Abstract
Purpose of Review
We highlight the clinical development of Poly (ADP-Ribose) polymerase (PARP) inhibitors in prostate cancer.
Recent Findings
Approximately 10 to 30% of metastatic prostate cancer patients carry germline or somatic mutations in DNA repair pathways. BRCA2 is the most commonly mutated gene in DNA damage repair pathways. Because of its critical function in homologous recombination repair (HRR) machinery, deleterious BRCA2 mutation enables synthetic lethality to a PARP inhibitor. Olaparib demonstrated clinical benefit in patients with deleterious mutations in HRR-related genes and most clearly in patients with BRCA2 mutations. Olaparib received the US FDA approval or mCRPC patients with a qualifying HRR gene mutation in May 2020. Rucaparib received an accelerated FDA approval for patients with BRCA1- or BRCA2-mutated mCRPC based on 43% objective response rate in a phase II study. To expand the application of a PARP inhibitor, several trials have evaluated various combination strategies with an androgen receptor signaling inhibitor, immunotherapy, radium-223, and others. While no PARP inhibitor combination regimen has been approved, promising data from a PARP inhibitor and an ASI combination have been reported.
Summary
PARP inhibitor represents a standard treatment for patient with mCRPC with germline or somatic mutations in BRCA2 and other HRR pathway genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most common cancer of men in the world, slightly behind lung cancer, with an annual estimated incidence of 1,414,259 in 2020, and 375,304 deaths according to GLOBOCAN 2020 [1]. In the USA, prostate cancer is the most common non-skin cancer with 209,512 cases annually. Incidence per 100,000 is 127.9 while mortality per 100,000 is 19.8. While not every prostate cancer case ends up in prostate cancer-related death, the disease status known as metastatic castration-resistant prostate cancer (mCRPC), the one that progresses with metastatic disease despite androgen deprivation therapy, kills approximately 370,000 men worldwide every year. The median overall survival for mCRPC ranges 33.7 to 36.2 months per a contemporary clinical trial [2]. Treatment options for mCRPC include androgen signaling inhibitors (ASI) (e.g., abiraterone and enzalutamide), cytotoxic chemotherapies (docetaxel and cabazitaxel), bone metastasis-targeted radiopharmaceutical (radium-223), and a cell-based immunotherapy (sipuleucel-T). While their respective pivotal trials showed OS benefit of 3–4 months, the application of these data has now become outdated because most of these agents are now used in the metastatic castration sensitive prostate cancer (mCSPC). With this background, the approval of two PARP inhibitors, olaparib and rucaparib, for mCRPC with a deleterious HRR gene alteration heralds the beginning of precision oncology in mCRPC. In this review, we will discuss the key milestones in the clinical development of PARP inhibitors and their future direction in prostate cancer.
Genomic Landscape of Metastatic Prostate Cancer
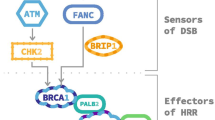
Several researchers spearheaded the efforts to sketch out the genomic landscape of metastatic prostate cancer by performing germline sequencing of metastatic prostate cancer patients [3•] and next-generation-sequencing of prostate tumor biopsies [4, 5]. Pritchard et al. showed that 11.8% of metastatic prostate cancer patients carry germline DNA-repair gene mutations [3•]. The most common germline mutations were BRCA2 (5.3%), ATM (1.6%), CHEK2 (1.9%), and BRCA1 (0.9%). Robinson et al. reported the integrative data from whole-exome, matched germline, and transcriptome sequencing from 150 patients with mCRPC and their tumor biopsy samples [4]. The integrative analysis showed 12.7% of the cases with loss of BRCA2, most of which were bi-allelic loss as a result of somatic mutation plus loss of heterozygosity or homozygous deletion. An expanded analysis identified at least 22.7% of cases with other DNA repair gene alteration, including bi-allelic loss of ATM, BRCA1, CDK12, FANCA, RAD51B, and RAD51C. The most commonly mutated genes in DNA repair pathway were BRCA2 (13.3%), ATM (7.3%), and CDK12 (4.7%). Similarly, Abida et al. identified 27% of patients harboring a germline or a somatic alteration in a DNA damage repair (DDR) gene, which may predict a response to a PARP inhibitor.
Role of PARP and Early Clinical Development of PARP Inhibitors
Poly (ADP-ribose) polymerases are a superfamily of proteins capable of ribosylation of protein targets, including themselves. PARPs are present throughout the cell and perform multiple cellular functions. Once bound to the target in the nucleus, poly-ADP-ribose (PAR) serves as a molecular signal to recruit DNA damage repair factors [6,7,8]. PARylation of the target factors is one of early key steps in base excision repair (BER) and single strand break repair (SSBR) pathways. Because of the critical role of PARP in DNA damage repair pathways, PARP inhibitors were first developed as sensitizers of DNA damaging therapies such as alkylating chemotherapy [9,10,11,12] and ionizing radiation therapy [13] to augment their anti-tumor activity. A phase I study evaluated AG014699, a first-generation PARP inhibitor, in combination with temozolomide, an alkylator, to establish a “PARP inhibitory dose” as a pharmacodynamic measure of DNA single-strand breaks [11]. Farmer, Bryant, and their colleagues first reported the therapeutic potential of a PARP inhibitor in BRCA-mutated tumor cells [14•, 15]. Both groups demonstrated that the BRCA-deficient cells are defective in homologous recombination repair and are highly sensitive to PARP inhibitors. Fong et al. first reported the clinical activity of a PARP inhibitor, olaparib, in cancer patients with germline BRCA mutation [16•]. This opened the floodgate of PARP inhibitor trials, which eventually led to their approval in ovarian, breast, pancreas, and prostate cancers.
Mechanism of Cytotoxicity of PARP Inhibitor
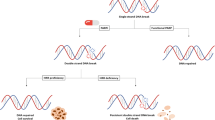
Cytotoxicity of PARP inhibitors has been described in two mechanisms — (1) catalytic inhibition of PARP, and (2) trapping of PARP-DNA complexes [17]. As shown in Fig. 1, the first mechanism (illustrated with a line 1 in Fig. 1A and B) refers to the catalytic inhibition of PARP’s PARylation, a critical step of BER and single strand break repair. Without BER and SSBR, SSBs go unrepaired, which eventually stalls and damages the replication fork (RF), requiring HR repair. In tumors with defective HRR, PARP inhibition disrupting SSBR and BER leads to effective apoptosis of cancer cells. This concept of disrupting two critical biological processes to induce selective cytotoxicity is known as synthetic lethality.
