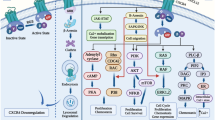
Abstract
Purpose of Review
To evaluate the clinical potential of chemokine receptor antagonists for the treatment of patients with cancer.
Recent findings
Chemokine receptors and their ligands can have a significant impact on the infiltration of cells into the tumor microenvironment. The receptors are increasingly being investigated as targets for the treatment of cancers. Recent studies are demonstrating the promise of chemokine receptor antagonists in this setting.
Summary
There are many chemokine receptors, and each can have different functions depending on the cellular context. Targeting chemokine receptors is a promising strategy in both pre-clinical research and clinical trials. Inhibiting chemokine receptors that either recruit suppressive cells or improve cancer mobility and viability while sparing those necessary for proper immune trafficking may prove to dramatically improve treatment responses. Further research in this area is warranted and has the potential to dramatically improve patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expression of chemokine receptors and their cognate ligands can dramatically alter the tumor microenvironment (TME), changing both the composition and function of the cells and leading to resistance to chemotherapy, radiation, and immunotherapy treatment strategies. Understanding and targeting the mechanisms underpinning this resistance is likely to lead to novel treatment options for patients with cancer. Here, we review the importance of chemokine receptors in cancer biology and the data evaluating the targeting of these receptors as anti-neoplastic agents.
Chemokine receptors are critical for recruiting lymphocytes and stromal cells to the site of disease, ensuring the induction of a proper response to any threat. These receptors are also expressed on cancer cells themselves altering their behavior to meet the demands of their environment. There are two major classes of chemokine receptors: typical, which are members of the GPCR superfamily, and atypical, which act through the beta-arrestin pathway, the former being more traditionally associated with inflammation and the latter acting in a regulatory fashion. Each class has been implicated in cancer, with unique effects associated with the individual receptors.
Typical chemokine receptors are further subdivided by structure, differentiated by the number and spacing of cysteine residues. The four classes are CXC, CC, CX3C, and XC, with X being any other amino acid. These receptors are Gα GPCRs consisting of three subunits (α, β, and y) which will change conformation upon binding of the correct chemokine. This causes the Gα and Gyβ subunits to separate, allowing the Gyβ subunit to initiate chemotaxis through either the phospholipase-C-DAG pathway or PI3K signaling. Several other pathways can also be activated upon chemokine binding, such as Ras, ERK, and the MAPK cascade, dramatically altering cell function.
Atypical chemokine receptors are not GPCRs and internalize upon binding to their ligands. They can act to transport chemokines across cellular boundaries, such as in and out of the bloodstream to allow lymphocytes to sample our blood and act accordingly if a pathogen is found. Additionally, these receptors work in concert with typical receptors to coordinate chemotaxis, recycling ligands on the trailing edge and focus signaling to the leading edge [1].
CXC Chemokine Receptors
The CXC (α) family of chemokines is named according to the structural arrangement indicating an amino acid separating the first two N-terminal cysteine residues. Associated CXC ligands play important roles in immune cell recruitment and trafficking among other functions.
CXC Receptor 2 (CXCR2)
CXCR2 is broadly expressed in a range of leukocytes, most notably in polymorphonuclear (PMN) cells. CXCR2 has been implicated in neutrophil trafficking, recruitment, and tumor development under inflammatory conditions [1]. Several agents have now been developed targeting CXCR2 including SX-682 [3•], reparixin [4], danirixin [5], elubrixin [6], and navarixin [7]. While these agents are showing promising anti-inflammatory activity, their anti-neoplastic effects are just beginning to be explored. CXCR2 is potentially a therapeutic target in natural killer (NK) cell–based immunotherapy [8, 9]. CXCR2 inhibitors have demonstrated anti-neoplastic activity in gastric cancer and pancreatic cancer mouse models.
CXC Receptor 3 (CXCR3)
CXCR3 is most notably expressed on activated T cells after activation in lymph nodes; its expression allows effector T cells to traffic to the sight of disease via CXCL9 and CXCL10 gradients. Outside of lymphocytes, however, expression of this chemokine receptor has been found in several cancer types. Recent studies have indicated that CXCR3 plays a role in the metastasis of a variety of tumors. AMG487 is a CXCR3 antagonist that has inhibited tumor metastasis across multiple injectable mouse tumor models, including osteosarcoma [10] and breast cancer [11]. Additionally, mice treated with AMG487 also showed increased levels of CD3 + CD4 + and CD3 + CD8 + cells, suggesting that AMG487 also improves host immune responses [12]. In colon cancer models, AMG487 resulted in a reduction in the number of pulmonary nodules but did not alter the number of liver metastases [13].
CXC Receptor 4 (CXCR4)
CXCR4 is involved in numerous cell signaling pathways, such as enhancing MAPK signaling, AKT signaling, and binding LPS to activate a bacterial immune response [14,15,16]. In cancer, overexpression of CXCR4 has been associated with a worse clinical course [17]. Plerixafor (AMD3100) is a selective CXCR4 antagonist that interferes with the CXCL12-CXCR4 chemokine axis [1]. Plerixafor has shown promise in reducing cell proliferation, migration, and inducing cell apoptosis in various cancer types [17]. Additionally, AMD3100 has been studied as an adjunct therapy to paclitaxel [18]. In combination, AMD3100 and paclitaxel significantly reduced cell proliferation in ID8 and TOV-112D cell lines compared with either drug alone. AMD3100 has also been shown to be a potent radiosensitizer in a triple-negative breast cancer cell line [19].
In a recent phase II clinical trial, the safety and efficacy of a combination treatment of CXCR4 antagonist motixafortide (BL-8040) and pembrolizumab in pancreatic ductal adenocarcinoma (PDAC) were evaluated [20••]. The combination of pembrolizumab and motixafortide results in a disease control rate of 34.5%, including one patient with a partial response in patients with treatment refractory disease. The treatment increased tumor CD8 T cell infiltration, decreased myeloid suppressor cells, and further decreased circulatory regulatory T cells. However, the median overall survival was only 3.3 months. In a second cohort, 22 patients received pembrolizumab, motixafortide, liposomal irinotecan, 5-fluorouracil, and leucovorin. The objective response rate (ORR) was 32% and disease control rate was 77%, which is dramatically improved upon what would be expected for this chemotherapy regimen alone. These are promising results that deserve validation in a randomized studied.
