Abstract
Purpose of Review
Management of parapharyngeal tumors is challenging due to the complex anatomic nature of the space and the wide range of pathologies encountered. This article will review the anatomy, common pathologies, and management of parapharyngeal masses. Surgical strategies are also reviewed.
Recent Findings
Masses of the parapharyngeal space are most commonly benign (80%). More recent longitudinal studies have shown that observation and non-surgical therapy are indicated in many cases. When surgery is indicated, innovative endoscopic and robotic-assisted techniques allow for improved visualization and complete tumor removal while avoiding significant blood loss, tumor spillage, and injury to surrounding nerves and vessels.
Summary
Management of parapharyngeal masses should consider morbidity of surgical resection versus the natural course of the disease. Surgical strategy is determined by location, size, and pathology. Adequate access is needed surgically to ensure complete resection and avoid tumor rupture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction to Parapharyngeal Tumors
The parapharyngeal space (PPS) is a complex anatomic potential space of the head and neck. Masses of this space are rare, making up only 0.5% of all head and neck tumors. PPS masses are most commonly benign (80%) and are made up of a variety of pathologies. Surgical excision is the mainstay of treatment for most diagnoses. The PPS is an anatomically complex region and the best surgical approach must be carefully selected prior to proceeding to the operating room in order minimize morbidity.
Anatomy
The parapharyngeal space is typically described as an inverted pyramid, with the skull base making up the floor of the pyramid and the greater cornu of the hyoid bone making up the apex. [1, 2] However, the shape is not truly pyramidal but rather runs as an elongated oblong potential space positioned between more substantial adjacent spaces. Medially, the space is bounded by the tensor veli palatine, the pharyngobasilar fascia, and superior constrictor muscles. This boundary separates the PPS from the retropharyngeal space. Laxity of the fascia separating these spaces in the elderly can result in the carotid artery protruding into the PPS. The pterygoids, parotid, mandibular condyle, and ramus of the masticated space form the anterior border superiorly. The stylomandibular ligament forms the lateral boundaries. This ligament separates the parotid, sometimes called the parotid space in radiology literature, from the PPS, and causes the classic dumbbell shape of parotid tumors of the PPS as the tumor grows through the constriction. The styloid process and ligament conceptually divide the PPS into two compartments: anterior and posteriorly. The anterior (pre-styloid) space contains fat and salivary tissue and is just posterior to the pterygoid muscle. The posterior (post-styloid) space contains the carotid artery and internal jugulars vein; cranial nerves IX, X, and XII; and lymph nodes. These compartments are important anatomically, radiologically, and clinically as tumor pathology can be predicted by the space it is within. [2]
Clinical Presentation
Due to the deep location of the PPS and the typically indolent nature of lesions in this area, the clinical presentation of PPS masses is often subtle. It is common for patients to present with incidentally noted PPS masses on imaging obtained for other reasons. Masses are often asymptomatic but can cause symptoms due to the space occupying effect [3]. The most frequent presenting clinical symptoms are a neck mass and/or intraoral asymmetry with protrusion the anterior tonsil pillar and/or palate. Patients can complain of pain, dysphagia, odynophagia, dysphonia, otalgia, snoring, or globus sensation. A serous effusion in the middle ear can be seen on the side of the lesion due to compression on the Eustachian tube [4]. Larger lesions can cause trismus, by physically impeding the mandible. Diagnosis is usually delayed, and parapharyngeal masses are typically at least 2–3 cm in size prior to detection. Cranial nerve dysfunction is often a late symptom due to mass effect [2, 5,6,7,8,9].
Differential of Parapharyngeal Tumors
Both benign and malignant tumors can be found within the parapharyngeal space, arising from the structures contained within, spreading from structures immediately adjacent to it, or metastasizing from a distance. Benign tumors are most common (80%), whereas malignant lesions are less likely (20%) [6, 10, 11]. Most commonly, benign tumors are salivary gland (50%) or neurogenic in origin (20%). A systematic review from 2014 showed over 70 different pathologies of the PPS reported in the literature [6].