Mechanisms of cytotoxicity of a PARP inhibitor. A Arrow 1 represents catalytic inhibition of PARPi on the catalytic domain to inhibit auto-modifying PARylation, which ultimately leads to lack of inhibition at the DNA BD with DNA. Arrow 2 represents binding of a PARPi to PARP enzyme causing allosteric changes on the DNA binding domain promoting the binding of PARP onto the DNA and preventing it. B Arrow 1 represents catalytic inhibition of PARP leading to interrupted BER and allowing SSDB un-repaired and prompting HRR machinery to repair. Arrow 2 represents binding of a PARPi at DNA damage site and its persistent trapping leading to replication fork failure
The second mechanism of cytotoxicity is mediated via allosteric effect from the binding of a PARP inhibitor to the catalytic domain on PARP enzyme. Murai et al.showed that, when a PARP inhibitor binds the catalytic domain on the enzyme, it not only inhibits PARylation, but also causes allosteric changes on the DNA binding domain of the enzyme and enhances its binding affinity to the DNA, preventing it from dissociation when it should, which ultimately leads to RF damage and double-stranded DNA breaks [17]. This latter allosteric effect depends on the rigidity and chemical structure of a PARP inhibitor. The bulkier the structure is, the greater is the allosteric effect with greater PARP-DNA trapping mediated cytotoxicity. This type of DNA damage requires utilization of additional repair pathways including Fanconi anemia pathway, template switching, ATM, replicative flap endonuclease, and polymerase-beta [17, 18]. Of the PARP inhibitors tested, BMN673 (aka, talazoparib) and niraparib were shown to have stronger potencies at PARP trapping than rucaparib and olaparib. Veliparib seems to be weaker than others [17, 18].
This non-catalytic inhibition of PARP activity and DNA repair was shown to be cytotoxic for healthy erythroid progenitor cells at concentrations inhibiting PAR synthesis [19]. This preclinical observation aligns with clinical experience of the differential effects of myelosuppression. Table 1 summarizes the hematologic toxicities of the clinically approved PARP inhibitors from their respective trials and FDA package inserts. Grade ≥ 3 anemia occurred > 20% across all PARP inhibitors. Grade ≥ 3 anemia occurred > 30% with niraparib and talazoparib, the agents that are shown to have greater PARP trapping potential. It is not known if greater PARP trapping capacity translates into improved clinical efficacy.
Clinical Activity of PARP Inhibitor as a Monotherapy in mCRPC
Olaparib
TOPARP was the first phase II study of olaparib in mCRPC that provided the promise of olaparib in mCRPC and paved the path toward its biomarker-development [24•]. This was a single-arm, two-part, phase II trial with an adaptive design. During the first part of the study (TOPARP-A), as without knowing a validated predictive biomarker at that time, the study accrued patients without a selective molecular biomarker. The study required archival tissue or fresh tumor biopsies for biomarker analyses with the sequencing of the DNA repair genes. The primary endpoint was the response rate, where a response was defined as an objective response by RECIST v1.1 criteria, a decline in the PSA level of 50% or more (PSA50) from the baseline, or a conversion of circulating tumor cells count (CTC) from 5 or more at baseline to less than 5 in 7.5 ml of blood (CTC < 5) during treatment with a confirmation assessment at least 4 weeks later. In overall population, the response rate was 33% (16 of 49 patients). Among patients with deleterious mutations in DNA repair genes, the response rate was 88% (14 of 16) whereas the response rate was only 6% (2 of 33) among those without the mutations. Radiographic progression-free survival (rPFS) and overall survival (OS) were significantly longer in DNA repair gene mutation positive group than in the negative group (median PFS: 9.8 vs 2.7 months; median OS: 13.8 vs 7.5 months). The results of this study provided an impetus for the pivotal phase III trial of olaparib in mCRPC in biomarker selected mCRPC. Table 2 summarizes the results.
PROFOUND was a randomized, open-label, phase III trial of olaparib versus a second-line ASI (e.g., abiraterone or enzalutamide) in men with mCRPC who received a first-line ASI and have a qualifying genetic alteration in prespecified 15 HRR-related genes [20•]. Assuming differential strengths as a predictive biomarker of each of these gene alterations, the investigator designed the study in two prospective cohorts based on the gene alteration. Cohort A (n = 245) consisted of patients with at least one alteration in BRCA1, BRCA2, or ATM. Cohort B (n = 142) consisted of patient with alterations in any of 12 other specified genes including (CDK12, CHEK2, PPP2R2A, RAD51B, RAD54L, and others). Patients were randomized in a 2:1 ratio to olaparib or the physician’s choice of enzalutamide or abiraterone (control). The primary end point was imaging-based progression-free survival (iPFS) in cohort A by blind independent central review (BICR). The study showed an improvement of median iPFS by 7.4 months vs. 3.6 months (hazard ratio (HR) of 0.34, 95% CI, 0.25 to 0.47, p < 0.001). The overall survival analysis in cohort A showed 4.4-month difference in median OS between olaparib arm (19.1 months) and control arm (14.7 month) with HR 0.69, 95% CI 0.5 to 0.97; p = 0.02 [27]. The median OS in overall population (cohorts A and B) was 17.3 months and 14.0 months in olaparib and control arms, respectively. This was not statistically significant. Another key secondary endpoint was the iPFS in overall population, combined cohorts A and B. With the iPFS data combined from cohorts A and B, the study continued to show a statistically significant improvement in iPFS. Median iPFS were 5.8 months versus 3.5 months (HR 0.49 (95% CI < 0.38–0.63), p < 0.001) [20•]. These data led to the US FDA approval of olaparib in patients with an alteration in the 15-gene panel used in the trial except the PPP2R2A. PPP2R2A, in fact, showed worse overall survival on its exploratory analysis (HR: 5.11 (1.10–35.73). Consistent with prior report, the most common grade ≥ 3 adverse event (AE) was anemia (21%) and fatigue (3%).
