Abstract
Purpose of Review
For patients with malignant pleural mesothelioma, prognosis is poor with extremely low 5-year survival rates and limited therapeutic options. Here, we review the current treatment landscape for mesothelioma and highlight promising future therapeutic directions.
Recent Findings
Evolving frontline therapeutic options for mesothelioma include VEGF inhibition in combination with chemotherapy and dual immune checkpoint inhibition, with synergisms between the therapies and response prediction via biomarkers also being explored. Evolving experimental treatments for mesothelioma include PARP and ALK inhibitors, dendritic and CAR T-cell therapies, anti-mesothelin vaccines, and oncolytic viral therapies, representing timely advances in the field.
Summary
The therapeutic landscape for malignant pleural mesothelioma is evolving and preferred treatment in the frontline and later settings will likely evolve with it. However, this does not preclude the evidence for including multi-modal therapies spanning angiogenesis and immune checkpoint inhibitors, and biomarker utilization, in current clinical trials and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesothelioma is an aggressive malignancy arising from cells of the mesothelium, a serous membrane that forms the outer linings of the thoracic and abdominal cavities (hence, pleural and peritoneal mesothelioma), but also the heart and testes (hence, pericardial and testicular/tunica vaginalis mesothelioma). This article focuses on malignant pleural mesothelioma (MPM), which accounts for 80–90% of reported mesothelioma cases (followed by peritoneal mesothelioma, which accounts for the near remainder of cases and will be briefly mentioned in this article as well).
Malignant pleural mesothelioma outcomes have been dismal, with 5-year survival rates of 5–10% [1]. Prognosis can be further stratified among the histologic subtypes along the continuum of pleural mesothelioma. The most common subtype, epithelioid, represents 50–70% of cases, resembles benign, reactive mesothelial cells, and is associated with the most favorable prognosis [2]. In contrast, the sarcomatoid subtype represents 10–20% of cases, comprises of spindle cells, and is invasive, typically resistant to cytotoxic therapy, and thus associated with the worst prognosis (median survival 4 months in some studies) [1, 3]. The remaining subtype, biphasic, represents 30–40% of cases, has both epithelioid and sarcomatoid features, and accordingly carries a prognosis between the two.
Other established and emerging prognostic factors include the European Organization for Research and Treatment of Cancer (EORTC) composite score which takes into account histology with age, gender, leukocyte count, and probability of diagnosis [4], the endoplasmic reticulum stress marker CHOP [5], the stromal marker CD31 [6], the monocarboxylate transporter 4 (MCT4) [7], and the epithelial-mesenchymal transition related molecules periostin and phosphatase and tensin homolog (PTEN) [8]. More recent studies have shown that expression of B7 homolog 1 (B7-H1; aka programmed cell death 1 ligand 1) [9], presence of weight loss, anemia, and low albumin [10], and mesothelioma prognostic test (MPT) poor risk combined with tumor volume greater than 200 cm3 [11] are associated with poor survival in MPM. Taking these tumor and patient factors into account allows for improved treatment stratification and prognostication compared to that of traditional clinical staging alone.
Despite insights gained from these various prognostic factors, high morbidity and mortality persist in MPM, necessitating the need for new directions in therapy. In this review, we will summarize current therapies in MPM, their strengths and weaknesses, the evolving therapeutic landscape, and its implications for current and future practice.
The Current Therapeutic Landscape
MPM can be difficult to identify early, as its early development is often asymptomatic. Instead, patients with MPM tend to present late in the disease course once dyspnea or chest pain has resulted, for example, due to tumor encasement of the lung, pleural effusion, and/or direct invasion of the tumor into the chest wall or mediastinum. These are the most common presenting symptoms, along with malaise, fatigue, anorexia, weight loss, and sweats, which often become more frequent with disease progression [12].
Due to this presentation pattern, the majority of patients with MPM do not present with early-stage disease and therefore are not amenable to local therapies such as surgery or radiation. For the minority of patients who do present with early-stage disease and have detailed preoperative staging and assessment of performance status and cardiopulmonary reserve and are ultimately deemed appropriate for surgery, current surgical options are discussed briefly below, along with radiotherapy approaches. This will be followed by a larger discussion of current systemic treatment options, keeping in mind that this is the treatment form applicable to the majority of patients with MPM.
Surgery and Radiotherapy
The spectrum of surgical approaches for MPM includes partial pleurectomy (partial removal of involved pleura), pleurectomy-decortication (removal of parietal and visceral pleura and any portions of involved lung), extended pleurectomy-decortication (removal of parietal and visceral pleura, visible tumor, pericardium, and hemidiaphragm), and extrapleural pneumonectomy (removal of the lung, pleura, pericardium, and hemidiaphragm with the goal of macroscopic complete resection (MCR)) [13, 14].
In this spectrum of surgical approaches, the most radical approach, extrapleural pneumonectomy (EPP), has 5-year survival rates of 14% with median survival of 18 months [15]. However, in the first randomized trial of EPP and postoperative radiotherapy versus no EPP/radiotherapy (both in the context of standard platinum based chemotherapy), the surgical group demonstrated shorter overall survival (OS) (14.4 versus 19.5 months) and significantly higher morbidity (surgical and radiation complications included reoperation, cardiopulmonary complications, infection, pneumonitis, ascites, pain, and death) [16]. While the results of this UK-based Mesothelioma and Radical Surgery (MARS) randomized feasibility study were negative, it has been argued that the considerable dropout rate in the originally screened group as well as the ultimate surgery and radiotherapy groups, and the more favorable biological disease in the nonsurgical group, may have problematically influenced the study outcome [17]. On the opposite end of the surgical spectrum, partial pleurectomy (PP), the least radical option, also did not result in improved survival (52% 1-year survival in PP versus 57% 1-year survival in talc pleurodesis) but saw more complications and longer hospital stays [18]. Consequently, the ongoing MARS2 study is investigating the only other radical treatment option remaining, extended pleurectomy-decortication (EPD) [19••]. It is comparing EPD versus no EPD, again in the context of a standard chemotherapy backbone, and will be the first randomized trial of its kind on a topic that has largely been contributed to by retrospective case series until now.
Radiotherapy trials have also been limited, with existing randomized data not yet showing improved survival such as in the phase II SAKK 17/04 trial where hemithoracic radiation after neoadjuvant chemotherapy and extrapleural pneumonectomy demonstrated median survival 19.3 months in the radiation group versus 20.8 months in the group without radiation, representing additional treatment burden without benefit [20]. Similarly, negative results were seen in randomized trials for prophylactic irradiation to prevent chest wall invasion after diagnostic/therapeutic procedures, as in the phase III Prophylactic Irradiation of Tracts (PIT) [21] and the phase III Surgical and Large-Bore Procedures in Malignant Pleural Mesothelioma and Radiotherapy trial (SMART trial) [22]. Ongoing randomized trials are evaluating the role of intensity-modulated radiotherapy [23] and radiotherapy in pain control [24]. If negative results are also seen in these studies, the role for routine radiotherapy will be further undermined.