Fifty percent of lesions in the PPS arise from the deep parotid lobe or minor salivary gland. These masses can extend through the stylomandibular ligament to give a dumbbell-shaped appearance on radiology. Ectopic rests of salivary tissue within the PPS are also possible origins. The majority of salivary neoplasms are pleomorphic adenoma and of all PPS lesions, pleomorphic adenomas are the most common (65%). However, any salivary neoplasm is possible [6, 10,11,12,13].
Neurogenic lesions are usually benign (95%) and are usually either paragangliomas (49%), schwannomas (31%), or neurofibromas (9%). Carotid body tumors are the most frequent paragangliomas, although glomus vagale can be found in the PPS as well as glomus jugulare extending from the temporal bone [1, 2]. Paragangliomas can be associated with syndromes including Von Hippel-Lindau, neurofibromatosis type 1, and MEN2a and 2b or can arise sporadically. Ten percent of paragangliomas are malignant, and 10% of patients with paragangliomas can have multicentric lesions [3]. Paragangliomas typically have slow, persistent growth of approximately 0.2 cm a year with a doubling time estimate of 4.2 years [4]. Surgery is the typical treatment for paragangliomas but due to risk of injury to cranial nerves IX, X, XI, and XII should only be offered in selected patients. If tumors are multicentric, surgery should also be considered carefully such as bilateral carotid body tumors with risks of bilateral vascular and cranial nerve injuries. Radiotherapy can halt tumor growth but is traditionally reserved for the elderly, medically frail, or those with high surgical risk [5].
Schwannomas are most commonly vagal or sympathetic chain in origin. They can cause mass effect on adjacent tissues and cause dysfunction of cranial nerves IX, X, and XII. They have slow growth and a low recurrence rate once resected [6]. Neurofibromas are typically multiple and associated with the nerve of origin. They have a risk of malignant transformation over time. Surgical excision is recommended for enlarging neurogenic lesions; however, the nerve of origin is usually non-functional after resection. Malignant nerve sheath tumors in the PPS are rare but possible (less than 5% of neurogenic lesions) [7].
Work Up
Obtaining cross-sectional imaging is the first step in work up of these tumors. MRI scans are the imaging of choice to evaluate lesions. The MRI scan can be characteristic for several lesions found within the PPS. For salivary lesions, they are typically T2 hyperintense and there can be a fat plane seen between the parotid and the mass. Schwannomas are usually T1 isointense or hypointense, with enhancement with the administration of gadolinium [8]. Imaging may predict the nerve of origin, with the pattern of vessel distribution around the nerve helpful to discern which nerve it is arising from. A vagal schwannoma can splay the carotid artery and the internal jugular vein, whereas a sympathetic chain schwannoma can displace both the carotid and jugular vein posteriorly without separating them [9]. This pattern is important to identify pre-operatively to determine post op dysfunction and to counsel patients accordingly [10].
If patients are suspected to have a paragangliomas, urine and plasma catecholamines should be evaluated [3]. Fine needle aspiration can be helpful in some situations but is not mandatory [11, 12]. Needle biopsy of schwannomas or paragangliomas is often non-diagnostic due to the large size of the spindle cells, and therefore you must have high suspicion for these lesions based on imaging alone. Transoral biopsy is not recommended due to bleeding risk and risk of tumor seeding [12, 13].
Management of Parapharyngeal Masses
Surgery is the mainstay of treatment of PPS masses, but the morbidity of surgery combined with the natural history of the disease and patient factors (age, function) should be considered in making a treatment plan. In the case of suspected malignancy, surgical treatment is typically required even with the risk of morbidity. For primary lymphoproliferative disease such as lymphoma, tissue diagnosis only is necessary and complete resection is avoided. The surgical treatment of benign lesions such as paragangliomas, schwannomas, and pleomorphic adenomas are considered on a case by case basis. Dysfunction from mass effect and impaired cranial nerve function with continued growth is weighed against the potential morbidity of resection. Carotid body tumors are easier to resect when smaller, with less risk of cranial nerve injury. It may be best to wait until patients develop complete cranial nerve palsy from the suspected paraganglioma prior to proceeding with resection. In the case of schwannomas, the growth rate may vary and these can be observed to confirm growth. Complete resection is typically recommended. This typically results in loss of function of the involved nerve, but early resection may avoid injury to other nearby nerves as the tumor grows. Complete resection will truncate the nerve, but enucleation has been shown to preserve nerve function in some cases but is not predictable. Thus, patients are counseled on the realistic risk of nerve dysfunction postoperatively [14••, 15, 16].