CC Chemokine Receptors
Numerous CC-type chemokine receptors have been implicated in cancer progression, growth, and resistance to treatment, with many acting through a stromal mediator to accomplish these effects. Inhibiting these interactions broadly, and in some cases more specifically, may prove to be beneficial in treating some cancers.
CC Receptor 1 (CCR1)
CCR1 is predominantly expressed on monocytes, T cells, dendritic cells, and neutrophils and can interact with the following ligands: CCL3, CCL5, CCL7, CCL14, CCL8, CCL15, and CCL23. Due to its wide variety of cognate ligands, this receptor has been found to function in several capacities in cancer progression, development, and survival, most notably in metastasis and immune therapy resistance. CCR1 has been implicated as important for metastatic establishment in multiple cancer models [21,22,23,24]. Jung, et al. demonstrated in a breast cancer model that the use of CCX9588, a CCR1 antagonist, was capable of reducing tumor growth and lung metastasis in an orthotopic mouse model [25]. When studied in combination with anti-PD-1 antibodies, synergistic activity was observed with significantly reduced tumor size and metastasis in mice. They also identified that CCX9588 reduced the number of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), a diverse subset of cells from the myeloid lineage that can be strongly immunosuppressive in cancer, possibly allowing for improved T cell function. Together indicate this could be a beneficial combination in subsets of patients with either high CCR1, CCL3, or CCL5 expression in their tumors.
CC Receptor 2 (CCR2)
CCR2 is predominantly associated with monocyte recruitment, which contributes to several different lymphocyte populations heavily involved in tumor growth and maintenance. The most notable of these populations are tumor-associated macrophages (TAMs). The CCL2-CCR2 axis is important for macrophage recruitment in esophageal cancer as TAMs are both important for tumor initiation and T cell suppression [26]. CCR2 or CCL2 knockouts dramatically reduced the incidence of gastric tumors and reduced the number of invading TAMs [26]. CCR2 aids in cell invasiveness through the induction of MMP2 and MMP9 by cancer cells, allowing for invasion and intravasation into circulation [27]. Once there, TAMs and MDSCs are recruited to the site of colonization and aid in cancer cell acclimation and survival [28]. However, along the way, CCR2 expressed on the cancer cells themselves or supporting cells have individual roles crucial for the cells to reach their final destination.
CCR2 also stimulates fibroblasts and MDSCs to traffic into the TME [29, 30]. MDSCs are also implicated in immune therapy resistance in cancer. Flore-Toro et al. report that CCR2 is important for MDSC recruitment in glioblastoma mouse models and that combining the CCR2 antagonist CCX872 with anti-PD-1 antibodies synergized to improve survival of glioma-bearing mice. Functional studies also revealed this dual therapy increased T cell function, with increases in IFNγ and a reduction in CD3 + /PD-1 + /Tim3 + cells [30].
CC Receptor 3 (CCR3)
CCR3 is highly expressed on several immune cell types, including eosinophils, basophils, and Th1 and Th2 CD4s. It is also expressed in airway epithelial cells, with a decent amount of research associating its expression with asthma. It also appears to play an important role in cancer, showing both pro-tumor and anti-tumor functions in many different cancers. Like many of the other chemokine receptors, however, it has several cognate ligands which make CCR3s function very situational.
CC Receptor 4 (CCR4)
CCR4 is a negative prognostic marker in several cancers, including gastric, breast, lung, and renal cells [31]. It is expressed on a variety of cells relevant to cancer biology, such as macrophages and dendritic cells, but its association with CD4 T cells appears to be especially important. It is found on Th2, Th17, and FOXP3 regulatory T cells, all of which have been implicated in immune suppression and cancer development. CCR4 blockade with the Affi-5 antibody in the RENCA mouse model of renal cancer carcinoma increased NK cells and Th1 cytokine levels while reducing the presence of immature myeloid cells [32]. Additional studies have also demonstrated the ability of CCR4 to recruit FOXP3 T regulator cells (T regs) to the site of the tumor [33]. Similarly, inhibiting the CCL17-CCR4 signaling axis with mogamulizumab, a humanized monoclonal CCR4 antibody, in a canine-engrafted mouse model reduced tumor growth and the number of tumor-infiltrating T regs [34]. This agent has now been FDA-approved for the treatment of mycosis fungoides and Sezary disease.
CC Receptor 5 (CCR5)
CCR5 is expressed by many different cell types making its role in cancer complicated. Immunologically, it is necessary for proper CD103 + dendritic cell trafficking within tumors, allowing for the activation and trafficking of CD8 T cells. However, CCR5 is also expressed on cancer cells. Two preclinical studies have shown that blocking CCR5, via the FDA-approved drug maraviroc, can prevent or at the very least reduce the size of metastases, decrease cell motility, and even induce apoptosis [35, 36]. Maraviroc was originally developed for the treatment of HIV, however is now being explored for its potential anti-neoplastic properties.
Clinically, maraviroc has also been studied in metastatic colorectal cancer (CRC) [37]. At the invasive margin of CRC metastases, T cells secrete CCL5 resulting in tumor cell proliferation, invasion, and increased production of matrix metalloproteinases by TAMs. Inhibition of CCL5/CCR5 signaling in CRC tumor explants result in morphologic changes consistent with tumor cell death and mitigation of tumor-promoting cytokines. Further investigation as part of a phase I clinic trial was performed in a total of 14 patients with late-line treatment-refractory metastatic CRC. Pre- and post-treatment biopsies were obtained for pharmacodynamic analyses and demonstrated that maraviroc as a single agent reduced tumor proliferation and reduced certain cytokines and morphologic changes consistent with tumor cell death. As a single agent, no objective responses were seen in these patients and the median PFS was only 1–1.5 months across two cohorts. Interestingly though, 5 patients went on to receive maraviroc in combination with chemotherapy, presumably chemotherapy they had previously been resistant to, and 3 out of these 5 patients had objective partial responses.
CC Receptor 6 (CCR6)
Unlike many of the other chemokine receptors, CCR6 has only one identified ligand, CCL20. It is predominantly expressed on immature DCs and several subsets of T cells and directs these cell subsets to the site of inflammation in skin epithelial and mucosal tissues. Also, CCR6 is expressed on cancer cells as well, aiding in survival and metastasis. As with other chemokine receptors, CCR6 appears to be associated with the severity of several cancers, correlating with more severe disease, playing a role in EMT transitions, and increasing metastatic potential [38,39,40,41].