Systemic Therapy
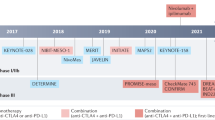
The first frontline standard of care systemic therapy regimen was defined by the phase III EMPHACIS study which showed superior median overall survival in the cisplatin with pemetrexed group (12.1 months) compared to the cisplatin alone group (9.3 months) and thus received Food and Drug Administration (FDA) approval in 2004 [25]. Similarly, cisplatin with raltitrexed was shown to be superior to cisplatin alone (11.4 versus 8.8 months median overall survival and no difference in health related quality of life measurement scales), confirming that cisplatin with an antifolate was superior to cisplatin alone in patients with MPM, without severe detriment to health related quality of life [26]. Just as single agent cisplatin fell to the background as standard of care treatment, the phase II CALGB 9530 study showed that single agent gemcitabine also had no role in MPM as its use in the frontline setting resulted in no complete or partial responses [27]. Having established the utility of frontline pemetrexed and platinum therapy, the phase II CALGB 30,901 study investigated the role of maintenance pemetrexed versus observation after frontline therapy and did not show significant increases in median progression free survival (PFS) or OS (3.4 versus 3 months, and 16.3 versus 11.8 months, respectively, both p-values > 0.6) [28], although the accrual goals for this study were not met.
In the continued search for targeted therapy, angiogenesis, a hallmark of cancer, has been targeted in mesothelioma with mixed results. Multikinase inhibitors that target multiple aspects of angiogenesis—such as cediranib (a TKI targeting VEGFR 1–3, c-Kit, and PDGFR-β) and nintedanib (a TKI targeting VEGFR 1–3, FGFR 1–3, and PDGFR α/β and Src-family members)—initially seemed effective when combined with chemotherapy in MPM, but ultimately were limited by toxicity and lack of reproducibility in larger studies, respectively [29, 30, 31, 32]. More promising results were seen in the phase III MAPS study, demonstrating that bevacizumab, a humanized anti–VEGF-A monoclonal antibody, when combined with chemotherapy in MPM in the frontline setting, improved survival compared to chemotherapy alone (median OS:18.8 months vs. 16.1 months, p < 0.02) [33]. These results led to bevacizumab’s inclusion into the US National Comprehensive Cancer Network (NCCN) guidelines as potential first-line treatment for unresectable MPM, though it has not gained FDA approval for MPM. Similarly, the phase II RAMES study demonstrated a survival advantage when ramucirumab, a humanized anti-VEGFR2 monoclonal antibody, was combined with gemcitabine, as compared to chemotherapy alone (median OS: 13.8 months vs. 7.5 months, p < 0.03), in patients with previously treated MPM (patients who had experienced disease progression during or after first-line pemetrexed/platinum-based chemotherapy) [34••]. However, as of March 2022, ramucirumab with gemcitabine has not yet been included in NCCN guidelines or gained FDA approval for this indication.
A promising target that has been transformative for various solid tumors is immune checkpoint inhibition (ICI), and multiple trials are showing clinical benefit of ICI in mesothelioma as well. While the phase III PROMISE-Meso trial did not show a survival benefit for pembrolizumab (PD-1 inhibitor) over standard chemotherapy for relapsed MPM [35•], the phase III CONFIRM trial showed a survival benefit for nivolumab (PD-1 inhibitor) over placebo for relapsed MPM (overall survival 10.2 months vs. 6.9 months, p < 0.01) [36•]. Furthering the argument for ICI incorporation into MPM treatment, the phase III CheckMate 743 trial showed an OS benefit for nivolumab plus ipilimumab (PD-1 plus CTLA-4 inhibitor) versus chemotherapy in patients with untreated, unresectable MPM (18.1 months vs. 14.1 months, p < 0.01) [37••]. The survival benefit was more pronounced in patients with non-epithelioid variants of mesothelioma, often with notable responses comparable to that seen in Fig. 1. Accordingly, this first-in-class regimen is now approved by the FDA for frontline use, marking a significant stride forward in MPM treatment. CM743 did not include a VEGF inhibitor in the control arm given the timing of the initiation of that trial and the lack of regulatory approval of bevacizumab, though combined VEGF and immune checkpoint inhibition is now being explored as will be discussed below.
Treatment response in a 61 year old male patient with malignant pleural mesothelioma, biphasic type, on dual immune checkpoint inhibitor (ICI) therapy. A Pre-treatment computed tomography (CT) chest at time of diagnosis shows a nodular pleural tumor in the right hemithorax. The tumor encased the lung from the apex to costophrenic angles with thickness greater than 2 cm in some areas, and nodular tumor directly invading mediastinal fat. B Pre-treatment positron emission tomography–computed tomography (PET-CT) following diagnosis of mesothelioma shows extensive hypermetabolic pleural-based soft tissue nodularity throughout the right hemithorax including the fissures and mediastinal fat. C Post-treatment CT chest, after 4 months of dual ICI therapy with nivolumab 360 mg every 3 weeks and ipilimumab 1 mg/kg every 6 weeks demonstrates that the previously extensive right sided pleural nodularity has significantly decreased and is now barely apparent. This patient’s presenting symptoms of dyspnea and chest wall pain resolved and he felt well overall. Treatment was complicated by acute kidney injury that was attributed to ICI-induced nephritis and improved with oral steroids
The Evolving Therapeutic Landscape
Chemoimmunotherapy
Building on the promising results of ICI therapy, the phase II DREAM study: Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma found that durvalumab, an anti-PD-L1 antibody, given during and after platinum-pemetrexed chemotherapy, resulted in 57% of patients achieving progression free survival at 6 months and median OS 18.4 months [38•], prompting a phase III study for further investigation. Accordingly, the phase III DREAM3R study: Durvalumab with chemotherapy as first line treatment in advanced pleural mesothelioma is recruiting patients to compare frontline durvalumab with chemotherapy followed by maintenance durvalumab vs. frontline chemotherapy alone followed by observation [39••]. It will also be interesting to see if this trial confirms findings seen in the recent phase 2 PrE0505 trial, where concurrent durvalumab with platinum-based chemotherapy reached a median survival of 20.4 months versus 12.1 months with historical control [40]. The PrE0505 trial also interestingly noted that patients with germline alterations in cancer predisposing genes, especially those involved in DNA repair, were more likely to achieve long-term survival [40]. The Canadian Cancer Trials Group is also investigating immunotherapy with standard chemotherapy in a randomized phase II/III study of pembrolizumab (a PD-1 inhibitor) with frontline chemotherapy vs. chemotherapy alone [41••]. Positive results would help prove the synergism between ICI therapy and chemotherapy that has become standard of care for other thoracic malignancies such as small cell lung cancer [42] and non-small cell lung cancer (NSCLC) [43].