Patient factors such as age and health status play an important role too given the predictable risk of injury to cranial nerve IX, X, and XII with resultant dysphagia and airway issues. Elderly patients are much less likely to compensate for nerve injuries, and tumor growth may be slow enough that observation may suffice or radiation to slow tumor growth may be an option [17]. Radiation for younger patients is not preferred due to the fact it only inhibits growth, and there is a potential risk of malignant transformation [14••].
Surgical Approaches to the Parapharyngeal Space
There are several surgical approaches described to access the PPS, and selection of the appropriate approach is challenging. Surgery must strike a balance between complete removal of the mass and minimizing functional and esthetic morbidity from surgery. The choice of the approach should be determined by the location of the lesion, histopathology, and tumor size. For instance, a schwannoma with a planned enucleation may require less access than a pleomorphic adenoma where a complete resection without disruption of the capsule is crucial.
Due to the complex anatomy of the PPS, the choice of approach can be guided by lesion location and surgeon experience. Select small pre-styloid lesions along the mid to inferior aspect of the mandibular ramus are amenable to transoral resection [18,19,20,21,22,23,24,25]. Both robotic and endoscopic assisted transoral techniques have been described. Such techniques allow for better visualization for tumors that were previously difficult to remove transorally alone [26,27,28]. An incision along the anterior tonsillar pillar exposes the medial pterygoid muscle, and dissection is carried from anterior and lateral to the parapharyngeal space. Exposure of the carotid in the neck may be necessary for full visualization and control [25, 29, 30].
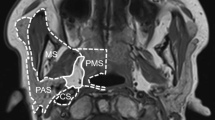
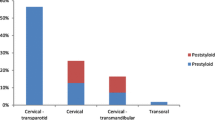
The transcervical approach is utilized in nearly 50% of cases in multiple systematic reviews of the PPS surgical literature [7, 16, 31,32,33,34,35,36, 37••]. This approach is useful for pre-styloid tumors located low within the parapharyngeal space. It provides visualization of the carotid artery and cranial nerves X, XI, and XII in this area. A trans-parotid approach can be used when needing to identify and protect the facial nerve prior to dissection of deep lobe parotid lesions and those deep in the PPS [18, 35, 36]. It is useful to provide access to the lateral aspect of both the pre- and post-styloid compartments, but limits access to the most cranial and caudal aspects of the PPS (see Fig. 1).
For tumors that are extending superiorly towards the skull base in the post-styloid space such as tumors entering the jugular bulb, one can use a combined transcervical and trans-mastoid approach [18, 35]. Removal of the mastoid tip allows for superior access of the PPS at the level of the skull base and direct access to the great vessels. Infratemporal or middle cranial fossa approaches can also be employed [35, 37••, 38, 39]. For lesions reaching the skull base along the greater sphenoid wing, subperiosteal elevation off the zygoma and removal of the zygoma bone can provide access to the superior aspect of the infratemporal fossa to augment a transcervical/trans-parotid approach but these generally are used for tumors of the infratemporal fossa not limited to the PPS.
Combined with these transcervical approaches, mandibular osteotomies are an option to improve access and visualization [35, 40, 41]. The mandibular ramus restricts superior and medial access to the parapharyngeal space. Osteotomies can be used in selected patients to allow for complete tumor removal and control of the vasculature. Situations would include patients with malignant neoplasms, recurrent neoplasms, large benign neoplasms, and highly vascular neoplasms with the need for vascular control. Single or double osteotomies can be used. A single parasymphyseal osteotomy can be sufficient for a small pre-styloid lesion. However, a single osteotomy puts traction on the temporomandibular joint and can cause disarticulation, edema, and occlusal discrepancies. If more access is needed, one can make a second posterior mandibular osteotomy in the ramus. Two osteotomies allow for displacement of the mandibular segment without disturbance of the temporomandibular joint. While useful in select situations, the mandibulotomy approach carries the additional risks of malocclusion, non-union, hardware infection, temporal mandibular joint arthrosis, and damage to the inferior alveolar nerve [41].