CC Receptor 7 (CCR7)
Dendritic cell uptake of antigens from the site of infection/inflammation, their subsequent trafficking to a neighboring lymph node, and presentation to naïve T cells are essential to the initiation of an adaptive immune response. CCR7 appears to be essential at multiple points in this process, showing an association with changes in cytoarchitecture and antigen endocytosis, a role in presentation to T cells, migration speed, and even survival [42]. It has also been found that CCR7 is essential in the overall process of lymph node migration for DCs [43, 44]. This brings us to how this receptor can aid in cancer development. Numerous publications have found CCR7 expression on cancer cells, many of which showing its presence associates with higher levels of metastasis [45,46,47]. A reduction in CCR7 has been shown to decrease cell migration, viability, and reduced several EMT markers [48,49,50]. Navarixin, a CXCR2 and CCR7 antagonist, is being evaluated clinically as a treatment for prostate cancer, and a combination of navarixin and pembrolizumab in patients with advanced/metastatic solid tumors is now underway (NCT03473925).
CC Receptor 8 (CCR8)
There are several important functions CCR8. In the setting of cancer, its primary role is in the trafficking of T regs to the TME. CCR8 is an important functional marker for tissue-resident FOXP3 T regs, with its expression on circulating T regs being much lower [51, 52]. In an allograft CT26 CRC model, a monoclonal antibody against CCR8 resulted in a reduction in tumor volume, higher frequencies of IFNγ + CD8 and CD4 T cells, and lower frequency of FOXP3 + T regs [53•]. Additionally, synergistic potential was observed when CCR8 blockade was paired with a listeria-based vaccine expressing an antigen derived from CT26 tumors [53•].
Several CCR8 antagonists have been created, such as the oral CCR8 antagonist AZ084 or the neutralizing antibody JTX-1811; however, it may also be relevant to consider blocking CCL-1 or CCL-18. CCL-1 is released from conventional T regs to increase the functional state of driver T regs, increasing the expression of IL-10, FOXP3, CD39, Granzyme B, and CCR8. CCL-18 is produced when IL-10 producing T regs acts on tumor-associated macrophages (which themselves also produce IL-10, which then circles back to further increase suppressive potential upon binding of CCR8 + T regs) [54,55,56].
CC Receptor 9 (CCR9)
CCR9 is predominantly found on naïve lymphocytes and GI tissues with prominent roles in thymocyte trafficking and differentiation, specifically for T cells, DCs, and macrophages [57], as well as proper immune responses in the small intestine [58]. CCR9 expression has also been found in several cancers, with involvement in several signaling pathways such as the PI3K-AKT pathway, the PI3K-B-Cat pathway, and STAT signaling [59]. All these pathways are crucial in several ways to cancer growth, invasiveness, and immune modulation. One approach was to block CCR9 signaling with a monoclonal antibody, 91R, in mouse leukemia xenografts, noting a significant reduction (85%) in tumor volume. The primary source of cell death was found to be associated with complement-dependent cytotoxicity, with their antibody showing a strong ability to induce complement-dependent cell lysis [60, 61].
CC Receptor 10 (CCR10)
CCR10 is important for the induction of a proper immune response in both skin and mucosal epithelium; however, the type of cell recruited to each of these tissues via CCR10 depends on the chemokine that is released. CCR10 has two cognate ligands, CCL28 and CCL27, which are expressed in mucosal tissues and skin epithelium, respectively. Mucosal-expressed CCL28 recruits IgA secreting plasma cells expressing CCR10 and the proper adhesion molecules to associate with gut epithelium. CCR10 + CLA + (cutaneous lymphocyte-associated antigen) CD8 + T cells however lack mucosal integrins and therefore cannot traffic via this axis and are instead attracted to skin epithelium via CCL27 secretion [62].
CCR10’s ability to either act in a pro-tumor or anti-tumor fashion is highly context specific. CCR10 is critical for proper T cell trafficking in skin epithelium via CCL27 so any attempt to block this pathway could dramatically favor tumor immune escape, a mechanism identified in skin cancer [63]. In a study using myeloma cells, however, CCL27-driven CCR10 signaling can induce drug resistance via IL-10 secretion by CCR10 + stromal cells [64]. Gastric cancer also benefits from CCR10 signaling, as beta-catenin-induced CCL28 expression correlated with increased tumor growth and T reg infiltration [65•]. In addition to immune-modulatory properties, CCR10 has been found to affect cancer cell invasiveness. This includes the attraction of lymphatic endothelial cells to the sight of the tumor via CCL27 and CCL28 expression by tumor cells [66]. CCR10 also appears to aid in breast cancer metastasis and avoidance of apoptosis induction via MAPK signaling [67]. In liver cancer, CCR10 was found to be important for inflammation-driven carcinogenesis, with high levels of TNF, CCR10, and CCL28 expression found in tetrachloromethane- and diethylnitrosamine-induced models of liver cancer. In these models, CCL28-CCR10 signaling led to increases in PI3K-AKT signaling, with a CCR10 knockout model showing a significant reduction in tumor formation and growth in-vivo. Akt inhibition was also found to reduce cell growth in vitro, offering a possible alternate route of inhibition [68]. There is however an exception to these findings, with CCL28-CCR10 signaling increasing the expression of RARβ in oral squamous cell carcinoma (OSCC) and a concurrent decrease in bone invasion. High levels of CCL28, CCR10, and RARβ in OSCC patients correlated with overall better survival and lower levels of bone invasion [69].
CX3C Chemokine Receptors
CX3CR1 is a chemokine receptor that has been shown to support tumorigenesis across multiple cancer types. In pancreatic cancer, overexpression of CX3CR1 in VCaP and PC-3 cells caused an increase in cellular proliferation and a decrease in cellular apoptosis [70]. In prostate cancer models, overexpressing CX3CR1 facilitated spine metastasis formation [71]. In breast cancer, CX3CL1 and CX3CR1 did not affect breast cancer cell proliferation but did promote the migration and invasion of CX3CR1-expressing MDA-MB-231 cells [72]. In osteosarcoma, MiR-485-5p inhibited metastasis and proliferation by targeting the CX3CL1/CX3CR1 axis [73]. CX3CR1/CX3CL1 axis also increases cell migration and invasion in gastric cancer [74]. Transwell migration and invasion assays showed that cells overexpressing CX3CR1 had a significant increase in migration and invasiveness. The group also showed the CX3CR1/CX3CL1 axis promotes gastric cancer cell proliferation and survival via activation of AKT. In a conflicting study, CX3CR1 expression correlated with a better patient prognosis by increasing the number of CD8 + T cells and natural killer cells [75].