Similarly, synergisms between ICI therapy, chemotherapy, and anti-angiogenesis therapy are also being explored. While MAPS and RAMES showed benefit of anti-angiogenesis therapy in combination with chemotherapy, the BEAT-meso study is investigating whether the addition of immunotherapy to chemotherapy and bevacizumab improves outcomes compared to chemotherapy with bevacizumab alone in the frontline MPM setting. This multicenter, randomized, phase III study is estimated to reach completion in 2024 [44••].
The positive outcomes of ICI therapy in MPM are somewhat unexpected since the tumor types that have seen clear survival benefit from ICI therapy typically have high tumor mutational burden, while MPM has a very low mutation burden [45, 46]. Thus, identifying biomarkers to predict outcomes and guide the use of ICI therapy in MPM would be of significant utility. To this aim, work has been done to investigate genomic structural variants in mesothelioma. Recently, it was shown that chromosomal rearrangements are present in mesothelioma and have neo-antigenic potential [47]. A more recent study investigating tumor junction burden and antigen presentation as biomarkers in MPM treated with ICI found that tumor junction burdens were not predictive of OS but an interaction between the junction burden and “the regulation of antigen processing and presentation of peptide antigen” gene set was predictive of overall survival [48•]. The recent PrE050 trial also identified that higher degrees of genomic instability were correlated with survival outcomes [40]. These findings suggest that further work in genomic approaches to evaluate junction burdens and antigen processing and presentation may help stratify and prognosticate patients being considered for ICI therapy.
Other Immune Targets
Dendritic and CAR T-Cell Therapies
A pivotal approach in engaging the immune system to target tumor cells has been seen with genetically engineered chimeric antigen receptor T (CAR T) cell therapy, which has achieved success in hematologic malignancies [49]. Initial CAR T-cell therapies for MPM targeted the tumor-associated antigen mesothelin (a cell surface glycoprotein expressed by all epithelioid but not sarcomatoid/biphasic mesotheliomas) [50], but struggled to achieve widespread clinical response [51] and in some cases, patients suffered life threatening anaphylactic reactions [52, 53]. However, CAR T-cell function was found to be dampened by PD-1 expression, and accordingly, enhanced by PD-1 blockade with pembrolizumab [54]. Building on this mechanism, a phase I trial evaluating intrapleural CAR T-cells with pembrolizumab achieved a median overall survival of 23.9 months (1-year overall survival, 83%) [55•], suggesting a more efficacious use for CAR T-cell therapy in the future of MPM therapy.
Another developing cellular therapy in MPM involves training dendritic cells (DCs) to promote an immunostimulatory response against selected antigens. With antigen sources spanning autologous tumor lysate [56], allogeneic tumor lysate [57], and combining dendritic cells with chemotherapy [58], clinical responses have been seen, and the currently recruiting phase II/III Dendritic Cell Immunotherapy for Mesothelioma (DENIM) trial will further clarify if there is a role for DCs in MPM [59].
Oncolytic Viral Therapies
Oncolytic viral therapies represent an appealing emerging therapy for mesothelioma as pleural/peritoneal disease involvement lends itself well to direct intratumoral injection of viral therapies. These viral therapies work through direct and indirect tumor activity, by lysing tumor cells but also by inducing immune responses. As early as 1994, human mesothelioma cell lines were found to be susceptible to adenovirus infection via a replication-deficient recombinant adenovirus carrying the Escherichia coli lacZ marker gene [60]. While this study showed the potential of viral vectors as vehicles for gene therapy in human mesothelioma, the ONCOS-102 study showed that ONCOS-102, a dual targeting, chimeric oncolytic adenovirus, coding for human GM-CSF, also held synergistic anti-tumor activity when combined with frontline chemotherapy [61]. Accordingly, there are now several ongoing clinical trials investigating adenoviral vectors as monotherapy [62, 63] or as multi-therapy such as ONCOS-102 with carboplatin/pemetrexed [64], interferon alpha-2b with celecoxib/gemcitabine [65] or with celecoxib/pemetrexed [66], and even with ICI therapy [67]. Herpes simplex virus type 1 [68], measles virus [69], vaccinia virus [70], Newcastle disease virus [71], retrovirus [72], and reovirus [73] have also been investigated in mesothelioma, with ongoing clinical trials mostly focusing on vaccinia virus [74, 75, 76].
Genomic Targets
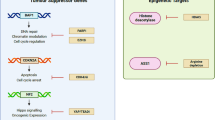
While MPM has a relatively low tumor mutational burden [45, 46], key tumor suppressor genes that are affected in MPM most commonly include BAP1, NF2, and CDKN2A. BAP1, or BRCA1-associated protein 1 carboxy-terminal hydrolase, was identified in 1998 as a nuclear protein that influenced activity of the BRCA1 protein [77], though the exact mechanism is still unclear [78, 79, 80]. It is clear, however, that individuals who inherit a BAP1 mutant allele are at risk for developing one or more malignancies, most commonly uveal or cutaneous melanoma, clear-cell renal cell carcinoma, and mesothelioma [81, 82, 83, 84, 85]. In mesothelioma, although the mechanism is unclear, BAP1 mutations have shown an association with better prognosis compared to sporadic disease, with as high as sevenfold improved survival [86, 87]. Due to the interaction between BAP 1 and BRCA1, studies are now investigating for the potential to treat with PARP inhibitors, with the Mesothelioma Stratified Therapy (MiST) nonrandomized phase II trial showing that PARP inhibition with rucaparib in refractory malignant mesothelioma of any type resulted in 58% disease control at 12 weeks, 23% at 24 weeks and was well tolerated [88•]. However, a similar nonrandomized phase II trial of PARP inhibition with olaparib interestingly showed decreased PFS and OS in the germline BAP1 mutation group compared to wild type (2.3 vs 4.1 months, and 4.6 vs 9.6 months) [89]. BAP 1 also interacts with polycomb repressor complex 2 (PRC2) which promotes tumor growth and invasion; thus, the PRC2 inhibitor tazetostat was investigated in a phase II trial and showed 51% disease control at 12 weeks [90].
NF2 encodes Merlin, which controls the expression of oncogenic genes via a pathway involving inhibition of transcriptional co-activators YAP and TAZ, which effect the Hippo pathway [91]. Many mesothelioma specimens have aberrant YAP activation [92], and targeting this via inhibition of Rho-associated kinase (ROCK), a downstream target of YAP, or via disruption of the YAP-TEA domain transcription factor interaction using verteporfin, has been shown to impede in vitro mesothelioma cell proliferation/invasion [93, 94].