Combined endoscopic approaches to the PPS have been reported in the literature. In 2015, Benet et al. reported on a combined endonasal-transcervical approach to a metastatic PPS papillary thyroid carcinoma [42]. A transnasal endoscopic approach allowed for exposure of the pterygopalatine and infratemporal fossa and skull base, where the tumor was extending medially. A transcervical approach can be combined with a transoral approach for very large PPS lesions and avoid need for more extensive osteotomies by allowing identification and control of the cranial nerves X, XI, and XII and the great vessels and posterior tumor dissection prior to anterior transoral tumor dissection [37••, 43•, 44,45,46,47,48].
Figure 1 provides a visual representation of regions of the PPS that can be accessed by each approach. Posterior lesions (red) can be accessed with trans-mastoid/transcervical approach. Tumors in the central region (green) are accessible via transcervical approach, but may require further access, such as a trans-parotid dissection, based on tumor pathology and need for en bloc resection. Tumors with superior extension can be aided with superior approach and removal of the zygoma working deep to the temporalis muscle, or via an endonasal trans-maxillary approach. Anterior lesions (yellow) can be accessible to transoral approach if distinct from the great vessels and anterior to the styloglossus muscle. The transoral approach can also be added to transcervical approach for larger lesions that may benefit from a multi-approach to avoid mandibular osteotomies.
Conclusion
Masses of the parapharyngeal space, while rare, are a challenge to manage due to the complex anatomy of the region. Management should consider morbidity of surgical resection versus the natural course of the disease. Surgical strategy is determined by location, size, and pathology. Adequate access is needed surgically to ensure complete resection and avoid tumor rupture.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Locketz GD, et al. Histopathologic classification of parapharyngeal space tumors: a case series and review of the literature. Eur Arch Otorhinolaryngol. 2016;273(3):727–34.
Sun F, et al. Surgical management of primary parapharyngeal space tumors in 103 patients at a single institution. Acta Otolaryngol. 2018;138(1):85–9.
Colen TY, et al. Catecholamine-secreting paragangliomas: recent progress in diagnosis and perioperative management. Skull Base. 2009;19(6):377–85.
Jansen JC, et al. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer. 2000;88(12):2811–6.
Mendenhall WM, et al. Head and neck paragangliomas. Head Neck. 2011;33(10):1530–4.
Sato Y, et al. Clinical diagnosis and treatment outcomes for parapharyngeal space schwannomas: a single-institution review of 21 cases. Head Neck. 2018;40(3):569–76.
Riffat F, et al. A systematic review of 1143 parapharyngeal space tumors reported over 20 years. Oral Oncol. 2014;50(5):421–30.
Gupta A, Chazen JL, Phillips CD. Imaging evaluation of the parapharyngeal space. Otolaryngol Clin N Am. 2012;45(6):1223–32.
Saito DM, et al. Parapharyngeal space schwannomas: preoperative imaging determination of the nerve of origin. Arch Otolaryngol Head Neck Surg. 2007;133(7):662–7.
Stambuk HE, Patel SG. Imaging of the parapharyngeal space. Otolaryngol Clin N Am. 2008;41(1):77–101.
Iglesias-Moreno MC, et al. Parapharyngeal space tumors: fifty-one cases managed in a single tertiary care center. Acta Otolaryngol. 2016;136(3):298–303.
Oliai BR, et al. "Parapharyngeal space" tumors: a cytopathological study of 24 cases on fine-needle aspiration. Diagn Cytopathol. 2005;32(1):11–5.
Bradley PJ, Bradley PT, Olsen KD. Update on the management of parapharyngeal tumours. Curr Opin Otolaryngol Head Neck Surg. 2011;19(2):92–8.
•• Moore MG, et al. Head and neck paragangliomas: an update on evaluation and management. Otolaryngol Head Neck Surg. 2016;154(4):597–605. This article is a review of the contemporary published literature on the management of head and neck paragangliomas, which are commonly encountered in the parapharyngeal space. Traditionally, management of paraganglioms invovled surgical removal. However, as this review highlights, more recently, the literature supports that up-front non-surgical management is often appropriate for paragangliomas of the head and neck. The authors offer recommendations on instances when surgery should be considered.
