Abstract
Amyotrophic lateral sclerosis (ALS), also called Lou Gehrig’s disease, is a fatal neuromuscular disorder characterized by degeneration of motor neurons and by skeletal muscle atrophy. Although the death of motor neurons is a pathological hallmark of ALS, the potential role of other organs in disease progression remains to be elucidated. Skeletal muscle and bone are the two largest organs in the human body. They are responsible not only for locomotion but also for maintaining whole body normal metabolism and homeostasis. Patients with ALS display severe muscle atrophy, which may reflect intrinsic defects in mitochondrial respiratory function and calcium (Ca) signaling in muscle fibers, in addition to the role of axonal withdrawal associated with ALS progression. Incidence of fractures is high in ALS patients, indicating there are potential bone defects in individuals with this condition. There is a lifelong interaction between skeletal muscle and bone. The severe muscle degeneration that occurs during ALS progression may potentially have a significant impact on bone function, and the defective bone may also contribute significantly to neuromuscular degeneration in the course of the disease. Due to the nature of the rapid and severe neuromuscular symptoms, a majority of studies on ALS have focused on neurodegeneration. Just a few studies have explored the possible contribution of muscle defects, even fewer on bone defects, and fewer still on possible muscle-bone crosstalk in ALS. This review article discusses current studies on bone defects and potential defects in muscle-bone crosstalk in ALS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neuromuscular system includes skeletal muscle and motor neurons in the spinal cord and brain that controls voluntary movements of the human body. Any condition that affects this system is defined as a neuromuscular disease. Amyotrophic lateral sclerosis (ALS) is one of the most severe neuromuscular diseases that is characterized by death of motor neurons and muscle atrophy. Most ALS patients die within 5 years after disease onset. The lifetime risk of ALS is about 1 in 472 in women and 1 in 350 in men [1]. ALS is an age-dependent disease. As the US population increases and ages, an increase in the prevalence of ALS can be anticipated. The majority of ALS cases are sporadic (SALS), with about 10 % being familial (FALS) [2]. Both SALS and FALS manifest similar pathological and clinical phenotypes, suggesting that different initiating causes lead to a mechanistically similar degenerative pathway in the neuromuscular system. There is no cure for ALS. Available treatments are designed to relieve symptoms and improve the quality of life for patients with ALS. Treatment with the only FDA-approved drug, Riluzole, extends patient life span only for a few months with little improvement in ALS symptoms [1]. Thus, there is an urgent need to further understand the pathogenic mechanisms responsible for this disease in order to develop entirely novel interventions for alleviating the disease progression and improving the quality of life for ALS patients.
Most of FALS are associated with mutations in the superoxide dismutase gene (SOD1) [2]. Mouse models expressing ALS-linked SOD1 mutations effectively recapitulate many features of the human disease and have been extensively used to investigate pathogenic mechanisms of ALS [3]. Lately, TDP-43 (TAR DNA-binding protein 43) was also found to be associated with both FALS and SALS [4]. While efforts have been made to model TDP-43-ALS in rodents, those animal models did not show consistent ALS phenotypes [4]. Thus, the classical ALS mouse model with the SOD1 mutation (i.e., G93A) that develops both neurodegeneration and muscle atrophy is still the most widely used animal model for understanding the pathogenesis of the disease and for the preclinical studies for potential therapeutic interventions for ALS [5]. While the majority of studies on ALS animal models focus on neurodegeneration, a few have explored the possible contribution of muscle defects and even fewer of bone defects and the possible muscle-bone crosstalk. Here, we review current studies on possible muscle-bone interaction in the course of ALS progression.
The Role of Skeletal Muscle in ALS
During ALS progression, the degeneration of motor neurons leads to axonal withdrawal from the neuromuscular junction leading to muscle atrophy. However, the massive and rapid decline of muscle function and muscle mass in ALS may indicate additional pathologies in addition to motor neuron denervation. Although the death of motor neurons is a pathological hallmark of ALS, defects present in other cell types may also actively contribute to the disease progression [6]. ALS has been described as a “distal axonopathy,” which affects the axon and the neuromuscular junction in the ALS transgenic mouse model at an age prior to significant loss of neuronal bodies and the onset of muscle atrophy [7, 8]. It is possible that an intrinsic muscle defect occurs early in the course of ALS that promotes or contributes to the motor axonal withdrawal. Recent studies have shown that defects in Ca signaling affect the integrity of the neuromuscular junction and contribute to muscle degeneration in the early stages of ALS progression [9•, 10•, 11].