CDKN2A encodes p16INK4a and p14ARF, which regulate the cell cycle by inhibiting cyclin-dependent kinase (CDK) 4 and CDK6-mediated phosphorylation of retinoblastoma protein and preventing p53 degradation, respectively [95, 96]. Many mesothelioma cases have CDKN2A deletion [97], and targeting this via CDK4/6 kinase inhibition with palbociclib showed synergistic ability to impede mesothelioma cell proliferation when combined with PI3K/AKT/mTOR inhibition [98]. CDKN2A loss has also been associated with shorter overall survival due to loss of the tumor suppressor p16ink4A, an endogenous suppressor of CDK4/6 [99]. Thus, the phase 2 MisT2 study investigated CDK4/6 inhibition with abemaciclib in p16ink4A-negative mesothelioma and found disease control at 12 weeks in 14 (54%) of 26 patients (95% CI 36–71) [99].
In addition to the above loss of function mutations in mesothelioma, there are also emerging case reports on oncogenic fusions, namely EWSR1 [100, 101] and ALK [102, 103] in peritoneal mesothelioma, which begs the question of whether ALK-inhibition can prove successful in mesothelioma even beyond case reports, as it notably has for NSCLC [104] and other tumors [105].
Cancer Vaccines
Just as CAR T-cell therapy investigated mesothelin as a target, cancer vaccines similarly aim to stimulate the immune system to destroy mesothelioma cells by targeting mesothelin. Monoclonal antibodies targeting mesothelin have shown acceptable tolerability and improved overall survival in phase I/II studies [106, 107], while vaccines with bacterial components (Listeria monocytogenes engineered to express human mesothelin [108] and Pseudomonas exotoxin A fused to anti-mesothelin antibody [109]) showed limited efficacy in phase I/II trials but more promising response activity when combined with chemotherapy [110, 111]. Wilms’ tumor 1 (WT1) peptide analog vaccines have also shown potential response activity with immunologic adjuvants (montanide and GM-CSF) compared to adjuvants alone [112]. mRNA vaccines [113] will hopefully provide a novel platform to explore cancer immunotherapy for mesothelioma.
Cell Proliferation and Motility Targets
One regulator of cancer cell proliferation and migration is focal adhesion kinase (FAK), which is attenuated by Merlin to inhibit cancer cell migration [114, 115]. Building on this mechanism, trials of FAK inhibitors alone [116] and with MEK inhibitor trametinib [117] saw improved median PFS in Merlin-negative tumors versus Merlin-positive tumors. Another tyrosine receptor kinase important in cell proliferation and motility is MET, which is overexpressed in mesothelioma [118, 119]. Since MET inhibition via tivantinib along with PI3K inhibition suppressed tumor growth and development [120], there is now a phase I/II trial of tivantinib with frontline chemotherapy for mesothelioma and NSCLC [121].
Conclusions
Therapeutic advancements for mesothelioma have been slow due to its relative rarity and interpatient heterogeneity. However, while the incidence of malignant mesothelioma is mildly decreasing in the USA and western countries due to work practices moving away from asbestos use, its use is growing in countries such as India and China [122], pointing to the continued need for clinical research in malignant mesothelioma to improve outcomes for our current and future patients.
Thus, the therapeutic advances discussed above, spanning immune checkpoint inhibition, angiogenesis inhibition, CAR-T and dendritic cell therapies, oncolytic viral therapies, anti-mesothelin therapies, PARP, CDK 4/6, and ALK inhibition, with ongoing research into synergisms between these groups and biomarker identification to further refine and prognosticate patients among these groups, represent timely advances in the field and exciting progress in this challenging disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Milano MT, Zhang H. Malignant pleural mesothelioma: a population-based study of survival. J Thorac Oncol. 2010;5:1841–8.
van Zandwijk N, Clarke C, Henderson D, Musk AW, Fong K, Nowak A, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis. 2013;5:E254-307.
Beckett P, Edwards J, Fennell D, Hubbard R, Woolhouse I, Peake MD. Demographics, management and survival of patients with malignant pleural mesothelioma in the National Lung Cancer Audit in England and Wales. Lung Cancer. 2015;88:344–8.
Fennell DA, Parmar A, Shamash J, Evans MT, Sheaff MT, Sylvester R, et al. Statistical validation of the EORTC prognostic model for malignant pleural mesothelioma based on three consecutive phase II trials. J Clin Oncol. 2005;23:184–9.
Dalton LE, Clarke HJ, Knight J, Lawson MH, Wason J, Lomas DA, et al. The endoplasmic reticulum stress marker CHOP predicts survival in malignant mesothelioma. Br J Cancer. 2013;108:1340–7.
Chia PL, Russell P, Asadi K, Thapa B, Gebski V, Murone C, et al. Analysis of angiogenic and stromal biomarkers in a large malignant mesothelioma cohort. Lung Cancer. 2020;150:1–8.
Dell’Anno I, Barone E, Mutti L, Rassl DM, Marciniak SJ, Silvestri R, et al. Tissue expression of lactate transporters (MCT1 and MCT4) and prognosis of malignant pleural mesothelioma (brief report). J Transl Med. 2020;18:341.
Schramm A, Opitz I, Thies S, Seifert B, Moch H, Weder W, et al. Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2010;37:566–72.
Mansfield AS, Roden AC, Peikert T, Sheinin YM, Harrington SM, Krco CJ, et al. B7–H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9:1036–40.
Harris EJA, Kao S, McCaughan B, Nakano T, Kondo N, Hyland R, et al. Prediction modelling using routine clinical parameters to stratify survival in Malignant Pleural Mesothelioma patients undergoing cytoreductive surgery. J Thorac Oncol. 2019;14:288–93.
Yeap BY, Rienzo AD, Gill RR, Oster ME, Dao MN, Dao NT, et al. Mesothelioma risk score: a new prognostic pretreatment, clinical-molecular algorithm for malignant pleural mesothelioma. J Thorac Oncol. 2021;16:1925–35.
Parker C, Neville E. Lung cancer * 8: management of malignant mesothelioma. Thorax. 2003;58:809–13.
Wolf AS, Flores RM. Updates in staging and management of malignant pleural mesothelioma. Surg Oncol Clin N Am. 2020;29:603–12.
Gelzinis TA. The 2019 ERS/ESTS/EACTS/ESTRO guidelines on the management of patients with malignant pleural mesothelioma. J Cardiothorac Vasc Anesth. 2021;35:378–88.
Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma. Ann Surg. 2014;260:577–82.
Treasure T, Lang-Lazdunski L, Waller D, Bliss JM, Tan C, Entwisle J, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763–72.
Janes SM, Alrifai D, Fennell DA. Perspectives on the treatment of malignant pleural mesothelioma. N Engl J Med. 2021;385:1207–18.
Rintoul RC, Ritchie AJ, Edwards JG, Waller DA, Coonar AS, Bennett M, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet. 2014;384:1118–27.
