with a decrease in B cell development in mice, rabbit, and humans (47, 52). Our observation of increased concentrations of inflammatory cytokines in the BM of Sost−/− mice would also be unexpected, if BM adipose tissue is reduced in the absence of Sost. Taken together, these papers suggest that osteocytes and BM adipocytes play direct roles in the maintenance of HSCs and B cell development, and that Sost is an important regulator of these cellular interactions in the bone. Further studies are necessary to identify the effects of constitutive marrow adipose tissue (cMAT) and regulated marrow adipose tissue (rMAT) on cellular metabolism during hematopoiesis (53), and whether cMAT and rMAT are differentially regulated by Sost. Since MSCs are a common progenitor of both adipocytes and osteocytes, it is also possible that SOST acts directly at the level of the MSC. It is tempting to speculate that deletion of Sost could affect MSC maintenance and function, which, in turn, could affect HSCs and B cells within the bone marrow niches (27), independently of the status of the BM adiposity and bone mass.
Conclusion
Successful clinical trials have led to the approval of the sclerostin antibody Romosozumab for the treatment of osteoporosis patients. Despite the promising effect on bone mass of this treatment, there is concern because defects in the immune development were identified in Sost-deficient mice. Increases in pro-inflammatory cytokines in the bone marrow of these mice as well as extramedullary hematopoiesis highlight the need for further exploration into the effects that inhibition of sclerostin has on bone marrow homeostasis. Monitoring of the three hallmarks of hematopoietic aging, anemia, thrombopoiesis, and immunosenescence, may be an important future clinical consideration for monitoring patients of Romosozumab.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–9.
van Bezooijen RL, Svensson JP, Eefting D, Visser A, van der Horst G, Karperien M, et al. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res. 2007;22:19–28.
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–4.
Gori F, Lerner U, Ohlsson C, Baron R. A new WNT on the bone: WNT16, cortical bone thickness, porosity and fractures. Bonekey Rep. 2015;4:669.
Yavropoulou MP, Xygonakis C, Lolou M, Karadimou F, Yovos JG. The sclerostin story: from human genetics to the development of novel anabolic treatment for osteoporosis. Hormones (Athens). 2014;13:323–37.
McClung MR. Sclerostin antibodies in osteoporosis: latest evidence and therapeutic potential. Ther Adv Musculoskelet Dis. 2017;9:263–70.
Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–83.
Schaniel C, Sirabella D, Qiu J, Niu X, Lemischka IR, Moore KA. Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood. 2011;118:2420–9.
Richter J, Traver D, Willert K. The role of Wnt signaling in hematopoietic stem cell development. Crit Rev Biochem Mol Biol. 2017;52:414–24.
McClung MR. Romosozumab for the treatment of osteoporosis. Osteoporos Sarcopenia. 2018;4:11–5.
Markham A. Romosozumab: first global approval. Drugs. 2019;79:471–6.
Denkinger MD, Leins H, Schirmbeck R, Florian MC, Geiger H. HSC aging and senescent immune remodeling. Trends Immunol. 2015;36:815–24.
Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37.
Cain CJ, Rueda R, McLelland B, Collette NM, Loots GG, Manilay JO. Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res. 2012;27:1451–61.
Yee, C. S., J. O. Manilay, J. C. Chang, N. R. Hum, D. K. Murugesh, J. Bajwa, M. E. Mendez, A. E. Economides, D. J. Horan, A. G. Robling, and G. G. Loots. 2018. Conditional deletion of Sost in MSC-derived lineages identifies specific cell-type contributions to bone mass and B-cell development. J Bone Miner Res 33: 1748–1759. This study demonstrated thatPrx1+mesenchymal stem cells significantly contribute to the paracrine pool of sclerostin in the bone, and that conditional deletion ofSostin Prx1-expressing cells recapitulates the increased bone mass phenotype observed in the globalSost−/−mouse. Furthermore, this study demonstrated thatSostspecifically in mesenchymal stem cells, rather thanSostin osteocytes, influences B lymphocyte development.