The US FDA’s labeled indication of olaparib in prostate cancer reads, “deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated” mCRPC. This rather seemingly general labeling without specifying gene alterations generated some discussion on the strength of each of the “qualifying” mutations used in the trial as a predictive biomarker. For instance, the number of patients with the following gene alterations in PROFOUND study was quite small. The number of participants with a mutation in the following genes were only 4 or less: BARD1, BRIP1, CHEK1, and RAD51D. Another point was that even for those with sizable number of mutation cases, the hazard ratio and their 95% confidence interval (CI) seemed underwhelming. For instance, HR with 95% CI for ATM, CDK12, and CHEK2 were as follows: 1.04 (0.61 to 1.87), 0.74 (0.44 to 1.31), and 0.87 (0.23 to 4.13), respectively. Based on these data along with exploratory analyses, the European Medicines Agency conferred the approval of olaparib limited to those with BRCA1 and BRCA2 mutations, who have progressed on a novel hormonal agent for mCRPC [28].
Taken together, while the study was positive in the combined cohort, the benefit of olaparib over a second-line ASI in non-BRCA-mutated mCRPC is debatable and is unknown over a taxane-based chemotherapy. In clinical practice, at least in the USA, for patients with non-BRCA-mutated mCRPC who progressed on a first-line ASI, the benefit of olaparib should be discussed in the context of alternative treatment options, such as a second-line ASI and taxane-based chemotherapy.
Rucaparib
TRITON2 was a single-arm, open-label phase II study of rucaparib in patients with mCRPC with a DDR gene alteration who have received one or two ASI and one prior taxane-based chemotherapy. The results were reported in two publications. The first was on the group of patients with BRCA1 or BRCA2 mutations [21] and the other was on the group with a non-BRCA, DDR gene alteration [29]. The study used the panel of 15 DDR genes: BRCA1, BRCA2, ATM, BARD1, BRIP1, CKD12, CHEK2, FANCA, NBN, PALB2, RAD51, RAD51B, RAD51C, RAD51D, and RAD54L. The study accrued 115 patients with BRCA alteration including 13 BRCA1 and 102 BRCA2, and 44 germline and 71 somatic mutations. Among the patients with measurable disease, rucaparib resulted in 43.5% ORR by BICR and 54.8% confirmed PSA response rate [21]. The response rates were similar for patients with germline or somatic BRCA mutation. The median radiographic PFS was 9.0 months (95% CI: 8.3–13.5).
The study also accrued 78 patients with a non-BRCA DDR gene alteration. The most common was ATM (n = 49), CDK12 (n = 15), CHEK2 (n = 12), and other DDR genes (n = 14). Clinical activity was observed only in a limited number of patients with an alteration in ATM (2/19, 11%) and CHEK2 (1/9, 11% ORR). None of 10 patients with CDK12 mutation responded. No radiographic or PSA response was seen in 11 patients with confirmed biallelic ATM loss, which undermined the role of ATM mutation as a predictive biomarker. A small number of patients with a deleterious alteration in PALB2, FANCA, BRIP1, or RAD51B achieved a response to rucaparib.
This set of data led to the US FDA’s approval of rucaparib on an accelerated pathway for mCRPC patients with somatic or germline BRCA1 or BRCA2 mutation who had one or two prior NHAs and one prior taxane-based chemotherapy [30].
TRITON3 is an ongoing, randomized phase III trial of rucaparib 600 mg BID versus physician choice of abiraterone, enzalutamide, or docetaxel in patients with mCRPC and a deleterious germline or somatic BRCA1, BRCA2, or ATM mutation (NCT02975934). The primary endpoint is rPFS assessed by BICR. Table 3 provides the selected list of PARP inhibitor trials in prostate cancer.
Talazoparib
Talazoparib is an approved therapy for germline BRCA mutation carrier with pre-treated HER2 negative locally advanced or metastatic breast cancer but remains to an investigational therapy in prostate cancer. Compared with other clinically available PARP inhibitors, talazoparib has the strongest PARP-trapping potential [18].
TALAPRO-1 was a single-arm, open-label phase II trial of talazoparib in men with mCRPC, who received one or two taxane-based regimens, and progressed on one or two ASI [22]. The study used an 11 DDR gene panel: ATM, ATR, BRCA1, BRCA2, CHEK2, FANCA, MLH1, MRE11A, NBN, PALB2, and RAD51. The primary endpoint was confirmed objective response rate by BICR. Of 1225 patient screened, 161 (11%) passed the prescreening with a qualifying mutation. Of those, 127 were enrolled and received talazoparib, including 52 patients with BRCA2 mutation alone, 15 with ATM mutation, 4 with BRCA1, 4 with PALB2 mutations, and 7 with co-occurring HRR mutations, and the rest had one of the other mutations. Among patients with measurable disease and a qualifying g mutation (n = 104), the ORR was 29.8% (31/104). The ORR were 46% (28/61), 25% (1/4), and 12% (1/17) in BRCA1 or BRCA2, PALB2, and ATM cohorts, respectively. No objective response was observed in rest of the patients. No difference in ORR was observed between germline and somatic HRR gene mutation groups. The median rPFS was 5.6 months in overall population (n = 104), 11.2 months in BRCA1 or BRCA2 group (n = 61), and 3.5 months in ATM group (n = 11). Median OS was 16.4 months, 24 months, 16 months, and 12.2 months, in overall population, BRCA1 or BRCA2 mutation group, PALB2 mutation group, and ATM mutation group, respectively. The most common grade 3–4 treatment-emergent adverse events were anemia (31%), thrombocytopenia (9%), and neutropenia (8%). No myelodysplastic syndrome or acute myeloid leukemia was observed while on study or by the end of the follow-up.
Based on the findings from TALAPRO-1, talazoparib is now under investigation in two phase III trials — TALAPRO-2 and TALAPRO-3. TALAPRO-2 is a randomized phase III trial of enzalutamide 160 mg plus talazoparib 0.5 mg daily versus enzalutamide plus placebo as a first-line treatment of mCRPC (NCT03395197). The DDR gene mutation status is used as a stratification factor, not as a selection criterion. The study has two co-primary endpoints: rPFS in unselected patients and rPFS in patients harboring DDR mutation. TALAPRO-3 is a randomized phase III trial of talazoparib plus enzalutamide versus placebo plus enzalutamide in men with DDR gene-mutated mCSPC (NCT04821622). The primary endpoint is the rPFS in the overall population. The qualifying mutation will be identified by either a liquid or soft tissue tumor biopsy using FoundationOne Liquid CDx or FoundationOne CDx, respectively.