••Lim E, Darlison L, Edwards J, Elliott D, Fennell DA, Popat S, et al. Mesothelioma and Radical Surgery 2 (MARS 2): protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma. BMJ Open. 2020;10:e038892. This study will be the first randomized trial to compare surgery in MPM, namely EPD vs. no EPD, with respect to overall survival, cost-effectiveness, and quality of life. Patients will be followed up at regular intervals for two years and the trial is currently recruiting. The study will also include an optional “informational study” focusing on the patient decision process to take part in research or not, with the aim of improving recruitment to clinical trials..
Stahel RA, Riesterer O, Xyrafas A, Opitz I, Beyeler M, Ochsenbein A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase II trial. Lancet Oncol. 2015;16:1651–8.
Bayman N, Appel W, Ashcroft L, Baldwin DR, Bates A, Darlison L, et al. Prophylactic irradiation of tracts in patients with malignant pleural mesothelioma: an open-label, multicenter, phase III randomized trial. J Clin Oncol. 2019;37:1200–8.
Clive AO, Taylor H, Dobson L, Wilson P, de Winton E, Panakis N, et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): a multicentre, open-label, phase III, randomised controlled trial. Lancet Oncol. 2016;17:1094–104.
Rimner A, Hu C, Rusch VW, Gill RR, Ii CBS, Zauderer M, et al. A phase III randomized trial of pleurectomy/decortication plus chemotherapy with or without adjuvant hemithoracic intensity-modulated pleural radiation therapy (IMPRINT) for Malignant pleural mesothelioma (MPM) (NRG LU-006). Int J Radiat Oncol Biol Phys. 2021;111:e463–4.
Ashton M, O’Rourke N, Macleod N, Laird B, Stobo J, Kelly C, et al. SYSTEMS-2: a randomised phase II study of radiotherapy dose escalation for pain control in malignant pleural mesothelioma. Clin Transl Radiat Oncol. 2017;8:45–9.
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44.
van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881–9.
Kindler HL, Millard F, Herndon JE, Vogelzang NJ, Suzuki Y, Green MR. Gemcitabine for malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B. Lung Cancer. 2001;31:311–7.
Dudek AZ, Wang X, Gu L, Duong S, Stinchcombe TE, Kratzke R, et al. Randomized study of maintenance pemetrexed versus observation for treatment of malignant pleural mesothelioma: CALGB 30901. Clin Lung Cancer. 2020;21:553-561.e1.
Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–400.
Garland LL, Chansky K, Wozniak AJ, Tsao AS, Gadgeel SM, Verschraegen CF, et al. Phase II study of cediranib in patients with malignant pleural mesothelioma: SWOG S0509. J Thorac Oncol. 2011;6:1938–45.
Tsao AS, Miao J, Wistuba II, Vogelzang NJ, Heymach JV, Fossella FV, et al. Phase II trial of cediranib in combination with cisplatin and pemetrexed in chemotherapy-naïve patients with unresectable malignant pleural mesothelioma (SWOG S0905). J Clin Oncol. 2019;37:2537–47.
Grosso F, Steele N, Novello S, Nowak AK, Popat S, Greillier L, et al. Nintedanib plus pemetrexed/cisplatin in patients with malignant pleural mesothelioma: phase II results from the randomized, placebo-controlled LUME-meso trial. J Clin Oncol. 2017;35:3591–600.
Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–14.
••Pinto C, Zucali PA, Pagano M, Grosso F, Pasello G, Garassino MC, et al. Gemcitabine with or without ramucirumab as second-line treatment for malignant pleural mesothelioma (RAMES): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2021;22:1438–47 This multi-center, randomized, double-blind, placebo-controlled phase II trial in Italy showed that gemcitabine plus ramucirumab resulted in significantly increased overall survival compared to gemcitabine plus placebo for patients with MPM that progressed on first-line treatment, with an acceptable safety profile (no treatment-related deaths; the most common serious adverse event was thromboembolism in 4% of the ramucirumab group vs. 2% of the placebo group)..
•Popat S, Curioni-Fontecedro A, Dafni U, Shah R, O’Brien M, Pope A, et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9–15) PROMISE-meso trial. Ann Oncol. 2020;31:1734–45. This multi-center, randomized, open label phase III trial in Spain, Switzerland, and the U.K. was the first randomized trial to evaluate pembrolizumab (anti-PD1 agent) in MPM patients who progressed on frontline chemotherapy. The trial showed that pembrolizumab led to higher objective response rate (22% versus 6%, p=0.004) but did not ultimately improve PFS or OS compared to institutional choice single-agent chemotherapy (gemcitabine or vinorelbine) in relapsed MPM patients with progression after/on previous platinum-based chemotherapy..
•Fennell DA, Ewings S, Ottensmeier C, Califano R, Hanna GG, Hill K, et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021;22:1530–40. This multi-center, randomized, double-blind, placebo-controlled phase III trial in the U.K. investigated the efficacy of nivolumab (anti-PD1 agent) after progression on frontline chemotherapy. The trial showed that PFS and OS were both improved in the nivolumab group compared to the placebo group (3 months versus 1.8 months, and 10.2 versus 6.9 months, respectively; both p-values< 0.01). With these results, CONFIRM became the first phase 3 trial to show improved survival for patients with pleural or peritoneal malignant mesothelioma who have progressed following platinum-based chemotherapy..
••Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–86. This multi-center, randomized, open-label phase III trial across 21 countries investigated the use of first line nivolumab plus ipilimumab (PD-1 plus CTLA-4 inhibition) versus standard first line platinum plus pemetrexed chemotherapy and found that nivolumab plus ipilimumab significantly extended overall survival (median OS 18.1 months versus 14.1 months; p=0.0020). These notable results led to approval of this first-in-class regimen in the USA as of October 2020, for previously untreated unresectable MPM..
•Nowak A, Kok P, Lesterhuis W, Hughes B, Brown C, Kao S, et al. OA08.02 DREAM - a phase 2 trial of durvalumab with first line chemotherapy in mesothelioma: final result. Journal of Thoracic Oncology. 2018;13:S338-9. This multi-center, open-label phase II trial in Australia examined the effect of durvalumab (anti-PD-L1 agent), given during and after first-line chemotherapy with cisplatin and pemetrexed in patients with advanced MPM. 57% of patients were alive and progression free at 6 months, prompting a randomized phase III trial DREAM3R..
••PrECOG, LLC. DREAM3R: DuRvalumab (MEDI4736) With chemotherapy as first line treatment in advanced pleural mesothelioma - a phase 3 randomised trial [Internet]. clinicaltrials.gov; 2022 Mar. Report No.: NCT04334759. Available from: . https://clinicaltrials.gov/ct2/show/NCT04334759This multi-center, randomized, open-label phase III trial in the U.S., Australia, and New Zealand builds on the DREAM trial and will explore durvalumab (anti-PD-L1 agent) with standard chemotherapy followed by maintenance durvalumab versus standard chemotherapy followed by observation, in the frontline setting for advanced MPM. The study is currently recruiting and estimated to reach completion December 2025.