Atypical Chemokine Receptors
ACKR1
The expression of ACKR1, also known as the Duffy antigen receptor for chemokines (DARC), has shown antitumor effects across multiple studies. ACKR1 expression inhibits tumorigenesis and metastasis in breast, prostate, and lung cancer models by interfering with angiogenesis [76,77,78]. A drug targeting ACKR1 expression has yet to be discovered, but manipulating its expression would seems to be promising in treating multiple cancer types.
ACKR2
Atypical chemokine receptor 2, also known as D6, is thought to slow tumor progression in various cancer types. In Kaposi sarcoma, forced ACKR2 expression reduced tumor size in vivo [79]. The reason for this reduced tumor size was shown to be a reduction in both M2-like TAMs and inflammatory cytokines CCL2, CCL3, and CCL5. These results were replicated in breast cancer as well [80]. Additionally, breast cancer samples an inverse correlation between ACKR2 expression and lymph node metastasis was found [80]. A protective role for ACKR2 was also observed in non-small cell lung cancer [81]. ACKR2 expression reduced cell proliferation by enhancing the clearance of chemokines CCL2, CCL4, and CCL5 and resulted in a 40% decrease in tumor volume over 40 days in mice transfected with A549 lung cancer cells constitutively expressing ACKR2 compared to mice that were not [81].
ACKR3
Unlike the other atypical chemokine receptors, ACKR3, also known as CXCR7, has been shown to promote tumorigenesis. High ACKR3 expression in glioblastoma patients is often associated with poor patient prognosis [82]. Using an antibody targeting ACKR3 (X7Ab) in combination with temozolomide extended survival in vivo in a GBM mouse model [82]. CCX771, a ACKR3 antagonist, negates many of the tumor-causing effects of ACKR3, including reduced blood vessel formation in vivo [83]. ACKR3 has been shown to increase survival, adhesiveness, and invasiveness of prostate cancer cells [84]. The combination of enzalutamide and the ACKR3 inhibitor CCX771 led to a reduction in EGFR and AKT phosphorylation and inhibition of prostate cancer tumor models [85].
ACKR4
Although less studied than the other atypical chemokine receptors, multiple studies have shown a protective role for ACKR 4, also known as CCX-CKR or CCRL1. In breast cancer, ACKR4 expression inhibited cell proliferation in vitro [86]. In vivo, ACKR4-transfected mice had significantly reduced tumor volumes and the total number of lung metastases compared to mock-transfected mice. In human breast cancer patients, ACKR4 expression had a significant negative correlation with lymph node metastasis and a positive correlation with patient survival [86]. Similar findings have also been observed in hepatocellular carcinoma [87].
Discussion
Chemokine receptors and their cognate ligands play a critical role in determining the makeup of the tumor microenvironment through their ability to recruit a myriad of immune and stromal cell populations to the site of a tumor. They can also dramatically alter proliferation as well as mobility, the latter implicating them as having a role in metastasis. Understanding which cell types each chemokine receptor is expressed on, its role or function in different settings, and identifying TME changes upon inhibition is crucial for understanding their place in cancer treatment.
Throughout this review, several drugs were named that have shown promising effects, such as preventing or reducing immunosuppressive cells from entering the TME, lessening the metastatic potential of tumor cells, and in some cases even reducing tumor cell viability. With this information, we can identify chemokine antagonists that would pair well with current treatment modalities, possibly improving responses in patients. For example, many immunotherapies are thought to fail because solid tumors are plagued with having immunosuppressive TMEs. Several drugs in this review showed the ability to prevent the migration of several immunosuppressive cell subsets and in vivo have shown synergistic ability when paired with immunotherapies.
Conclusion
Taking current drug combinations to clinical trials is the next logical step but several other chemokine receptors do not have drugs available for treatment in patients. Identifying which chemokine receptor inhibitors are most likely to improve outcomes for patients, by either slowing disease progression or improving treatment responses to current therapies, should be the focus moving forward.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sokol, Caroline L., Zamaneh Mikhak, and Andrew D. Luster. “Chemokines.” In Middleton’s allergy: principles and practice, 9th ed., 95–109.e1. Philadelphia, PA, 2020. https://www-clinicalkey-com.ezproxy.library.wisc.edu/#!/content/book/3-s2.0-B9780323544245000071?scrollTo=%23hl0000619. Accessed 2 Feb 2021.
Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. “Immunological decision-making: how does the immune system decide to mount a helper T-cell response?” Immunology. 2008;123(3):326–38. https://doi.org/10.1111/j.1365-2567.2007.02719.x.
• Greene S, Robbins Y, Mydlarz W, Huynh A, Schmitt N, Friedman J, Horn L, Palena C, et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clin Cancer Res. March 2020;26(6):1420–31. https://doi.org/10.1158/1078-0432.CCR-19-2625.. (NK cell-based immunotherapies are an exciting treatment prospect for patients. This study identifies CXCR1/2 as a target for enhancing these therapies.)
Wang J, Hu W, Wang K, Yu J, Luo B, Luo G, Wang W, Wang H, et al. Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. Int J Oncol. February 2016;48:1341–52. https://doi.org/10.3892/ijo.2016.3371.
Lazaar AL, Miller BE, Tabberer M, Yonchuk J, Leidy N, Ambery C, Bloomer J, et al. Effect of the CXCR2 antagonist danirixin on symptoms and health status in COPD. Eur Respir J. 2018;52:1801020. https://doi.org/10.1183/13993003.01020-2018.
Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, Tal-Singer R, Lazaar AL. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J Cyst Fibros. September 2012;12(3):241–8. https://doi.org/10.1016/j.jcf.2012.08.016.
Hastrup N, Khalilieh S, Dale DC, Hanson LG, Magnusson P, Tzontcheva A, Tseng J, Huyck S, et al. The effects of the CXCR2 antagonist, MK-7123, on bone marrow functions in healthy subjects. Cytokine. 2015;72(2):197–203. https://doi.org/10.1016/j.cyto.2015.01.002.
Greene S, Robbins Y, Mydlarz W, Huynh A, Schmitt N, Friedman J, Horn L, Palena C, et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clin Cancer Res. March 2020;26(6):1420–31. https://doi.org/10.1158/1078-0432.CCR-19-2625.