Niraparib
Niraparib (MK4827) is an oral PARP-1 and PARP-2 inhibitor with a maximum inhibitory concentration of 2.8 nmol/L for PARP-1 and 2.1 nmol/L for PARP-2 [31]. Compared with veliparib and olaparib, niraparib has shown superior potency in trapping PARP [17]. Niraparib remains to be an investigational therapy in prostate cancer whereas it is approved as a maintenance therapy after the first-line or platinum-based chemotherapy in ovarian cancer without a biomarker requirement.
During an early stage of the phase I study of niraparib, it was enriched with BRCA1 or BRCA2 mutation carriers. Most of the mutation carriers were with ovarian or breast cancers. As expected, objective responses were seen in 40 to 50% of these patients. The study also included 23 sporadic prostate cancer patients. Nine (43%) were able to receive maximally tolerated dose (MTD) (300 mg/day), and median duration for stable disease was 254 days. None of these prostate cancer patients had a radiographic objective response. One patient had > 50% decrease in PSA, and three patients had significant decreases in circulating tumor cells (CTC). No correlation was observed between PTEN or ERG rearrangements and treatment benefits [32].
GALAHAD is an open-label phase II trial of niraparib in mCRPC patients with DNA-repair gene defect (DRD) that received at least one taxane-based treatment and at least on ASI. The biomarker, DRD, was defined as a pathogenic mutation in an 8-gene panel: BRCA1, BRCA2, ATM, FANCA, PALB2, CHEK2, BRIP1, or HDAC2 using plasma circulating tumor DNA (Resolution, Bioscience, Redmond, WA). Of all enrolled patients (n = 207), 29% patients were with mutations in BRCA1 or BRCA2, the primary analysis cohort. In patients with biallelic BRCA1 or BRCA2 mutations, the ORR was 41.4%, and the CTC < 5 rate was 49% (25 of 51). CTC0 rate, defined as rate of CTC conversion from ≥ 5 to 0 CTC, was 20% (10 of 51). They also showed a correlation of CTC0 or CTC < 5 with OS. Most common reported grade 3–4 adverse events in BRCA population (n = 60) were anemia (35%), neutropenia (10%), thrombocytopenia (8.3%), and hypertension (5.3%). Two fatalities were reported from adverse events: urosepsis and seizure, which were deemed related to study drug [23].
Clinical Investigation of PARP Inhibitor Combination in mCRPC
To expand the application of a PARP inhibitor, various combination strategies have been examined including AR signaling inhibitors, immunotherapy, and other investigational therapies.
Rationale for Combining PARP Inhibitor plus AR Signaling Inhibitor
Schiewer and colleagues at Knudsen’s lab first reported the interaction between PARP-1 and androgen receptor [29]. They showed that tumorigenic effects of PARP-1 in AR-positive prostate cancer cell lines. PARP-1 is recruited to the sites AR function and promotes the AR occupancy and AR function leading resulting in tumorigenic effects in the absence of the DNA damage. Brenner and colleagues also showed that ETS-positive prostate cancer xenografts were preferentially sensitive to PARP inhibition and TMPRSS2:ERG fusion induces DNA damage which is potentiated by PARP inhibition [33]. These set of data generated a hypothesis that there may be added benefit of PARP inhibitor to the ASI in AR-driven prostate cancer even in the absence of DDR mutation.
ABT888 (Veliparib) plus Abiraterone
Veliparib and abiraterone combination was tested in a randomized, biomarker-stratified, phase II trial compared against abiraterone and prednisone in mCRPC. The patients were stratified by ETS fusion status. The study did not confirm the preclinical hypothesis. The study did not show significant difference in confirmed PSA response rate between the arms. ETS fusion status did not predict PSA response either. An exploratory analysis, however, showed the patients with DRD, defined as presence of a deleterious mutation in BRCA1, BRCA2, ATM, FANCA, PALB2, RAD51B, or RAD51C, had superior clinical outcome in terms of PSA response rate, radiographic response rate, and PFS compared with those without DRD [26].
Olaparib plus Abiraterone
Olaparib and abiraterone was evaluated in a placebo-controlled randomized phase II trial against abiraterone in post-docetaxel setting [34]. The study showed olaparib/abiraterone (O/A) showed a superior median rPFS compared with placebo/abiraterone (P/A) in overall population (13.8 versus 8.2 months, HR = 0.65 95% CI 0.44–0.97). The difference was even greater for DDR-deficient subgroups — 17.8 months versus 6.5 months (HR = 0.74, p = 0.58) in O/A versus P/A, respectively. In DDR-proficient subgroups, there was a trend toward a rPFS improvement favoring O/A arm with 5.3-month difference without statistical significance (HR = 0.52, p = 0.11). Based on the data, the PROPEL study, a phase III randomized controlled trial of abiraterone with olaparib or placebo was conducted (NCT03732820) as a first-line treatment for mCRPC. The recent press release indicated that the study met the primary endpoint of rPFS at the interim analysis [35]. The publication of the full data is eagerly awaited.
Niraparib plus Abiraterone
BEDIVERE was a phase I study to evaluate the recommended phase 2 dose of niraparib combined with abiraterone (1000 mg; prednisone 10 mg) (AAP) or apalutamide 240 mg. Because of dose-limiting toxicities observed at 300-mg dose level of niraparib, and lower niraparib exposure given with apalutamide, niraparib 200 mg was determined as a recommended phase 2 dose with AAP. The common AEs with this regimen were thrombocytopenia (26.3%) and hypertension (21.1%) [36]. MAGNITUDE is an ongoing phase III randomized trial of abiraterone with niraparib or with placebo as a first-line treatment for mCRPC (NCT03748641). The study will have two parallel cohorts: with DRD (n = 400) and without DRD (n = 600). DRD status will be determined by plasma and tissue assays with a marker panel consisting of BRCA1, BRCA2, FANCA, PALB2, CHEK2, BRIP1, HDAC2, and ATM.