Skeletal muscle comprises approximately 40 % of whole-body lean mass [12] and is substantially affected in ALS. Accumulating evidence supports the concept that muscle plays an active role in ALS progression [9•, 13, 14]. Individual muscle fibers communicate with motor neurons at the site of the neuromuscular junction. The degeneration of motor neurons limits neuron-to-muscle signaling leading to severe muscle atrophy in ALS, while the retrograde signaling from muscle-to-neuron, which is important for axonal growth and neuromuscular junction maintenance [15], is also lost in ALS during disease progression. Expression of muscle-specific IGF-1 in G93A mice was shown to enhance motor neuron survival, delaying disease onset and progression [16]. The gene expression profile of ALS muscle is significantly different from that of the muscle with axotomy-induced denervation [13], suggesting there are muscle defects that are independent of axonal withdrawal. Overexpression of ALS-associated mutation SOD1G93A in skeletal muscle of normal mice directly leads to an abnormal mitochondrial network and dynamics in the absence of motor neuron degeneration [9•]. In further support of an active role of muscle in ALS progression, published studies have shown that transgenic mice with muscle-specific overexpression of the SOD1 mutation (G93A) developed age-related neurologic and pathologic phenotypes consistent with ALS [17, also see 18]. Thus, studies of ALS animal models (mimic familial ALS) support the hypothesis that skeletal muscle is likely one of the primary targets of ALS-associated mutations. In the case of sporadic ALS, there are no identified genetic causes. However, the pathologic insults that damage motor neurons could play a similar role in skeletal muscle. It is likely that skeletal muscle experiences double pathological insults both intrinsic and extrinsic during ALS progression leading to severe atrophy in a very short period of time (3–5 years) after onset of the ALS symptoms. This severe muscle degeneration in ALS may not only feedback to damage neuronal function, but likely has a significant impact on bone function.
Potential Muscle-Bone Crosstalk in ALS
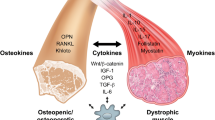
Bone is a metabolically active organ and constantly undergoes resorption and formation called bone remodeling. Bone remodeling and maintenance is necessary not only to maintain the structural integrity of the skeleton but also for homeostasis of the whole body [19]. Skeletal muscle plays an important role in bone remodeling. There is a lifelong interaction between skeletal muscle and bone [20–24, 25•, 26]. Skeletal muscle acts as an important source of osteogenic growth factors, such as fibroblast growth factor (FGF-2) and insulin-like growth factor (IGF-1) [27], thus providing important anabolic stimuli for bone remodeling [28, 29, 30•, 31]. Muscle-derived osteogenic stem cells play a significant role in maintaining bone homeostasis [32, 33•, 34•, 35]. Most importantly, the shape of bones is constantly regulated by the muscle force, and the muscle-induced mechanical load regulates key aspects of bone morphogenesis [26, 30•, 31, 36, 37].
Patients with muscle atrophy such as Duchenne muscular dystrophy (DMD) and spinal muscular atrophy (SMA) show reduced bone density and higher incidence of bone fractures [38, 39, 40•, 41]. In the case of ALS, the severe muscle atrophy could lead to rapidly reduced mechanical load to the skeleton and may potentially lead to impairment of bone morphology and function as the disease progresses. As ALS patients experience rapid muscle paralysis and death, the neuromuscular system becomes so overwhelmed. Therefore, little attention has been paid to the bone defects in ALS patients and the potential role of the defective bone in ALS disease progression. There are only a few studies covering this area. In an early clinical study, Sato Y et al. found reduced bone density with hypovitaminosis D and compensatory hyperparathyroidism in these patients, indicating the development of osteopenia and impaired bone integrity in ALS patients [42]. Not surprising, the incidence of fractures was also found to be high in ALS patients [43].