Ijichi H, Chytil A, Agnieszka GE, Aakre ME, Bierie B, Tada M, Mohri D, Miyabayashi K, et al. Inhibiting CXCR2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Investig. October 2011;121(10):4106–17. https://doi.org/10.1172/JCI42754.
Pradelli E, Karimdjee-Soilihi B, Michiels JF, Ricci JE, Millet MA, Vandenbos F, Sullivan TJ, Collins TL, Johnson MG, Medina JC, Kleinerman ES, Schmid-Alliana A, Schmid-Antomarchi H. Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. Int J Cancer. December 2009;125(11):2586–94. https://doi.org/10.1002/ijc.24665.
Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Can Res. August 2006;66(15):7701–7. https://doi.org/10.1158/0008-5472.CAN-06-0709.
Zhu G, Yan HH, Pang Y, Jian J, Achyut BR, Liang X, Weiss JM, Wiltrout RH, Hollander MC, Yang L. CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumor cell migration and promotion of host anti-tumor immunity. Oncotarget. 2015;6(41):43408–19. https://doi.org/10.18632/oncotarget.6125.
Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, Martini V, Birnbaum D, Scoazec JY, Abello J, Al Saati T, Johnson MG, Sullivan TJ, Medina JC, Collins TL, Schmid-Alliana A, Schmid-Antomarchi H. Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br J Cancer. June 2009;100(11):1755–64. https://doi.org/10.1038/sj.bjc.6605078.
Cao Y, Hunter ZR, Liu X, Xu L, Yang G, Chen J, Patterson CJ, et al. “The WHIM-like CXCR4S338X somatic mutation activates AKT and ERK, and promotes resistance to ibrutinib and other agents used in the treatment of Waldenstrom’s macroglobulinemia.” Leukemia. 2015;29(1):169–76. https://doi.org/10.1038/leu.2014.187.
“CXCR4-Lo: molecular cloning and functional expression of a novel human CXCR4 splice variant.” Accessed February 8, 2021. https://www.uniprot.org/citations/10452968.
Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. April 2001;2(4):338–45. https://doi.org/10.1038/86342.
Mao TL, Fan KF, Liu CL. Targeting the CXCR4/CXCL12 axis in treating epithelial ovarian cancer. Gene Ther. September 2017;24:621–9. https://doi.org/10.1038/gt.2017.69.
Reeves PM, Abbaslou MA, Kools FRW, Poznansky MC. CXCR4 blockade with AMD3100 enhances taxol chemotherapy to limit ovarian cancer cell growth. Anticancer Drugs. October 2017;28(9):935–42. https://doi.org/10.1097/CAD.0000000000000518.
Zhou KX, Xie LH, Peng X, Guo QM, Wu QY, Wang WH, Zhang GL, Wu JF, et al. CXCR4 antagonist AMD3100 enhances the response of MDA-MB-231 triple-negative breast cancer cells to ionizing radiation. Cancer Lett. April 2018;418:196–203. https://doi.org/10.1016/j.canlet.2018.01.009.
•• Bockorny B, Semenisty V, Macarulla T, Erkut B, Wolpin BM, Stemmer SM, Golan T, Geva R, et al. “BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT Trial. Nat Med. June 2020;26:878–85. https://doi.org/10.1038/s41591-020-0880-x.T.. (his report outlines promising results using CXCR4 blockade in combination regimens.)
Dairaghi DJ, Babatunde OO, Gupta A, McCluskey B, Miao S, Powers JP, Seitz LC, et al. “CCR1 blockade reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease.” Blood. 2012;120(7):1449–57. https://doi.org/10.1182/blood-2011-10-384784.
Kitamura T, Teruaki F, Pius L, Laszlo R, Hiroki H, Kizaka-Kondoh S, Masahiro A, Makoto MT. “Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model.” Proc Natl Acad Sci. 9 2010;107(2):13063–8. https://doi.org/10.1073/pnas.1002372107.
Kiyasu Y, Kenji K, Hideyo H, Ryotaro O, Keita H, Hideyuki M, Gen N, et al. “Disruption of CCR1-mediated myeloid cell accumulation suppresses colorectal cancer progression in mice.” Cancer Lett. 2020;487:53–62. https://doi.org/10.1016/j.canlet.2020.05.028.
Kitamura T, Bin-Zhi Q, Soong D, Luca C, Noy R, Sugano G, Yu K, Li J, Pollard JW. “CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages.” J Exp Med. 2015;212(7):1043–59. https://doi.org/10.1084/jem.20141836.
Jung H, Bischof A, Ebsworth K, Ertl L, Schall T, Israel C. “Combination therapy of chemokine receptor inhibition plus PDL-1 blockade potentiates anti-tumor effects in a murine model of breast cancer.” J Immuno Ther Cancer. 2015;3(2):P227. https://doi.org/10.1186/2051-1426-3-S2-P227.
Yang H, Qiannan Z, Miao X, Lei W, Xuewei C, Yongquan F, Yongning L, Xin Z, Wenming C, Xudong J. “CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis.” Mol Cancer. 2020;19(1):41. https://doi.org/10.1186/s12943-020-01165-x.
Lim SY, Arseniy EY, Gordon-Weeks AN, Muschel RJ. “Targeting the CCL2-CCR2 signaling axis in cancer metastasis.” Oncotarget. 2016;7(19):28697. https://doi.org/10.18632/oncotarget.7376.
Wolf, Monika Julia, Alexandra Hoos, Judith Bauer, Steffen Boettcher, Markus Knust, Achim Weber, Nicole Simonavicius, et al. “Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and P38MAPK pathway.” Cancer Cell 22, no. 1 (July 2012): 91–105. https://doi.org/10.1016/j.ccr.2012.05.023.
Brummer G, Acevedo DS, Qingting H, Portsche M, Bin Fang W, Yao M, Zinda B, et al. “Chemokine signaling facilitates early-stage breast cancer survival and invasion through fibroblast-dependent mechanisms.” Mol Cancer Res. 2018;16(2):296–308. https://doi.org/10.1158/1541-7786.MCR-17-0308.
Flores-Toro J, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Israel FC, Rajinder S, et al. “CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas.” Proc Natl Acad Sci. 2020;117(2):1129–38. https://doi.org/10.1073/pnas.1910856117.