Forde PM, Anagnostou V, Sun Z, Dahlberg SE, Kindler HL, Niknafs N, et al. Durvalumab with platinum-pemetrexed for unresectable pleural mesothelioma: survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat Med. 2021;27:1910–20.
••Canadian Cancer Trials Group. A phase II/III randomized study of pembrolizumab in patients with advanced malignant pleural mesothelioma [Internet]. clinicaltrials.gov; 2022 Jan. Report No.: NCT02784171. Available from: https://clinicaltrials.gov/ct2/show/NCT02784171.This multi-center, randomized, open-label phase II/III trial in Canada, France, and Italy is investigating pembrolizumab (anti-PD1 agent) in combination with frontline chemotherapy and as single agent maintenance therapy versus frontline chemotherapy alone. The study is active but not recruiting, and estimated completion is December 2022.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. New England Journal of Medicine [Internet]. 2018 [cited 2022 Mar 23]; Available from: https://www.nejm.org/doi/https://doi.org/10.1056/nejmoa1809064
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–92.
••European Thoracic Oncology Platform. A multicentre randomised phase III trial comparing atezolizumab plus bevacizumab and standard chemotherapy versus bevacizumab and standard chemotherapy as first-line treatment for advanced malignant pleural mesothelioma [Internet]. clinicaltrials.gov; 2021 Apr. Report No.: NCT03762018. Available from:https://clinicaltrials.gov/ct2/show/NCT03762018. This multi-center, randomized, open-label phase III trial in Belgium, France, Italy, Spain, Switzerland, and the U.K. is studying atezolizumab (anti-PD-L1 agent) with bevacizumab (anti-VEGF-A) plus chemotherapy versus bevacizumab plus chemotherapy without immune checkpoint inhibitor therapy in the frontline setting for advanced MPM. The study is currently recruiting and estimated to reach completion in January 2024.
Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–16.
Hmeljak J, Sanchez-Vega F, Hoadley KA, Shih J, Stewart C, Heiman D, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;8:1548–65.
Mansfield AS, Peikert T, Smadbeck J, Udell J, Garcia-Rivera E, Elsbernd L, et al. Neoantigenic potential of complex chromosomal rearrangements in mesothelioma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer [Internet]. 2019 [cited 2022 Mar 23];14. Available from: https://pubmed.ncbi.nlm.nih.gov/30316012/
•Kosari F, Disselhorst M, Yin J, Peikert T, Udell J, Johnson S, et al. Tumor junction burden and antigen presentation as predictors of survival in mesothelioma treated with immune checkpoint inhibitors. J Thorac Oncol. 2022;17:446–54. This study performed genomic analysis on pleural biopsies of mesothelioma after at least one of line of therapy (n=44) before treatment with single agent nivolumab (anti-PD1 agent) or together with ipilimumab (anti-CTLA-4 agent). They identified junctions resulting from chromosomal rearrangements and antigen processing and presentation gene set expression and estimated associations with OS using Cox models. They found that tumor junction burdens were not predictive of OS, but the “regulation of antigen processing and presentation of peptide antigen” gene set revealed an interaction with tumor junction burden and was predictive of OS. However, this interaction was not predictive of OS in a different cohort of MPM patients who did not receive immune checkpoint inhibitor (ICI) therapy. These results suggest that genomic analysis represents a tool for stratification and prognostication in MPM patients being considered for ICI therapy..
Park, J.H, Geyer M.B., et al. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date | Blood | American Society of Hematology [Internet]. [cited 2022 Mar 9]. Available from: https://ashpublications.org/blood/article/127/26/3312/35352/CD19-targeted-CAR-T-cell-therapeutics-for
Ordóñez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–7.
Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31.
Klampatsa A, Haas AR, Moon EK, Albelda SM. Chimeric antigen receptor (CAR) T cell therapy for malignant pleural mesothelioma (MPM). Cancers (Basel). 2017;9.
Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–20.
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–44.
•Adusumilli PS, Zauderer MG, Rivière I, Solomon SB, Rusch VW, O’Cearbhaill RE, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti–PD-1 agent pembrolizumab regional CAR T-cell therapy for mesothelioma. Cancer Discov. 2021;11:2748–63. This single-center, open-label, dose-escalating, first-in-human, phase I trial of interpleural delivery of mesothelin-targeted CAR T cells in combination with pembrolizumab (anti-PD1 agent) in patients with previously treated MPM and showed median OS 23.9 months with acceptable safety profile. These findings support the continued investigation of combination immunotherapy with ICI and CAR T cell therapies..
Hegmans JP, Veltman JD, Lambers ME, de Vries IJM, Figdor CG, Hendriks RW, et al. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med. 2010;181:1383–90.
Aerts JGJV, de Goeje PL, Cornelissen R, Kaijen-Lambers MEH, Bezemer K, van der Leest CH, et al. Autologous dendritic cells pulsed with allogeneic tumor cell lysate in mesothelioma: from mouse to human. Clin Cancer Res. 2018;24:766–76.
Cornelissen R, Hegmans JPJJ, Maat APWM, Kaijen-Lambers MEH, Bezemer K, Hendriks RW, et al. Extended tumor control after dendritic cell vaccination with low-dose cyclophosphamide as adjuvant treatment in patients with malignant pleural mesothelioma. Am J Respir Crit Care Med. 2016;193:1023–31.
Belderbos RA, Baas P, Berardi R, Cornelissen R, Fennell DA, van Meerbeeck JP, et al. A multicenter, randomized, phase II/III study of dendritic cells loaded with allogeneic tumor cell lysate (MesoPher) in subjects with mesothelioma as maintenance therapy after chemotherapy: DENdritic cell Immunotherapy for Mesothelioma (DENIM) trial. Transl Lung Cancer Res. 2019;8:280–5.
Smythe WR, Kaiser LR, Hwang HC, Amin KM, Pilewski JM, Eck SJ, et al. Successful adenovirus-mediated gene transfer in an in vivo model of human malignant mesothelioma. Ann Thorac Surg. 1994;57:1395–401.
Kuryk L, Haavisto E, Garofalo M, Capasso C, Hirvinen M, Pesonen S, et al. Synergistic anti-tumor efficacy of immunogenic adenovirus ONCOS-102 (Ad5/3-D24-GM-CSF) and standard of care chemotherapy in preclinical mesothelioma model. Int J Cancer. 2016;139:1883–93.