Rucaparib plus Enzalutamide
RAMP was a phase Ib trial that assessed PK, safety, and preliminary efficacy of rucaparib with enzalutamide in mCRPC patients. The overall safety profile of rucaparib 600 mg BID combined with enzalutamide 160 mg once daily was consistent with observed in monotherapy. Preliminary efficacy was observed in 4 of 8 patients with confirmed PSA response (≥ 50% reduction) and 1 of 1 measurable disease with radiographic complete response. This combination is investigated in a double-blinded placebo-controlled, phase III trial, CASPAR trial, in the frontline setting for mCRPC (NCT04455750) [37].
PARP Inhibitor plus Immunotherapy
PARP inhibitors have been combined with several immune checkpoint inhibitors (ICI) in clinical trials in mCRPC and other solid tumors. The activity of an ICI monotherapy in prostate cancer is limited to patients with mismatch repair deficiency (MMRd), and the prevalence of which is ~ 5% [3•]. Otherwise, the activity of an ICI is limited to a small subset of biomarker-unselected patients with mCRPC [38]. One of the preclinical data supporting the evaluation of PARP inhibitor combined with an immunotherapy is the work by Ding L et al. [39]. She and her colleagues showed STING pathway mediated intratumoral immune activation generated by the PARP inhibitors can be extended via PD-1 blockade.
Olaparib was tested in combination with durvalumab [40] and pembrolizumab [41].
Olaparib plus Durvalumab
Durvalumab and olaparib combination was evaluated in patients with advanced solid tumors including 17 patients with mCRPC in a phase I study [40]. Nine (53%) of 17 patients had a PSA50 response or radiographic response. Of these 17 patients, 11 had a BRCA2 mutation: 4 with indel-frameshift, 1 “pathogenic mutation,” 1 “deep deletion,” and 4 “shallow deletion.” Four of 4 (100%) indel-frameshift BRCA2 mutation, 1 of 1 “deep deletion,” and 1 of 1 “pathogenic mutation” responded, and 1 of 4 (25%) “shallow deletion” of BRCA2 cases responded. One responder had a NBN mutation, and the other responder did not a detectable DDR alteration [40].
Olaparib plus Pembrolizumab
Pembrolizumab and olaparib combination was tested in cohort A of KEYNOTE-365 trial, a phase Ib trial of pembrolizumab with different combination in mCRPC [41]. ORR and PSA response rate was 8% and 9%, in molecularly unselected, docetaxel-pretreated mCRPC. The full data are yet to be published. This regimen is being evaluated in a phase III trial in unselected mCRPC patients progressed after one ASI and chemotherapy, compared against a ASI with OS as the primary endpoint [42].
Rucaparib plus Nivolumab
Rucaparib and nivolumab were tested as a cohort of CheckMate9KD trial, a phase II trial investigating various combinations of nivolumab for mCRPC. Results of cohort A2 of CheckMate 9KD, presented at 2021 ESMO, showed rucaparib and nivolumab resulted in 25% (5/20) ORR and 41.9% (13/31) PSA50 RR in homologous repair deficient (HRD) biomarker positive mCRPC and 5.3% ORR and 14.3% PSA50RR in HRD biomarker negative [43].
Taken together, while the clinical activities of ICI and PARP inhibitor combination have been reported in patients with HRD biomarker positive patients, only modest activities have been reported in unselected, or HRD biomarker-negative patients. It is unknown how much benefit an ICI adds to a PARP inhibitor in HRD biomarker-positive mCRPCP. Further studies are warranted to investigate the efficacy of the ICI/PARP inhibitor combination over PARP inhibitor in HRD biomarker selected patients.
PARP Inhibitor plus Radium-223 (Radioisotope Therapy)
Because of its inherent genotoxic property inducing DNA damage, radium-223 has been investigated in combination with PARP inhibitors. Both olaparib [44] and niraparib [45] are investigated in a phase I/II trial combined with radium223. Kelly et al. reported that the MTD of niraparib combined with 55kbBq of Radium223 was 100 mg daily for chemotherapy-exposed patients and 200 mg daily for chemotherapy-naïve patients. Three (10%) of 30 patient ≥ 50% PSA decline at 12 weeks. Most common treatment-related grade ≥ 3 adverse events were lymphopenia (13%), neutropenia (10%), anemia (10%), and hypertension (10%) and thrombocytopenia (7%) [45]. Olaparib is investigated in combination with Radium223 in COMRADE trial (NCT03076203).
PARP Inhibitor plus VEGFR Inhibitor
NCI 9984 was a randomized phase II study of olaparib with or with cediranib in patients with mCRPC. The study showed olaparib combined with cediranib led to superior rPFS over olaparib alone [46]. The biomarker analysis showed that the margin of the benefit was driven primarily by those with HRD mCRPC, not by those with HRP mCRPC [47], warranting further investigation of this combination in a biomarker selected group.
Conclusion
PARP inhibitors have emerged as a new standard treatment for mCRPC harboring deleterious mutations in HRR pathway genes. While the activity of a PARP inhibitor in BRCA2 and BRCA1 mutation is most clear, the evidence of its activity in other HRR genes has been debated. This is expected given the different roles of these HRR factors in the HRR repair pathway. While the loss of function mutation in BRCA2 seems to the most bona fide event predicting PARP inhibitor sensitivity, loss of other HRR factors, such as ATM and CDK12, does not appear to confer such great sensitivity to PARP inhibitors. The clinical use of PARP inhibitors in non-BRCA2-mutated mCRPC should be discussed in the context of alternative treatment options, such as taxane-based chemotherapy.
To date, no PARP inhibitor combination has been approved. Various combination strategies have been examined with an ASI, an ICI, and radium-223. Most of the phase III trial of PARP inhibitor plus ASI combination trials are using a HRD biomarker as a stratification factor not as an eligibility criterion to see if the benefit of the combination extends beyond the biomarker-positive group. Of those trials, PROPEL trial, a phase III study of olaparib and abiraterone combination, has met the primary endpoint. Publication of the full data is eagerly awaited. Trials of other novel DNA pathway targeting agents or other combination strategies are needed to overcome PARP inhibitor-resistant, HRD mCRPC.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB Jr, Oudard S, Steuber T, Suzuki H, Wu D, Yeruva K, De Porre P, Brookman-May S, Li S, Li J, Thomas S, Bevans KB, Mundle SD, McCarthy SA, Rathkopf DE. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22(11):1541–59. https://doi.org/10.1016/s1470-2045(21)00402-2.
• Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, Elemento O, Rubin MA, Robinson D, Lonigro R, Hussain M, Chinnaiyan A, Vinson J, Filipenko J, Garraway L, Taplin ME, AlDubayan S, Han GC, Beightol M, Morrissey C, Nghiem B, Cheng HH, Montgomery B, Walsh T, Casadei S, Berger M, Zhang L, Zehir A, Vijai J, Scher HI, Sawyers C, Schultz N, Kantoff PW, Solit D, Robson M, Van Allen EM, Offit K, de Bono J, Nelson PS. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–53. https://doi.org/10.1056/NEJMoa1603144.PubMedPMID:27433846;PMCID:4986616. This work was the report of germline sequencing of metastatic prostate cancer patients showing that 12% of metastatic prostate cancer patients carry germline DNA-repair gene mutations. The frequencies of these germline mutations among men with metastatic disease did not differ significantly according to age at diagnosis or family history of prostate cancer. This work provided a rationale for a NCCN guideline recommendation of routine germline sequencing in patients with metastatic prostate cancer.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28. https://doi.org/10.1016/j.cell.2015.05.001.PubMedPMID:26000489;PMCID:4484602.
Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, Danila D, Rathkopf D, Morris M, Slovin S, McLaughlin B, Curtis K, Hyman DM, Durack JC, Solomon SB, Arcila ME, Zehir A, Syed A, Gao J, Chakravarty D, Vargas HA, Robson ME, Joseph V, Offit K, Donoghue MTA, Abeshouse AA, Kundra R, Heins ZJ, Penson AV, Harris C, Taylor BS, Ladanyi M, Mandelker D, Zhang L, Reuter VE, Kantoff PW, Solit DB, Berger MF, Sawyers CL, Schultz N, Scher HI. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017. https://doi.org/10.1200/PO.17.00029.
Daniels CM, Ong SE, Leung AK. The promise of proteomics for the study of ADP-ribosylation. Mol Cell. 2015;58(6):911–24. https://doi.org/10.1016/j.molcel.2015.06.012.
Vyas S, Chesarone-Cataldo M, Todorova T, Huang YH, Chang P. A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nat Commun. 2013;4:2240. https://doi.org/10.1038/ncomms3240.
Sousa FG, Matuo R, Soares DG, Escargueil AE, Henriques JAP, Larsen AK, Saffi J. PARPs and the DNA damage response. Carcinogenesis. 2012;33(8):1433–40. https://doi.org/10.1093/carcin/bgs132.
Kawamitsu H, Miwa M, Tanaka Y, Sakamoto H, Terada M, Hoshi A, Sugimura T. Inhibitors of poly(adenosine diphosphate ribose) polymerase potentiate the antitumor activity of bleomycin against Ehrlich ascites carcinoma. J Pharmacobiodyn. 1982;5(11):900–4. https://doi.org/10.1248/bpb1978.5.900.
Park SD, Kim CG, Kim MG. Inhibitors of poly(ADP-ribose) polymerase enhance DNA strand breaks, excision repair, and sister chromatid exchanges induced by alkylating agents. Environ Mutagen. 1983;5(4):515–25. https://doi.org/10.1002/em.2860050402.
Plummer R, Jones C, Middleton M, Wilson R, Evans J, Olsen A, Curtin N, Boddy A, McHugh P, Newell D, Harris A, Johnson P, Steinfeldt H, Dewji R, Wang D, Robson L, Calvert H. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2008;14(23):7917–23. https://doi.org/10.1158/1078-0432.CCR-08-1223.
Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, Durkacz BW, Hostomsky Z, Kumpf RA, Kyle S, Li J, Maegley K, Newell DR, Notarianni E, Stratford IJ, Skalitzky D, Thomas HD, Wang LZ, Webber SE, Williams KJ, Curtin NJ. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96(1):56–67. https://doi.org/10.1093/jnci/djh005.
Ben-Hur E, Utsumi H, Elkind MM. Inhibitors of poly (ADP-ribose) synthesis enhance radiation response by differentially affecting repair of potentially lethal versus sublethal damage. Br J Cancer Suppl. 1984;6:39–42.
• Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. https://doi.org/10.1038/nature03445. This study, in concordance with the paper published by Bryant et al. in the same issue of Nature in 2005, demonstrated the synergistic effect PARP1 depletion and BRCA deficiency have on BRCA-1 and BRCA-2 KO stem cells, laying the groundwork for the concept known as synthetic lethality.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. https://doi.org/10.1038/nature03443.
• Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. https://doi.org/10.1056/NEJMoa0900212. This was the first report of a PARP inhibitor trial that provided clinical evidence of its synthetic lethality in germline BRCA mutation carriers. Radiologic or tumor marker response was observed in patients with breast, ovarian, and prostate cancers foreshadowing the efficacy in future studies.
Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588–99. https://doi.org/10.1158/0008-5472.CAN-12-2753.
Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, Morris J, Teicher B, Doroshow JH, Pommier Y. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13(2):433–43. https://doi.org/10.1158/1535-7163.MCT-13-0803.
Hopkins TA, Ainsworth WB, Ellis PA, Donawho CK, DiGiammarino EL, Panchal SC, Abraham VC, Algire MA, Shi Y, Olson AM, Johnson EF, Wilsbacher JL, Maag D. PARP1 trapping by PARP inhibitors drives cytotoxicity in both cancer cells and healthy bone marrow. Mol Cancer Res. 2019;17(2):409–19. https://doi.org/10.1158/1541-7786.MCR-18-0138.
• de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–102. https://doi.org/10.1056/NEJMoa1911440. This is the report of phase III trial of olaparib versus a second-line ASI demonstrating the improvement of rPFS in patients with BRCA1, BRCA2, or ATM mutations (cohort A), which was the primary analysis cohort. Analysis of combined cohorts A and B which included mutations in 11 other DNA repair genes continued to show a significant improvement in rPFS over a second-line ASI.
Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, Sautois B, Vogelzang NJ, Bambury RM, Voog E, Zhang J, Piulats JM, Ryan CJ, Merseburger AS, Daugaard G, Heidenreich A, Fizazi K, Higano CS, Krieger LE, Sternberg CN, Watkins SP, Despain D, Simmons AD, Loehr A, Dowson M, Golsorkhi T, Chowdhury S, TRITON2 investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38(32):3763–72. https://doi.org/10.1200/JCO.20.01035.
de Bono JS, Mehra N, Scagliotti GV, Castro E, Dorff T, Stirling A, Stenzl A, Fleming MT, Higano CS, Saad F, Buttigliero C, van Oort IM, Laird AD, Mata M, Chen HC, Healy CG, Czibere A, Fizazi K. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22(9):1250–64. https://doi.org/10.1016/S1470-2045(21)00376-4.
Smith MR, Fizazi K, Sandhu SK, Kelly WK, Efstathiou E, Lara P, Yu EY, George DJ, Chi KN, Saad F, Summa J, Freedman JM, Mason G, Espina BM, Zhu E, Ricci DS, Snyder LA, Simon JS, Cheng S, Scher HI. Niraparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD): correlative measures of tumor response in phase II GALAHAD study. J Clin Oncol. 2020;38(6_suppl):118. https://doi.org/10.1200/JCO.2020.38.6_suppl.118.
• Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–708. https://doi.org/10.1056/NEJMoa1506859. This work is the first report of a phase II PARP inhibitor trial in mCRPC showing 88% response rate among patients with DNA repair defect. This work laid the foundation for biomarker development of PARP inhibitors in mCRPC and paved the way for the pivotal phase III trial.
Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, Syndikus I, Ralph C, Jain S, Varughese M, Parikh O, Crabb S, Robinson A, McLaren D, Birtle A, Tanguay J, Miranda S, Figueiredo I, Seed G, Bertan C, Flohr P, Ebbs B, Rescigno P, Fowler G, Ferreira A, Riisnaes R, Pereira R, Curcean A, Chandler R, Clarke M, Gurel B, Crespo M, Nava Rodrigues D, Sandhu S, Espinasse A, Chatfield P, Tunariu N, Yuan W, Hall E, Carreira S, de Bono JS. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):162–74. https://doi.org/10.1016/S1470-2045(19)30684-9.
Hussain M, Daignault-Newton S, Twardowski PW, Albany C, Stein MN, Kunju LP, Siddiqui J, Wu YM, Robinson D, Lonigro RJ, Cao X, Tomlins SA, Mehra R, Cooney KA, Montgomery B, Antonarakis ES, Shevrin DH, Corn PG, Whang YE, Smith DC, Caram MV, Knudsen KE, Stadler WM, Feng FY, Chinnaiyan AM. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J Clin Oncol. 2018;36(10):991–9. https://doi.org/10.1200/jco.2017.75.7310.
Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Roubaud G, Ozguroglu M, Kang J, Burgents J, Gresty C, Corcoran C, Adelman CA, de Bono J, Investigators PRT. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345–57. https://doi.org/10.1056/NEJMoa2022485.
Agency EM. An overview of Lynparza and why it is authorised in the EU: European Medicines Agency; 2020 [updated 11/2020; cited 2021 9/12/2021]. Available from: https://www.ema.europa.eu/en/documents/overview/lynparza-epar-medicine-overview_en.pdf.
Abida W, Campbell D, Patnaik A, Shapiro JD, Sautois B, Vogelzang NJ, Voog EG, Bryce AH, McDermott R, Ricci F, Rowe J, Zhang J, Piulats JM, Fizazi K, Merseburger AS, Higano CS, Krieger LE, Ryan CJ, Feng FY, Simmons AD, Loehr A, Despain D, Dowson M, Green F, Watkins SP, Golsorkhi T, Chowdhury S. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase II TRITON2 study. Clin Cancer Res. 2020;26(11):2487–96. https://doi.org/10.1158/1078-0432.CCR-20-0394.
Administration FAD. FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer: Food and Drug Administration; 2020 [updated 05/15/2020; cited 2021 09/12/2021]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate.
Jones P, Altamura S, Boueres J, Ferrigno F, Fonsi M, Giomini C, Lamartina S, Monteagudo E, Ontoria JM, Orsale MV, Palumbi MC, Pesci S, Roscilli G, Scarpelli R, Schultz-Fademrecht C, Toniatti C, Rowley M. Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem. 2009;52(22):7170–85. https://doi.org/10.1021/jm901188v.
Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, Hylands L, Riisnaes R, Forster M, Omlin A, Kreischer N, Thway K, Gevensleben H, Sun L, Loughney J, Chatterjee M, Toniatti C, Carpenter CL, Iannone R, Kaye SB, de Bono JS, Wenham RM. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–92. https://doi.org/10.1016/s1470-2045(13)70240-7.
Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, Patel S, Wang X, Liang H, Yu J, Palanisamy N, Siddiqui J, Yan W, Cao X, Mehra R, Sabolch A, Basrur V, Lonigro RJ, Yang J, Tomlins SA, Maher CA, Elenitoba-Johnson KS, Hussain M, Navone NM, Pienta KJ, Varambally S, Feng FY, Chinnaiyan AM. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19(5):664–78. https://doi.org/10.1016/j.ccr.2011.04.010.
Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, Jassem J, Flechon A, Redfern C, Goessl C, Burgents J, Kozarski R, Hodgson D, Learoyd M, Saad F. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975–86. https://doi.org/10.1016/S1470-2045(18)30365-6.
Kemp A. Lynparza in combination with abiraterone significantly delayed disease progression in all-comers in PROpel phase III trial in 1st-line metastatic castration-resistant prostate cancer: AstraZeneca; 2021 [updated 9/24/202112/28/2021]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2021/lynparza-propel-trial-meets-primary-endpoint.html.
Saad F, Chi KN, Shore ND, Graff JN, Posadas EM, Lattouf JB, Espina BM, Zhu E, Yu A, Hazra A, De Meulder M, Mamidi R, Bradic B, Francis P, Hayreh V, Kalebasty Rezazadeh A. Niraparib with androgen receptor-axis-targeted therapy in patients with metastatic castration-resistant prostate cancer: safety and pharmacokinetic results from a phase 1b study (BEDIVERE). Cancer Chemother Pharmacol. 2021;88(1):25–37. https://doi.org/10.1007/s00280-021-04249-7.