Karasaki T, Guangliang Q, Masaki A, Yanbin S, Aya S-U, Eiichi S, Kosuke K, et al. “High CCR4 expression in the tumor microenvironment is a poor prognostic indicator in lung adenocarcinoma.” J Thorac Dis. 2018;10(8):4741–50. https://doi.org/10.21037/jtd.2018.07.45.
Berlato C, Moddasar NK, Tiziana S, Thompson R, Maniati E, Montfort A, Jangani M, et al. “A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer.” J Clin Invest. n.d.;127(3):801–13. https://doi.org/10.1172/JCI82976.
Sun W, Wei-Jin L, Fan-Qin W, Thian-Sze W, Wen-Bin L, Xiao-Lin Z, Jian L, Wei-Ping W. Blockade of MCP-1/CCR4 signaling-induced recruitment of activated regulatory cells evokes an antitumor immune response in head and neck squamous cell carcinoma. Oncotarget. 2016;7(25):37714–27. https://doi.org/10.18632/oncotarget.9265.
Maeda S, Kohei M, Akiko I, Tomohiro Y, Naoaki M. “CCR4 blockade depletes regulatory T cells and prolongs survival in a canine model of bladder cancer.” Cancer Immunol Res. 2019;7(7):1175–87. https://doi.org/10.1158/2326-6066.CIR-18-0751.
Velasco-Velazquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, Pestell RG. “CCR5 antagonist blocks metastasis of basal breast cancer cells.” Cancer Res. 2012;72(15):3839–50. https://doi.org/10.1158/0008-5472.CAN-11-3917.
Pervaiz A, Michael Z, Saqib M, Doaa MA, Berger MR, Hassan A, et al. Cellular Oncol. 2019;42(1):93–106. https://doi.org/10.1007/s13402-018-0415-3.
Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 2016;29:587–601. https://doi.org/10.1016/j.ccell.2016.03.005.
Rubie C, Oliveira Frick V, Ghadjar P, Wagner M, Grimm H, Vicinus B, Justinger C, Graeber S, Schilling MK. “CCL20/CCR6 expression profile in pancreatic cancer.” J Transl Med. 2010;8(1):45. https://doi.org/10.1186/1479-5876-8-45.
Zhang X-P, Zhi-Juan Hu, Ai-Hong M, Guo-Chen D, Qing-Tao Z, Jing Y. “Role of CCL20/CCR6 and the ERK signaling pathway in lung adenocarcinoma.” Oncology Lett. 2017;14(6):8183–9. https://doi.org/10.3892/ol.2017.7253.
Kapur N, Hina M, Clarence E, Clark I, Uma K, Derrick JB, James WL, Shailesh S. “CCR6 expression in colon cancer is associated with advanced disease and supports epithelial-to-mesenchymal transition.” Br J Cancer. 2016;114(12):1343–51. https://doi.org/10.1038/bjc.2016.113.
Liu J, Fang K, Zhenyao X, Zhaoyuan L, Lingyun Z, Sha Y, Zhe W, Hong W, Honglin W. “CCR6 is a prognostic marker for overall survival in patients with colorectal cancer, and its overexpression enhances metastasis in vivo.” Plos One. 2014;9(6):e101137. https://doi.org/10.1371/journal.pone.0101137.
Sánchez-Sánchez N, Lorena R-B, Rodríguez-Fernández JL. “The multiple personalities of the chemokine receptor CCR7 in dendritic cells.” J Immunol. 2006;176(9):5153–9. https://doi.org/10.4049/jimmunol.176.9.5153.
Jang MH, Nagako S, Toshiyuki T, Takako H, Takachika H, Kazuo T, Zijin G, et al. “CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes.” J Immunol. 2006;176(2):803–10. https://doi.org/10.4049/jimmunol.176.2.803.
Hirao M, Nobuyuki O, Kazumasa H, Watkins SC, Kouji M, Robbins PD, Lotze MT, Hideaki T. “CC chemokine receptor-7 on dendritic cells is induced after interaction with apoptotic tumor cells: critical role in migration from the tumor site to draining lymph nodes.” Cancer Res. 2000;60(8):2209–17.
Tutunea-Fatan E, Mousumi M, Xiping X, Peeyush KL. “The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis.” Mol Cancer. 2015;14(1):35. https://doi.org/10.1186/s12943-015-0306-4.
Zhou S, Shuchang X, Huihong T, Zhiwei Z, Guolin C, Zhiqiang Z, Yaoqin Y. “CCR7 expression and intratumoral FOXP3+ regulatory T cells are correlated with overall survival and lymph node metastasis in gastric cancer.” Plos One. 2013;8(9):e74430. https://doi.org/10.1371/journal.pone.0074430.
An S, Karthik T, Ying W, Ligeng X, Mengying H, Jingjing L, Wantong S, et al. “Locally trapping the C-C chemokine receptor type 7 by gene delivery nanoparticle inhibits lymphatic metastasis prior to tumor resection.” Small. 2019;15(9):1805182. https://doi.org/10.1002/smll.201805182.
Xu B, Minjie Z, Wencai Q, Jueming Y, Qiming F. “CCR7 mediates human breast cancer cell invasion, migration by inducing epithelial–mesenchymal transition and suppressing apoptosis through AKT pathway.” Cancer Med. 2017;6(5):1062–71. https://doi.org/10.1002/cam4.1039.
Zhang L, Xuyang X, Hui A, Jian W, Yanmei M, Yi-Hua Q. “Inhibition of CCR7 promotes NF-ΚB-dependent apoptosis and suppresses epithelial-mesenchymal transition in non-small cell lung cancer.” Oncology Rep. 2017;37(5):2913–9. https://doi.org/10.3892/or.2017.5524.
Kobayashi D, Masataka E, Hirotaka O, Hironobu H, Masayuki M, Haruko H. “Regulation of CCR7-dependent cell migration through CCR7 homodimer formation.” Sci Rep. 2017;7(1):8536. https://doi.org/10.1038/s41598-017-09113-4.
Yano H, Lawrence PA, Workman CJ, Vignali Dario AA. “Intratumoral regulatory T cells: markers, subsets and their impact on anti-tumor immunity.” Immunol. 2019;157(3):232–47. https://doi.org/10.1111/imm.13067.
Plitas G, Konopacki C, Kenmin Wu, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. November 2016;45(5):1122–34. https://doi.org/10.1016/j.immuni.2016.10.032.