As bone is an important organ that produces muscle trophic factors, impaired bone homeostasis could exacerbate muscle degeneration and accelerate disease progression. Bone marrow mesenchymal stem cells (MSCs) have the capacity to differentiate into various cell types, including myoblasts and neuron-like cells. Bone produces various trophic factors including VEGF, FGF-2, BMPs, and IGF-1 that are critical for maintaining the homeostasis of neurons and muscles. It has been shown that the paracrine release of VEGF by MSCs in bone marrow improves muscle regeneration [44, 45•]. Interestingly, MSCs from the bone marrow of ALS patients have diminished stem cell capacity and produce fewer trophic factors, and those defects correlate with disease progression [46•, 47]. In addition, intraspinal infusion of autologous bone marrow mononuclear cells (BMNCs) prevents spinal motor neuron degeneration in ALS patients [48]. Results from those studies suggest a possible interaction between muscle and bone during ALS disease progression. However, the molecular mechanism underlying the potential muscle-bone crosstalk remains largely unknown.
Bone Defects Have Been Demonstrated in an ALS Mouse Model
There are three types of bone cells critical for bone remodeling: [1] the bone-forming cells (osteoblasts) found at the surface of the bone. They are responsible for forming new bone by producing collagen and other components of bone matrix; [2] the bone-resorbing cells (osteoclasts) are also found at the surface of the bone. When activated, they initiate bone resorption by releasing hydrogen ions and lysosomal enzymes that degrade all components of bone matrix; [3] osteocytes, the bone cells embedded in the bone matrix [19]. Osteocytes are thought to coordinate the function of osteoblasts and osteoclasts by translating mechanical stimuli into biochemical signals that ultimately regulate bone remodeling [20, 49–54, 55•]. It was not known how those three types of bone cells respond to ALS pathology and what the possible molecular basis is for the bone defects observed in ALS patients.
In a recent study, Zhu et al., for the first time, systematically explored whether and how bone homeostasis is impaired during ALS disease progression [56••]. Using the classic ALS mouse model with overexpression of ALS mutation SOD1G93A (G93A), they analyzed the activity of osteoblasts, osteoclasts, and osteocytes during ALS disease progression at stages with or without muscle atrophy. They have found that the G93A mice with muscle atrophy have dramatically reduced trabecular and cortical bone mass, while the young G93A mice before disease onset without muscle atrophy show normal bone density. These observations indicate that reduced muscle loading is the key cause of osteopenia in the ALS mouse model. The reduced bone density in G93A mice is associated with impaired function of osteoblasts and osteocytes, along with striking acceleration of osteoclast formation in bone.
Molecular Mechanisms Underlying the Bone Defects in ALS
Zhu et al. also evaluated the potential cellular and molecular pathways responsible for the bone defects during ALS progression [56••]. As demonstrated in their study, primary culture of osteoblasts derived from G93A mice with muscle atrophy showed dramatically reduced expression of the osteoblast differentiation markers Runx2 and osterix at both mRNA and protein levels. The formation and expansion of bone marrow mesenchymal stem cells (BMSCs) and osteoprogenitors were also significantly reduced in bone marrow cultures. Both abnormalities could be responsible for reduced numbers of osteoblasts resulting in less bone formation. The Wnt/β-catenin signaling pathway has been found to play a critical role in mediating the mechanical regulation of bone homeostasis [20, 49-54, 55•, 57] and is also required for the differentiation of bone marrow mesenchymal stem cells towards the osteoblastic lineage and bone formation [37, 58–61]. Indeed, the level of β-catenin was found to be drastically reduced in BMSCs and osteoblasts from G93A mice at the disease stage characterized by muscle atrophy. As it is known that AKT/ERK/MAPK pathway is involved in osteoblast survival, proliferation, and differentiation [62], defective activation of the AKT pathway was also found in these mice that may explain the impaired osteoblast function through negative impact on Runx2 activity [63–65].
In the same study, Zhu et al. also found that osteoclast formation was strikingly enhanced in primary bone marrow monocyte cultures and bone resorption was elevated in G93A mice with severe muscle atrophy. They discovered increases in osteoclast differentiation marker genes including those encoding TRAP (Trap), cathepsin K (CatK), matrix metallopeptidase 9 (Mmp-9), and receptor activator of nuclear factor kappa-B (Rank). These results suggest that muscle atrophy induced an intrinsic activation of the osteoclast differentiation program in G93A mice. In addition, there was an increased expression of Rankl in osteocytes derived from G93A mice with muscle atrophy. Rankl is a key molecule that promotes bone resorption by binding and activating the RANK receptor on osteoclast precursors [66, 67] and may contribute to the enhanced osteoclast formation and bone resorption in G93A mice. Together, the results suggest that muscle-generated mechanical force is required to inhibit osteoclast formation and bone resorption, which is lost with ALS progression.