University of Pennsylvania. A phase I clinical trial of repeated dose intrapleural adenoviral-mediated interferon-beta (BG00001, Ad.hIFN-β for pleural malignancies [Internet]. clinicaltrials.gov; 2020 Mar. Report No.: NCT00299962. Available from: https://clinicaltrials.gov/ct2/show/NCT00299962
Abramson Cancer Center of the University of Pennsylvania. A pilot study of repeated dose intrapleural adenoviral-mediated interferon-alpha (SCH 721015, Ad.hIFN-a2b) gene transfer for malignant pleural mesothelioma [Internet]. clinicaltrials.gov; 2015 Sep. Report No.: NCT01212367. Available from: https://clinicaltrials.gov/ct2/show/NCT01212367
Targovax Oy. A randomised phase II open-label study with a phase Ib safety lead-in Cohort of ONCOS-102, an immune-priming GM-CSF coding oncolytic adenovirus, and pemetrexed/cisplatin in patients with unresectable malignant pleural mesothelioma [Internet]. clinicaltrials.gov; 2020 Oct. Report No.: NCT02879669. Available from: https://clinicaltrials.gov/ct2/show/NCT02879669
Trizell Ltd. A phase 3, open-label, randomized, parallel group study to evaluate the efficacy and safety of intrapleural administration of adenovirus-delivered interferon alpha-2b (rAd-IFN) in combination with celecoxib and gemcitabine in patients with malignant pleural mesothelioma [Internet]. clinicaltrials.gov; 2021 Oct. Report No.: NCT03710876. Available from: https://clinicaltrials.gov/ct2/show/NCT03710876
Abramson Cancer Center of the University of Pennsylvania. A pilot and feasibility trial evaluating two different chemotherapy regimens in combination with intrapleural adenoviral-mediated interferon-alpha gene transfer for malignant pleural mesothelioma [Internet]. clinicaltrials.gov; 2020 Mar. Report No.: NCT01119664. Available from: https://clinicaltrials.gov/ct2/show/NCT01119664
Momotaro-Gene Inc. A phase 2, open-label, single-center study of MTG201 in combination with nivolumab in patients with relapsed malignant pleural mesothelioma [Internet]. clinicaltrials.gov; 2019 Oct. Report No.: NCT04013334. Available from: https://clinicaltrials.gov/ct2/show/NCT04013334
Adusumilli PS, Stiles BM, Chan M-K, Mullerad M, Eisenberg DP, Ben-Porat L, et al. Imaging and therapy of malignant pleural mesothelioma using replication-competent herpes simplex viruses. J Gene Med. 2006;8:603–15.
Li H, Peng K-W, Dingli D, Kratzke RA, Russell SJ. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17:550–8.
Kelly KJ, Woo Y, Brader P, Yu Z, Riedl C, Lin S-F, et al. Novel oncolytic agent GLV-1h68 is effective against malignant pleural mesothelioma. Hum Gene Ther. 2008;19:774–82.
Silberhumer GR, Brader P, Wong J, Serganova IS, Gönen M, Gonzalez SJ, et al. Genetically engineered oncolytic Newcastle disease virus effectively induces sustained remission of malignant pleural mesothelioma. Mol Cancer Ther. 2010;9:2761–9.
Kawasaki Y, Tamamoto A, Takagi-Kimura M, Maeyama Y, Yamaoka N, Terada N, et al. Replication-competent retrovirus vector-mediated prodrug activator gene therapy in experimental models of human malignant mesothelioma. Cancer Gene Ther. 2011;18:571–8.
Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–72.
Memorial Sloan Kettering Cancer Center. Phase I study of intra-pleural administration of GL-ONC1, a genetically modified vaccinia virus, in patients with malignant pleural effusion: primary, metastases and mesothelioma [Internet]. clinicaltrials.gov; 2022 Feb. Report No.: NCT01766739. Available from: https://clinicaltrials.gov/ct2/show/NCT01766739
Kelly K. An Open Label, Non-randomized phase 1b study to investigate the safety and effect of the oncolytic virus GL-ONC1 administered intravenously prior to surgery to patients with solid organ cancers undergoing surgery for curative-intent or palliative resection [Internet]. clinicaltrials.gov; 2020 Mar. Report No.: NCT02714374. Available from: https://clinicaltrials.gov/ct2/show/NCT02714374
Wales Cancer Trials Unit. A phase II trial to assess the safety, immunological activity of TroVax® plus pemetrexed/cisplatin in patients with malignant pleural mesothelioma [Internet]. clinicaltrials.gov; 2013 Mar. Report No.: NCT01569919. Available from: https://clinicaltrials.gov/ct2/show/NCT01569919
Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–112.
Nishikawa H, Wu W, Koike A, Kojima R, Gomi H, Fukuda M, et al. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res. 2009;69:111–9.
Hauri S, Comoglio F, Seimiya M, Gerstung M, Glatter T, Hansen K, et al. A high-density map for navigating the human polycomb complexome. Cell Rep. 2016;17:583–95.
Carbone M, Harbour JW, Brugarolas J, Bononi A, Pagano I, Dey A, et al. Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov. 2020;10:1103–20.
Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer. 2013;13:153–9.
Carbone M, Adusumilli PS, Alexander HR, Baas P, Bardelli F, Bononi A, et al. Mesothelioma: scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin. 2019;69:402–29.
Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5.
Carbone M, Ferris LK, Baumann F, Napolitano A, Lum CA, Flores EG, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179.
Haugh AM, Njauw C-N, Bubley JA, Verzì AE, Zhang B, Kudalkar E, et al. Genotypic and phenotypic features of BAP1 cancer syndrome: a report of 8 new families and review of cases in the literature. JAMA Dermatol. 2017;153:999–1006.
Pastorino S, Yoshikawa Y, Pass HI, Emi M, Nasu M, Pagano I, et al. A subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other germline mutations. JCO. 2018;36:3485–94.
Baumann F, Flores E, Napolitano A, Kanodia S, Taioli E, Pass H, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis. 2015;36:76–81.
•Fennell DA, King A, Mohammed S, Branson A, Brookes C, Darlison L, et al. Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): an open-label, single-arm, phase 2a clinical trial. Lancet Respir Med. 2021;9:593–600. This single-center, open-label, phase II trial in the U.K. investigated the use of rucaparib (PARP inhibition) in patients with cytoplasmic-BAP1-deficient or BRCA1-deficient mesothelioma (pleural or peritoneal or other primary localization), after at least one course of systemic therapy. Disease control rate at 12 weeks was 58%, at 24 weeks was 23%, and toxicities were manageable. This data supports further investigation of PARP inhibition in mesothelioma.
Ghafoor A, Mian I, Wagner C, Mallory Y, Agra MG, Morrow B, et al. Phase 2 study of olaparib in malignant mesothelioma and correlation of efficacy with germline or somatic mutations in BAP1 gene. JTO Clin Res Rep. 2021;2: 100231.