• Villarreal, Daniel O., Andrew L’Huillier, Susan Armington, Cristina Mottershead, Elena V. Filippova, Brandon D. Coder, Robert G. Petit, and Michael F. Princiotta. “Targeting CCR8 induces protective antitumor immunity and enhances vaccine-induced responses in colon cancer.” Cancer Research 78, no. 18 (September 15, 2018): 5340–48. https://doi.org/10.1158/0008-5472.CAN-18-1119. (This study highlights the ability of chemokine antagonism to shift the immune landscape of a tumor to be more amenable to immunotherapies.)
Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. STEM CELLS. 2013;31(9):1980–91. https://doi.org/10.1002/stem.1432.
Ge, Xiaoxu, Yamei Zhao, Chao Chen, Jian Wang, and Lifeng Sun. “<p>Cancer immunotherapies targeting tumor-associated regulatory T cells</P>.” OncoTargets and Therapy. Dove Press, December 13, 2019. https://doi.org/10.2147/OTT.S231052.
Hoelzinger DB, Shannon ES, Noweeda M, Dominguez AL, Manrique SZ, Lustgarten J. “Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses.” J Immunol. 2010;184(12):6833–42. https://doi.org/10.4049/jimmunol.0904084.
Wang C, Zhenghuan L, Zhihui X, Xian W, Dongyang Z, Ziqi Z, Jianqin W. “The role of chemokine receptor 9/chemokine ligand 25 signaling: from immune cells to cancer cells (review).” Oncology Lett. 2018;16(2):2071–7. https://doi.org/10.3892/ol.2018.8896.
Kunkel EJ, James JC, Guttorm H, Junliang P, Boisvert J, Roberts AI, Ebert EC, et al. “Lymphocyte Cc chemokine receptor 9 and epithelial thymus-expressed chemokine (Teck) expression distinguish the small intestinal immune compartment.” J Exp Med. 2000;192(5):761–8.
Tu Z, Ruijing X, Jie X, Kingsley MT, Xinzhou D, Meng X, Pan L, Meng W, Qiuping Z. “CCR9 in cancer: oncogenic role and therapeutic targeting.” J Hematol Oncol. 2016;9(1):10. https://doi.org/10.1186/s13045-016-0236-7.
Chamorro S, Vela M, Franco-Villanueva A, Carramolino L, Gutiérrez J, Gómez L, Lozano M, et al. “Antitumor effects of a monoclonal antibody to human CCR9 in leukemia cell xenografts.” MAbs. 2014;6(4):1000–12. https://doi.org/10.4161/mabs.29063.
Somovilla-Crespo, Beatriz, Maria Teresa Martín Monzón, Maria Vela, Isabel Corraliza-Gorjón, Silvia Santamaria, Jose A. Garcia-Sanz, and Leonor Kremer. “92R monoclonal antibody inhibits human CCR9+ leukemia cells growth in NSG mice xenografts.” Frontiers in Immunology 9 (2018). https://doi.org/10.3389/fimmu.2018.00077.
Kogan, Avi N, and Ulrich H von Andrian. “Chapter 10 - Lymphocyte Trafficking.” In Microcirculation (Second Edition), edited by Ronald F. Tuma, Walter N. Durán, and Klaus Ley, 449–82. San Diego: Academic Press, 2008. https://doi.org/10.1016/B978-0-12-374530-9.00012-7.
Pivarcsi A, Müller A, Hippe A, Rieker J, van Lierop A, Steinhoff M, Seeliger S, et al. “Tumor immune escape by the loss of homeostatic chemokine expression.” Proc Natl Acad Sci. 2007;104(48):19055–60. https://doi.org/10.1073/pnas.0705673104.
Thangavadivel S, Zelle-Rieser C, Olivier A, Postert B, Untergasser G, Kern J, Brunner A, et al. “CCR10/CCL27 crosstalk contributes to failure of proteasome-inhibitors in multiple myeloma.” Oncotarget. 2016;7(48):78605–18. https://doi.org/10.18632/oncotarget.12522.
• Ji, Lu, Wei Qian, Liming Gui, Zhongzhong Ji, Pan Yin, Guan Ning Lin, You Wang, Bin Ma, and Wei-Qiang Gao. “Blockade of β-catenin–induced CCL28 suppresses gastric cancer progression via inhibition of Treg cell infiltration.” Cancer Research 80, no. 10 (May 15, 2020): 2004–16. https://doi.org/10.1158/0008-5472.CAN-19-3074. (This study demonstrates that chemokine antagonism can be achieved indirectly, allowing for more unique approaches to treatment.)
Karnezis T, Farnsworth RH, Harris NC, Williams SP, Caesar C, Byrne DJ, Herle P, et al. “CCL27/CCL28–CCR10 chemokine signaling mediates migration of lymphatic endothelial cells.” Cancer Res. 2019;79(7):1558–72. https://doi.org/10.1158/0008-5472.CAN-18-1858.
Yang XL, Kai YL, Feng JL, Hui MS, Zhou LO. “CCL28 promotes breast cancer growth and metastasis through MAPK-mediated cellular anti-apoptosis and pro-metastasis.” Oncology Rep. 2017;38(3):1393–401. https://doi.org/10.3892/or.2017.5798.
Wu Q, Jin-xian C, Yu C, Li-li C, Xiao-zhong W, Wu-hua G, Jian-feng Z. “The chemokine receptor CCR10 promotes inflammation-driven hepatocarcinogenesis via PI3K/Akt pathway activation.” Cell Death Dis. 2018;9(2):1–18. https://doi.org/10.1038/s41419-018-0267-9.
Park J, Xianglan Z, Sun KL, Na-Young S, Seung S, Ki RK, Jae Hoon S, Kwang-Kyun P, Won-Yoon C. CCL28-induced RARβ expression inhibits oral squamous cell carcinoma bone invasion. J Clin Investigation. 2019;129(12):5381–99. https://doi.org/10.1172/JCI125336.
Liu P, Liang Y, Jiang L, Wang H, Wang S, Dong J. CX3CL1/fractalkine enhances prostate cancer spinal metastasis by activating the Src/FAK pathway. Int J Oncol. July 2018;53(4):1544–56. https://doi.org/10.3892/ijo.2018.4487.
Tang J, Xiao L, Cui R, Li D, Zheng X, Zhu L, Sun H, Pan Y, Du Y, Yu X. CX3CL1 increases invasiveness and metastasis by promoting epithelial-to-mesenchymal transition through the TACE/TGF-α/EGFR pathway in hypoxic androgen-independent prostate cancer cells. Oncol Rep. 2016;35(2):1153–62. https://doi.org/10.3892/or.2015.4470.