Horowitz MC, Fretz JA. Sclerostin: a new mediator of crosstalk between the skeletal and immune systems. J Bone Miner Res. 2012;27:1448–50.
Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2011;12:49–60.
Lo Celso C, Scadden DT. The haematopoietic stem cell niche at a glance. J Cell Sci. 2011;124:3529–35.
Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–46.
Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–30.
Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–5.
Funk PE, Varas A, Witte PL. Activity of stem cell factor and IL-7 in combination on normal bone marrow B lineage cells. J Immunol. 1993;150:748–52.
Cordeiro Gomes A, Hara T, Lim VY, Herndler-Brandstetter D, Nevius E, Sugiyama T, et al. Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity. 2016;45:1219–31.
Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6:107–16.
Yu VW, Lymperi S, Oki T, Jones A, Swiatek P, Vasic R, et al. Distinctive mesenchymal-parenchymal cell pairings govern B cell differentiation in the bone marrow. Stem Cell Reports. 2016;7:220–35.
Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–73.
Aurrand-Lions M, Mancini SJC. Murine Bone marrow niches from hematopoietic stem cells to B cells. Int J Mol Sci. 2018;19.
Loots, G. G., A. G. Robling, J. C. Chang, D. K. Murugesh, J. Bajwa, C. Carlisle, J. O. Manilay, A. Wong, C. E. Yellowley, and D. C. Genetos. 2018. Vhl deficiency in osteocytes produces high bone mass and hematopoietic defects. Bone 116: 307–314. This study revealed a novel influence ofVhlin osteocytes and maintenance of bone through regulation of canonical Wnt signaling. Conditional deletion ofVhlin osteocytes using Dmp-Cre results in a high bone mass phenotype and reducedSostexpression. In addition, B cell development inVhl-conditional knockout mice is severely reduced and myelopoiesis was increased, and more extensive than the effect observed in globalSost−/−mice.
Fujiwara Y, Piemontese M, Liu Y, Thostenson JD, Xiong J, O’Brien CA. RANKL (receptor activator of NFkappaB ligand) produced by osteocytes is required for the increase in B cells and Bone loss caused by estrogen deficiency in mice. J Biol Chem. 2016;291:24838–50.
Panaroni C, Fulzele K, Saini V, Chubb R, Pajevic PD, Wu JY. PTH signaling in Osteoprogenitors is essential for B-lymphocyte differentiation and mobilization. J Bone Miner Res. 2015;30:2273–86.
Saito K. Effects of hyperoxia on phospholipid metabolism and on intracellular structure of cultured type II pneumocytes. Kokyu To Junkan. 1986;34:1079–85.
Cain CJ, Manilay JO. Hematopoietic stem cell fate decisions are regulated by Wnt antagonists: comparisons and current controversies. Exp Hematol. 2013;41:3–16.
Chen D, Li Y, Zhou Z, Wu C, Xing Y, Zou X, et al. HIF-1alpha inhibits Wnt signaling pathway by activating Sost expression in osteoblasts. PLoS One. 2013;8:e65940.
Stegen S, Stockmans I, Moermans K, Thienpont B, Maxwell PH, Carmeliet P, et al. Osteocytic oxygen sensing controls bone mass through epigenetic regulation of sclerostin. Nat Commun. 2018;9:2557.
Zuo GL, Zhang LF, Qi J, Kang H, Jia P, Chen H, et al. Activation of HIFa pathway in mature osteoblasts disrupts the integrity of the osteocyte/canalicular network. PLoS One. 2015;10:e0121266.
Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–5.
Chow, A., J. Mason, L. Coney, J. Bajwa, C. Carlisle, A. Zaslavsky, Y. Pellman, M. E. García-Ojeda, A. Economides, G. G. Loots, and J. O. Manilay. 2018. Sclerostin deficiency alters peripheral B lymphocyte responses in mice. bioRxiv: 357772.
McClung MR, Brown JP, Diez-Perez A, Resch H, Caminis J, Meisner P, et al. Effects of 24 months of treatment with Romosozumab followed by 12 months of Denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33:1397–406.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35.
Pronk CJ, Veiby OP, Bryder D, Jacobsen SE. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: involvement of two distinct receptors. J Exp Med. 2011;208:1563–70.