Zauderer MG, Szlosarek P, Le Moulec S, Popat S, Taylor P, Planchard D, et al. Phase 2, multicenter study of the EZH2 inhibitor tazemetostat as monotherapy in adults with relapsed or refractory (R/R) malignant mesothelioma (MM) with BAP1 inactivation. JCO. 2018;36:8515–8515.
Sato T, Sekido Y. NF2/merlin inactivation and potential therapeutic targets in mesothelioma. Int J Mol Sci. 2018;19.
Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–16.
Miyanaga A, Masuda M, et al. Hippo pathway gene mutations in malignant mesothelioma: revealed by RNA and targeted exon sequencing. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer [Internet]. 2015 [cited 2022 Mar 10];10. Available from: https://pubmed.ncbi.nlm.nih.gov/25902174/
Zhang W-Q, Dai Y-Y, Hsu P-C, Wang H, Cheng L, Yang Y-L, et al. Targeting YAP in malignant pleural mesothelioma. J Cell Mol Med. 2017;21:2663–76.
Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108–13.
Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–7.
Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75:264–9.
Bonelli M, Digiacomo G, Fumarola C, et al. Combined inhibition of CDK4/6 and PI3K/AKT/mTOR pathways induces a synergistic anti-tumor effect in malignant pleural mesothelioma cells. Neoplasia (New York, NY) [Internet]. 2017 [cited 2022 Mar 10];19. Available from: https://pubmed.ncbi.nlm.nih.gov/28704762/
Fennell DA, King A, Mohammed S, Greystoke A, Anthony S, Poile C, et al. Abemaciclib in patients with p16ink4A-deficient mesothelioma (MiST2): a single-arm, open-label, phase 2 trial. Lancet Oncol. 2022;23:374–81.
Panagopoulos I, Thorsen J, Gorunova L, Micci F, Haugom L, Davidson B, et al. RNA sequencing identifies fusion of the EWSR1 and YY1 genes in mesothelioma with t(14;22)(q32;q12). Genes Chromosomes Cancer. 2013;52:733–40.
Desmeules P, Joubert P, Zhang L, Al-Ahmadie HA, Fletcher CD, Vakiani E, et al. A subset of malignant mesotheliomas in young adults are associated with recurrent EWSR1/FUS-ATF1 fusions. Am J Surg Pathol. 2017;41:980–8.
Loharamtaweethong K, Puripat N, Aoonjai N, Sutepvarnon A, Bandidwattanawong C. Anaplastic lymphoma kinase (ALK) translocation in paediatric malignant peritoneal mesothelioma: a case report of novel ALK-related tumour spectrum. Histopathology. 2016;68:603–7.
Hung YP, Dong F, Watkins JC, Nardi V, Bueno R, Dal Cin P, et al. Identification of ALK rearrangements in malignant peritoneal mesothelioma. JAMA Oncol. 2018;4:235–8.
Awad DMM, Shaw DAT. ALK inhibitors in non–small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12:429.
Mansfield AS, Wei Z, Mehra R, Shaw AT, Lieu CH, Forde PM, et al. Crizotinib in patients with tumors harboring ALK or ROS1 rearrangements in the NCI-MATCH trial. NPJ Precis Oncol. 2022;6:13.
Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–8.
Hassan R, Kindler HL, Jahan T, Bazhenova L, Reck M, Thomas A, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res. 2014;20:5927–36.
Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–68.
Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9.
Hassan R, Alley E, Kindler H, Antonia S, Jahan T, Honarmand S, et al. Clinical Response of live-attenuated, Listeria monocytogenes expressing mesothelin (CRS-207) with chemotherapy in patients with malignant pleural mesothelioma. Clin Cancer Res. 2019;25:5787–98.
Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147.
Krug LM, Tsao AS, Kass S, Rusch VW, Travis WD, Panageas K, et al. Randomized, double-blinded, phase II trial of a WT1 peptide vaccine as adjuvant therapy in patients with malignant pleural mesothelioma (MPM). JCO. 2011;29:TPS139.
Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Molecular Cancer [Internet]. 2021 [cited 2022 Mar 24];20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7905014/
Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015;146:132–49.
Poulikakos PI, Xiao G-H, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–8.
Soria JC, Gan HK, Blagden SP, Plummer R, Arkenau HT, Ranson M, et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann Oncol. 2016;27:2268–74.
Mak G, Soria J-C, Blagden SP, Plummer R, Fleming RA, Nebot N, et al. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br J Cancer. 2019;120:975–81.
Jagadeeswaran R, Ma PC, Seiwert TY, Jagadeeswaran S, Zumba O, Nallasura V, et al. Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res. 2006;66:352–61.
Bois MC, Mansfield AS, Sukov WR, Jenkins SM, Moser JC, Sattler CA, et al. c-Met expression and MET amplification in malignant pleural mesothelioma. Ann Diagn Pathol. 2016;23:1–7.
Kanteti R, Dhanasingh I, Kawada I, Lennon FE, Arif Q, Bueno R, et al. MET and PI3K/mTOR as a potential combinatorial therapeutic target in malignant pleural mesothelioma. PLoS ONE. 2014;9: e105919.
Santoro A. Phase I-Ib study of the combination of tivantinib plus pemetrexed and carboplatin as first-line therapy in patients with advanced or metastatic cancer suitable for a carboplatin and pemetrexed regimen as part of their specific therapy [Internet]. clinicaltrials.gov; 2021 Jan. Report No.: NCT02049060. Available from: https://clinicaltrials.gov/ct2/show/NCT02049060
Frank AL, Joshi TK. The global spread of asbestos. Ann Glob Health. 2014;80:257–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nirosha D. Perera declares no conflict of interest. A.S.Mansfield reports grants and other support from the NCI, DoD, the Mark Foundation, Bristol-Myers Squibb, Novartis, and Verily; other support to his institution from Rising Tide, TRIPTYCH Health Partners Expert Think Tank, Janssen, BeiGene, Chugai Pharmaceutical (Roche), Ideology Health, Miami Int’l Mesothelioma Symposium Presenter, AXIS Medical Education CME Presentation, Johnson & Johnson Global Services, Intellisphere CME Presentation, Answers In CME, Roche, AbbVie Advisory Board, AstraZeneca Advisory Board, Bristol-Myers Squibb Advisory Board; personal fees from Antoni van Leeuwenhoek Kanker Instituutt–CME Presenter; and Genentech/Roche Advisory Board outside the submitted work; and is a Mesothelioma Applied Research Foundation–Non-remunerated Director.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lung Cancer
Rights and permissions
About this article
Cite this article
Perera, N.D., Mansfield, A.S. The Evolving Therapeutic Landscape for Malignant Pleural Mesothelioma. Curr Oncol Rep 24, 1413–1423 (2022). https://doi.org/10.1007/s11912-022-01302-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01302-3