Rao A, Morris D, Assikis VJ, Jha GG, Ryan CJ, Ablaza A-J, Habeck J, Loehr A, Xiao J, Gangolli EA. Rucaparib plus enzalutamide in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): pharmacokinetics (PK) and safety data from the phase Ib RAMP study. J Clin Oncol. 2021;39(6_suppl):79. https://doi.org/10.1200/JCO.2021.39.6_suppl.79.
Petrylak DP, Loriot Y, Shaffer DR, Braiteh F, Powderly J, Harshman LC, Conkling P, Delord J-P, Gordon M, Kim JW, Sarkar I, Yuen K, Kadel EE, Mariathasan S, O’Hear C, Narayanan S, Fassò M, Carroll S, Powles T. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: a phase I study. Clin Cancer Res. 2021;27(12):3360–9. https://doi.org/10.1158/1078-0432.Ccr-20-1981.
Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, Li BB, Xie S, Liu JF, Stover EH, Howitt BE, Bronson RT, Lazo S, Roberts TM, Freeman GJ, Konstantinopoulos PA, Matulonis UA, Zhao JJ. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. 2018;25(11):2972-80.e5. https://doi.org/10.1016/j.celrep.2018.11.054.
Karzai F, VanderWeele D, Madan RA, Owens H, Cordes LM, Hankin A, Couvillon A, Nichols E, Bilusic M, Beshiri ML, Kelly K, Krishnasamy V, Lee S, Lee MJ, Yuno A, Trepel JB, Merino MJ, Dittamore R, Marté J, Donahue RN, Schlom J, Killian KJ, Meltzer PS, Steinberg SM, Gulley JL, Lee JM, Dahut WL. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer. 2018;6(1):141. https://doi.org/10.1186/s40425-018-0463-2.
Yu EY, Piulats JM, Gravis G, Laguerre B, Arranz Arija JA, Oudard S, Fong PCC, Kolinsky MP, Augustin M, Feyerabend S, Kam AE, Gurney H, Tafreshi A, Retz M, Berry WR, Mar N, Wu H, Schloss C, Poehlein CH, De Bono JS. KEYNOTE-365 cohort A updated results: pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38(6_suppl):100. https://doi.org/10.1200/JCO.2020.38.6_suppl.100.
Yu EY, Park SH, Huang Y-H, Bennamoun M, Xu L, Kim J, Antonarakis ES. Phase III study of pembrolizumab (pembro) plus olaparib versus enzalutamide (enza) or abiraterone acetate (abi) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) who progressed on chemotherapy: KEYLYNK-010. J Clin Oncol. 2020;38(6_suppl):TPS256-TPS. https://doi.org/10.1200/JCO.2020.38.6_suppl.TPS256.
Petrylak DP, Perez-Gracia JL, Lacombe L, Bastos DA, Mahammedi H, Kwan EM, Zschäbitz S, Armstrong AJ, Pachynski RK, Goh JC, Burotto M, Gravis G, McCune SL, Vázquez Limón JC, Retz M, Saad F, Amin NP, Li J, Unsal-Kacmaz K, Fizazi K. 579MO CheckMate 9KD cohort A2 final analysis: nivolumab (NIVO) + rucaparib for chemotherapy (CT)-naïve metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2021;32:S629–30. https://doi.org/10.1016/j.annonc.2021.08.1092.
Shaya J, Xie W, Saraiya B, Parikh M, Folefac E, Olson AC, Choudhury AD, Einstein DJ, Heath EI, Parikh RA, Kunos C, Ivy SP, LoRusso P, Kurzrock R, Shapiro G, McKay RR. A phase I/II study of combination olaparib and radium-223 in men with metastatic castration-resistant prostate cancer with bone metastases (COMRADE): a trial in progress. J Clin Oncol. 2021;39(6_suppl):TPS182-TPS. https://doi.org/10.1200/JCO.2021.39.6_suppl.TPS182.
Kelly WK, Leiby B, Einstein DJ, Szmulewitz RZ, Sartor AO, Yang ES-H, Sonpavde G. Radium-223 (Rad) and niraparib (Nira) treatment (tx) in castrate-resistant prostate cancer (CRPC) patients (pts) with and without prior chemotherapy (chemo). J Clin Oncol. 2020;38(15_suppl):5540. https://doi.org/10.1200/JCO.2020.38.15_suppl.5540.
Kim JW, McKay RR, Taplin M-E, Davis NB, Monk P, Appleman LJ, Lara P, Vaishampayan UN, Zhang J, Paul AK, Bubley G, Allen EMV, Huang Y, Zhang Z, Loda M, Shapiro G, LoRusso P, Ivy SP, Petrylak DP. Randomized phase II study of olaparib with or without cediranib in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38(6_suppl):111. https://doi.org/10.1200/JCO.2020.38.6_suppl.111.
McKay RR, Radke MR, Shyr Y, Zhao S, Taplin M-E, Davis NB, Monk P, Appleman LJ, Lara PLN, Vaishampayan UN, Zhang J, Paul AK, Bubley G, Huang Y, Shapiro G, LoRusso P, Ivy SP, Petrylak DP, Swisher EM, Kim JW. Biomarker analysis from a randomized phase II study of olaparib with or without cediranib in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2021;39(6_suppl):7. https://doi.org/10.1200/JCO.2021.39.6_suppl.7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Serhan Unlu declares that he has no conflict of interest.
Joseph W. Kim (JWK) is the study chair of one of the two NCI sponsored trials of olaparib, one of which is discussed in this review (NCI 9984).
JWK also has received consulting fees from the following companies: Voluntis, Sanofi, EMD Serono and Clovis Oncology.
JWK also received research funding from the following companies: Immune Design, Hummingbird, ADCT Therapeutics, Exelexis, Regeneron, Genetech/Roche, Cosmo Technologies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Genitourinary Cancers
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Unlu, S., Kim, J.W. Emerging Role of PARP Inhibitors in Metastatic Prostate Cancer. Curr Oncol Rep 24, 1619–1631 (2022). https://doi.org/10.1007/s11912-022-01305-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01305-0