Kennedy, D. E., and K. L. Knight. 2017. Inflammatory changes in bone marrow microenvironment sssociated with declining B lymphopoiesis. J Immunol 198: 3471–3479. This study revealed that in rabbits, increases in myelopoiesis and bone marrow fat levels correspond with an early block of B lymphopoiesis and have been shownin vitroto inhibit B lymphopoiesis. This article also concludes that two inflammatory molecules produced by myeloid cells, IL-1β and S100A9, are increased in the BM during B lymphopoiesis arrest and inhibit B lymphopoiesisin vitro.
Chang, J. C., B. A. Christiansen, D. K. Murugesh, A. Sebastian, N. R. Hum, N. M. Collette, S. Hatsell, A. N. Economides, C. D. Blanchette, and G. G. Loots. 2018. SOST/Sclerostin improves posttraumatic osteoarthritis and inhibits MMP2/3 expression after injury. J Bone Miner Res 33: 1105–1113. Chang et al. demonstrated thatSostactivation in response to joint injury is TNFα and NF-κB dependent, and that in PTOA, SOST functions as a protective molecule to prevent cartilage degradation in subsequent traumatic injury by downregulating Wnt-dependent catabolic enzymes.
Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12.
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–7.
van Lierop AH, Hamdy NA, van Egmond ME, Bakker E, Dikkers FG, Papapoulos SE. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res. 2013;28:848–54.
MacNabb C, Patton D, Hayes JS. Sclerostin antibody therapy for the treatment of osteoporosis: clinical prospects and challenges. J Osteoporos. 2016;2016:6217286.
Horowitz MC, Berry R, Holtrup B, Sebo Z, Nelson T, Fretz JA, et al. Bone marrow adipocytes. Adipocyte. 2017;6:193–204.
Lassailly F, Foster K, Lopez-Onieva L, Currie E, Bonnet D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood. 2013;122:1730–40.
Geerman S, Hickson S, Brasser G, Pascutti MF, Nolte MA. Quantitative and qualitative analysis of bone marrow CD8(+) T cells from different bones uncovers a major contribution of the bone marrow in the vertebrae. Front Immunol. 2015;6:660.
Fairfield, H., C. Falank, E. Harris, V. Demambro, M. McDonald, J. A. Pettitt, S. T. Mohanty, P. Croucher, I. Kramer, M. Kneissel, C. J. Rosen, and M. R. Reagan. 2018. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol 233: 1156–1167. Fairfield et al. first demonstrated a direct link between sclerostin produced by osteocytes and the promotion of the development of bone marrow adipose tissue by SOSTin vitroandin vivo, in mice. This paper reveals another possible alteration of the bone marrow niche that could affect hematopoiesis and immune cell lineages when sclerostin is depleted.
Li S, Huang B, Jiang B, Gu M, Yang X, Yin Y. Sclerostin antibody mitigates estrogen deficiency-inducted marrow lipid accumulation assessed by proton MR spectroscopy. Front Endocrinol (Lausanne). 2019;10:159.
Kennedy DE, Witte PL, Knight KL. Bone marrow fat and the decline of B lymphopoiesis in rabbits. Dev Comp Immunol. 2016;58:30–9.
Turner RT, Martin SA, Iwaniec UT. Metabolic coupling between bone marrow adipose tissue and hematopoiesis. Curr Osteoporos Rep. 2018;16:95–104.
Authors’ Roles
Drafting and revising manuscript: CD and JOM; approving final version of manuscript: JOM.
Funding
This work was supported by University of California (UC), Merced faculty research funding, National Institutes of Health Award 1R15HL121786-01A1, Halcyon-Dixon Trust award to JOM, and UC Graduate Student Fellowships to CD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards and institutional approvals.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteoimmunology
Rights and permissions
About this article
Cite this article
Donham, C., Manilay, J.O. The Effects of Sclerostin on the Immune System. Curr Osteoporos Rep 18, 32–37 (2020). https://doi.org/10.1007/s11914-020-00563-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00563-w