Because the function of osteocytes reflects the functional coordination between osteoblasts and osteoclasts by translating mechanical stimuli into biochemical signals that ultimately regulate bone remodeling [20, 49–54, 55•], Zhu et al. also analyzed the function of osteocytes in G93A mice [56••]. It is known that osteocyte-derived sclerostin can be induced by mechanical unloading and inhibits Wnt/β-catenin signaling [68]. The study did reveal an increase in sclerostin protein in osteocytes embedded in the bone matrix of G93A mice. In addition, Rankl mRNA and protein were increased in bone and osteocytes, suggesting an overall enhancement of bone resorption and inhibition of bone formation by osteocytes in G93A mice.
Reduced Mechanical Load Due to Muscle Atrophy Is Likely the Key Player for Bone Defects in ALS Mouse Model
It has been suggested that muscle can affect bone independent of loading through the production of factors that support bone formation and maintenance [20, 69]. However, in the study of Zhu et al. [56••], even though there was significant reduction in bone mass in long bones, there were no detectable defects in the calvarial bone of G93A mice even at an age with severe muscle atrophy. As the calvaria is not a loaded bone, it was assumed that the effects of ALS disease was due to lack of muscle loading of bone and not due to a lack of osteogenic factors produced by muscle. The young G93A mice without muscle atrophy have normal bone mass with no detectable abnormality in the function of osteoblasts and osteoclasts. These data suggest that reduced mechanical load due to muscle atrophy should be the key player for the bone defects observed in ALS mouse model.
However, as with the skeletal muscle, the bone from G93A mice also carries ALS mutation SOD1G93A, it is not known if this ALS mutation can have a direct effect on bone as the mutation is globally expressed. Interestingly, the primary cultures derived from G93A bone show abnormal function of osteoblast and osteoclasts in the absence of the muscle influence. This result suggests that the reduced muscle mechanical load on bone may reprogram the gene expression network in bone during ALS progression. How the primary cultured bone cells derived from ALS mice keep the memory of the impact from the muscle unloading is a mystery. Or the mutation in bone cells makes cells more susceptible to the effect of lack of loading and/or reprogramming. A third possible explanation is that the timing for muscle atrophy due to the mutation in muscle is similar to the timing for bone defects due to the expression of the mutation in bone. Further mechanistic study is required to understand this phenomenon.
Conclusion
Since the first ALS mutation was identified and the first ALS animal model was generated in 1993 [5], the understanding of ALS pathogenesis has advanced significantly, especially with respect to motor neuron degeneration. However, despite extensive research efforts focusing on the degeneration of motor neurons, there has been no significant improvement in the treatment of ALS. It is possible that ALS is not only a motor neuron disease but also a systemic disorder, in which the dysfunction of multiple organs may exacerbate neuronal degeneration. The important role of muscle-bone crosstalk is beginning to be recognized in both health and diseases. It has been hoped that this review article will draw attention from the ALS research field, encouraging investigators to think “out of the box” by evaluating the possible roles of other organ systems, especially the role of muscle and bone, which may significantly contribute to ALS pathogenesis and progression. Understanding the functional crosstalk between muscle and bone during the progression of ALS will help to identify potential new therapeutic targets for treating and/or improving the quality and length of life for ALS patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Alonso A, Logroscino G, Jick SS, Hernan MA. Incidence and lifetime risk of motor neuron disease in the United Kingdom: a population-based study. Eur J Neurol. 2009;16:745–51.
Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–23.
McGoldrick P, Joyce PI, Fisher EM, Greensmith L. Rodent models of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2013;1832:1421–36.
Baloh RH. How do the RNA-binding proteins TDP-43 and FUS relate to amyotrophic lateral sclerosis and frontotemporal degeneration, and to each other? Curr Opin Neurol. 2012;25:701–7.
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–5.
Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59.
Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–40.
Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–42.
Luo G, Yi J, Ma C, Xiao Y, Yi F, Yu T, et al. Defective mitochondrial dynamics is an early event in skeletal muscle of an amyotrophic lateral sclerosis mouse model. PLoS One. 2013;8:e82112. ALS-linked mutation directly impairs mitochondrial dynamics in skeletal muscle. The study supports that the skeletal muscle defects caused by ALS mutation may actively contribute to ALS pathogenesis.