Liang Y, Yi L, Liu P, Jiang L, Wang H, Annan Hu, Sun C, Dong J. CX3CL1 involves in breast cancer metastasizing to the spine via the Src/FAK signaling pathway. J Cancer. September 2018;9(19):3603–12. https://doi.org/10.7150/jca.26497.
Wang FR, Xu SH, Wang BM, Wang F. “MiR-485–5p inhibits metastasis and proliferation of osteosarcoma by targeting CX3CL1.” Eur Rev Med Pharmacol Sci. 2018;22(21):7197–204. https://doi.org/10.26355/eurrev_201811_16253.
Wei LM, Cao S, Yu WD, Liu YL, Wang JT. Overexpression of CX3CR1 is associated with cellular metastasis, proliferation and survival in gastric cancer. Oncol Rep. February 2015;33(2):615–24. https://doi.org/10.3892/or.2014.3645.
Hyakudomi M, Matsubara T, Hyakudomi R, Yamamoto T, Kinugasa S, Yamanoi A, Maruyama R, Tanaka T. Increased expression of fractalkine is correlated with a better prognosis and an increased number of both CD8+ T cells and natural killer cells in gastric adenocarcinoma. Ann Surg Oncol. June 2008;15(6):1775–82. https://doi.org/10.1245/s10434-008-9876-3.
Wang J, Ou ZL, Hou YF, Luo JM, Shen ZZ, Ding J, Shao ZM. “Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential.” Oncogene. 2006;25(54):7201–11. https://doi.org/10.1038/sj.onc.1209703.
Shen H, Schuster R, Stringer KF, Waltz SE, Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) regulates prostate tumor growth. FASEB J. January 2006;20(1):59–64. https://doi.org/10.1096/fj.05-4764com.
Addison, C. L., Belperio, J. A., Burdick, M. D., and Strieter, R. M. “Overexpression of the duffy antigen receptor for chemokines (DARC) by NSCLC tumor cells results in increased tumor necrosis.” BMC Cancer 4, no. 28 (June 2004). https://doi.org/10.1186/1471-2407-4-28.
Savino B, Caronni N, Anselmo A, Pasqualini F, Borroni EM, Basso G, Celesti G, Laghi L, Tourlaki A, Boneschi V, Brambilla L, Nebuloni M, Vago G, Mantovani A, Locati M, Bonecchi R. ERK-dependent downregulation of the atypical chemokine receptor D6 drives tumor aggressiveness in Kaposi sarcoma. Cancer Immunol Res. July 2014;2(7):679–89. https://doi.org/10.1158/2326-6066.CIR-13-0202.
Wu FY, Ou ZL, Feng LY, Luo JM, Wang LP, Shen ZZ, Shao ZM. Chemokine decoy receptor d6 plays a negative role in human breast cancer. Mol Cancer Res. August 2008;6(8):1276–88. https://doi.org/10.1158/1541-7786.MCR-07-2108.
Wu FY, Fan J, Tang L, Zhao YM, Zhou CC. Atypical chemokine receptor D6 inhibits human non-small cell lung cancer growth by sequestration of chemokines. Oncol Lett. July 2013;6(1):91–5. https://doi.org/10.3892/ol.2013.1358.
Salazar N, Carlson JC, Huang K, Zheng Y, Oderup C, Gross J, Jang AD, Burke TM, Lewén S, Scholz A, Huang S, Nease L, Kosek J, Mittelbronn M, Butcher EC, Tu H, Zabel BA. A chimeric antibody against ACKR3/CXCR7 in combination with TMZ activates immune responses and extends survival in mouse GBM models. Molecular Therapy: The Journal of the American Society of Gene Therapy. May 2018;26(5):1354–65. https://doi.org/10.1016/j.ymthe.2018.02.030.
Qian T, Liu Y, Dong Y, Zhang L, Dong Y, Sun Y, Sun D. CXCR7 regulates breast tumor metastasis and angiogenesis in vivo and in vitro. Mol Med Rep. March 2018;17(3):3633–9. https://doi.org/10.3892/mmr.2017.8286.
Luo Y, Azad AK, Karanika S, Basourakos SP, Zuo X, Wang J, Yang L, Yang G, Korentzelos D, Yin J, Park S, Zhang P, Campbell JJ, Schall TJ, Cao G, Li L, Thompson TC. Enzalutamide and CXCR7 inhibitor combination treatment suppresses cell growth and angiogenic signaling in castration-resistant prostate cancer models. Int J Cancer. May 2018;142(10):2163–74. https://doi.org/10.1002/ijc.31237.
Rafiei S, Gui B, Wu J, Liu XS, Kibel AS, Jia L. Targeting the MIF/CXCR7/AKT signaling pathway in castration-resistant prostate cancer. Mol Cancer Res. September 2017;17(1):263–76. https://doi.org/10.1158/1541-7786.MCR-18-0412.
Feng LY, Ou ZL, Wu FY, Shen ZZ, Shao ZM. Involvement of a novel chemokine decoy receptor CCX-CKR in breast cancer growth, metastasis and patient survival. Clin Cancer Res. May 2009;15(9):2962–70. https://doi.org/10.1158/1078-0432.CCR-08-2495.
Shi JY, Yang LX, Wang ZC, Wang LY, Zhou J, Wang XY, Shi GM, Ding ZB, Ke AW, Dai Z, Qiu SJ, Tang QQ, Gao Q, Fan J. CC chemokine receptor-like 1 functions as a tumour suppressor by impairing CCR7-related chemotaxis in hepatocellular carcinoma. J Pathol. March 2015;235(4):546–58. https://doi.org/10.1002/path.4450.
Funding
This project was supported by Funk Out Cancer and the University of Wisconsin Carbone Cancer Center (P30 CA014520).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sean Kraus declares that he has no conflict of interest.
Thomas Kolman declares that he has no conflict of interest.
Austin Yeung declares that he has no conflict of interest.
Dustin Deming has received research funding from Bristol-Myers Squibb and Genentech, and has received compensation from Bristol-Myers Squibb, Genentech, and Merck for service as a consultant.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Evolving Therapies
Rights and permissions
About this article
Cite this article
Kraus, S., Kolman, T., Yeung, A. et al. Chemokine Receptor Antagonists: Role in Oncology. Curr Oncol Rep 23, 131 (2021). https://doi.org/10.1007/s11912-021-01117-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01117-8