Yi J, Ma C, Li Y, Weisleder N, Rios E, Ma J, et al. Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (E-C) coupling. J Biol Chem. 2011;286:32436–43. First study demonstrates that defective mitochondrial control in Ca signaling plays a key role in skeletal muscle degeneration during ALS progression.
Zhou J, Yi J, Fu R, Liu E, Siddique T, Rios E, et al. Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis. J Biol Chem. 2010;285:705–12.
Neel BA, Lin Y, Pessin JE. Skeletal muscle autophagy: a new metabolic regulator. Trends Endocrinol Metab. 2013;24:635–43.
Gonzalez de Aguilar JL, Niederhauser-Wiederkehr C, Halter B, De Tapia M, Di Scala F, Demougin P, et al. Gene profiling of skeletal muscle in an amyotrophic lateral sclerosis mouse model. Physiol Genomics. 2008;32:207–18.
Xiao Y, Ma C, Yi J, Wu S, Luo G, Xu X, et al. Suppressed autophagy flux in skeletal muscle of an amyotrophic lateral sclerosis mouse model during disease progression. Physiol Rep. 2015;3.
Nguyen QT, Son YJ, Sanes JR, Lichtman JW. Nerve terminals form but fail to mature when postsynaptic differentiation is blocked: in vivo analysis using mammalian nerve-muscle chimeras. J Neurosci. 2000;20:6077–86.
Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, et al. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–9.
Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. 2010;19:2284–302.
Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–36.
Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;45:1353–8.
Bonewald LF, Kiel DP, Clemens TL, Esser K, Orwoll ES, O’Keefe RJ, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2013;28:1857–65.
Edwards MH, Gregson CL, Patel HP, Jameson KA, Harvey NC, Sayer AA, et al. Muscle size, strength, and physical performance and their associations with bone structure in the Hertfordshire Cohort Study. J Bone Miner Res. 2013;28:2295–304.
Hamrick M. JMNI special issue: basic science and mechanisms of muscle-bone interactions. J Musculoskelet Neuronal Interact. 2010;10:1–2.
Kaji H. Interaction between muscle and bone. J Bone Metab. 2014;21:29–40.
Lang TF. The bone-muscle relationship in men and women. J Osteoporos. 2011;2011:702735.
Lebrasseur NK, Achenbach SJ, Melton LJ, Lebrasseur NK, Achenbach SJ, Melton 3rd LJ, et al. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res. 2012;27:2159–69. A recent study on potential muscle-bone interaction.
Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, et al. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2014;97:119–29.
Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10:64–70.
Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009;17:861–73.
Kahn J, Shwartz Y, Blitz E, Krief S, Sharir A, Breitel DA, et al. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell. 2009;16:734–43.
Sharir A, Stern T, Rot C, Shahar R, Zelzer E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development. 2011;138:3247–59. A recent study provides the evidence on the role of muscle contraction in regulating bone morphology.
Shwartz Y, Farkas Z, Stern T, Aszodi A, Zelzer E. Muscle contraction controls skeletal morphogenesis through regulation of chondrocyte convergent extension. Dev Biol. 2012;370:154–63.
Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–100.
Li H, Johnson NR, Usas A, Lu A, Poddar M, Wang Y, et al. Sustained release of bone morphogenetic protein 2 via coacervate improves the osteogenic potential of muscle-derived stem cells. Stem Cells Transl Med. 2013;2:667–77. A recent study on possible muscle-bone interaction in osteogenesis.
Oishi T, Uezumi A, Kanaji A, Yamamoto N, Yamaguchi A, Yamada H, et al. Osteogenic differentiation capacity of human skeletal muscle-derived progenitor cells. PLoS One. 2013;8:e56641. A recent study on the possible role of muscle-derived progenitor cells in osteogenesis.
Sun JS, Wu SY, Lin FH. The role of muscle-derived stem cells in bone tissue engineering. Biomaterials. 2005;26:3953–60.
Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravit Space Biol Bull. 2003;16:45–54.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75.
Khatri IA, Chaudhry US, Seikaly MG, Browne RH, Iannaccone ST. Low bone mineral density in spinal muscular atrophy. J Clin Neuromuscul Dis. 2008;10:11–7.
Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop. 2000;20:71–4.
Rufo A, Del Fattore A, Capulli M, Carvello F, De Pasquale L, Ferrari S, et al. Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J Bone Miner Res. 2011;26:1891–903. A recent study on the potential muscle-bone interaction in muscular dystrophic diseases.
Vestergaard P, Glerup H, Steffensen BF, Rejnmark L, Rahbek J, Moseklide L. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J Rehabil Med. 2001;33:150–5.
Sato Y, Honda Y, Asoh T, Kikuyama M, Oizumi K. Hypovitaminosis D and decreased bone mineral density in amyotrophic lateral sclerosis. Eur Neurol. 1997;37:225–9.
Sato Y, Honda Y, Iwamoto J. Etidronate for fracture prevention in amyotrophic lateral sclerosis: a randomized controlled trial. Bone. 2006;39:1080–6.
Majka SM, Jackson KA, Kienstra KA, Majesky MW, Goodell MA, Hirschi KK. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–9.
Sassoli C, Pini A, Chellini F, Mazzanti B, Nistri S, Nosi D, et al. Bone marrow mesenchymal stromal cells stimulate skeletal myoblast proliferation through the paracrine release of VEGF. PLoS One. 2012;7:e37512. A recent study on the potential role of bone in myogenesis.
Koh SH, Baik W, Noh MY, Cho GW, Kim HY, Kim KS, et al. The functional deficiency of bone marrow mesenchymal stromal cells in ALS patients is proportional to disease progression rate. Exp Neurol. 2012;233:472–80. A recent study on the deficiency of bone marrow mesenchymal stromal cells in ALS patients.
Cho GW, Noh MY, Kim HY, Koh SH, Kim KS, Kim SH. Bone marrow-derived stromal cells from amyotrophic lateral sclerosis patients have diminished stem cell capacity. Stem Cells Dev. 2010;19:1035–42.
Blanquer M, Moraleda JM, Iniesta F, Gomez-Espuch J, Meca-Lallana J, Villaverde R, et al. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: a pilot safety study. Stem Cells. 2012;30:1277–85.
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–61.
Di Monaco M, Vallero F, Di Monaco R, Tappero R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr. 2011;52:71–4.
Galli C, Passeri G, Macaluso GM. Osteocytes and WNT: the mechanical control of bone formation. J Dent Res. 2010;89:331–43.
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–17.
Zhao L, Shim JW, Dodge TR, Robling AG, Yokota H. Inactivation of Lrp5 in osteocytes reduces young’s modulus and responsiveness to the mechanical loading. Bone. 2013;54:35–43.
Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31:1075–81.
Javaheri B, Stern AR, Lara N, Dallas M, Zhao H, Liu Y, et al. Deletion of a single beta-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2014;29:705–15. A recent molecular mechanism study on muscle-bone interaction.
Zhu K, Yi J, Xiao Y, Lai Y, Song P, Zheng W, et al. Impaired bone homeostasis in amyotrophic lateral sclerosis mice with muscle atrophy. J Biol Chem. 2015. The first study to explore the muscle-bone crosstalk in ALS disease progression.
Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–15.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205.
Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50.
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–7.
ten Dijke P, Krause C, de Gorter DJ, Lowik CW, van Bezooijen RL. Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J Bone Joint Surg Am. 2008;90 Suppl 1:31–5.
Kawamura N, Kugimiya F, Oshima Y, Ohba S, Ikeda T, Saito T, et al. Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS One. 2007;2:e1058.
Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 2007;176:709–18.
Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–9.
Kim HJ, Kim JH, Bae SC, Choi JY, Kim HJ, Ryoo HM. The protein kinase C pathway plays a central role in the fibroblast growth factor-stimulated expression and transactivation activity of Runx2. J Biol Chem. 2003;278:319–26.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–4.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41.
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6:e25900.
Brotto M, Johnson ML. Endocrine crosstalk between muscle and bone. Curr Osteoporos Rep. 2014;12:135–41.
Compliance with Ethics Guidelines
Conflict of Interest
The authors of this paper declare they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the Topical Collection on Muscle and Bone
Rights and permissions
About this article
Cite this article
Zhou, J., Yi, J. & Bonewald, L. Muscle-Bone Crosstalk in Amyotrophic Lateral Sclerosis. Curr Osteoporos Rep 13, 274–279 (2015). https://doi.org/10.1007/s11914-015-0281-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-015-0281-0