Carlson ML, et al. Natural history of glomus jugulare: a review of 16 tumors managed with primary observation. Otolaryngol Head Neck Surg. 2015;152(1):98–105.
Eisele DW, Richmon JD. Contemporary evaluation and management of parapharyngeal space neoplasms. J Laryngol Otol. 2013;127(6):550–5.
Mendenhall WM, et al. Radiotherapy for parapharyngeal space tumors. Am J Otolaryngol. 2019;40(2):289–91.
Cohen SM, Burkey BB, Netterville JL. Surgical management of parapharyngeal space masses. Head Neck. 2005;27(8):669–75.
Carrau RL, Myers EN, Johnson JT. Management of tumors arising in the parapharyngeal space. Laryngoscope. 1990;100(6):583–9.
Carrau RL, Johnson JT, Myers EN. Management of tumors of the parapharyngeal space. Oncology (Williston Park). 1997;11(5):633–40 discussion 640, 642.
Bozza F, et al. Surgical management of parapharyngeal space tumours: results of 10-year follow-up. Acta Otorhinolaryngol Ital. 2009;29(1):10–5.
Hussain A, Ah-See KW, Shakeel M. Trans-oral resection of large parapharyngeal space tumours. Eur Arch Otorhinolaryngol. 2014;271(3):575–82.
Dimitrijevic MV, et al. Parapharyngeal space tumors: 61 case reviews. Int J Oral Maxillofac Surg. 2010;39(10):983–9.
Ducic Y, Oxford L, Pontius AT. Transoral approach to the superomedial parapharyngeal space. Otolaryngol Head Neck Surg. 2006;134(3):466–70.
Betka J, et al. Transoral and combined transoral-transcervical approach in the surgery of parapharyngeal tumors. Eur Arch Otorhinolaryngol. 2010;267(5):765–72.
Chan JY, et al. Transoral robotic surgery of the parapharyngeal space: a case series and systematic review. Head Neck. 2015;37(2):293–8.
Panda S, et al., Transoral robotic surgery for the parapharyngeal space: expanding the transoral corridor. J Robot Surg, 2019.
Meng LZ, et al. Early experience in endoscopic transoral resection for parapharyngeal space tumors. Ear Nose Throat J. 2018;97(4–5):E5–e9.
Mendelsohn AH. Transoral robotic assisted resection of the parapharyngeal space. Head Neck. 2015;37(2):273–80.
Markou K, et al. Transoral resection of giant parapharyngeal space tumors via a combined surgical approach. Iran J Otorhinolaryngol. 2019;31(103):87–96.
Basaran B, et al. Parapharyngeal space tumours: the efficiency of a transcervical approach without mandibulotomy through review of 44 cases. Acta Otorhinolaryngol Ital. 2014;34(5):310–6.
Ijichi K, Murakami S. Surgical treatment of parapharyngeal space tumors: a report of 29 cases. Oncol Lett. 2017;14(3):3249–54.
Singh M, Gupta SC, Singla A. Our experiences with parapharyngeal space tumors and systematic review of the literature. Indian J Otolaryngol Head Neck Surg. 2009;61(2):112–9.
Kuet ML, et al. Management of tumors arising from the parapharyngeal space: a systematic review of 1,293 cases reported over 25 years. Laryngoscope. 2015;125(6):1372–81.
Pradhan P, et al. Surgical management of parapharyngeal space tumours in a single tertiary care center. Indian J Otolaryngol Head Neck Surg. 2018;70(4):531–7.
van Hees T, et al. Tumors of the parapharyngeal space: the VU University medical center experience over a 20-year period. Eur Arch Otorhinolaryngol. 2018;275(4):967–72.
•• Ferrari M, et al. Surgical anatomy of the parapharyngeal space: multiperspective, quantification-based study. Head Neck. 2019;41(3):642–56. This anatomic study of human cadavers compares the main surgical approaches to the parapharyngeal space. This provides a comprehensive review of the objective strengths and limitations of each approach, with detailed human cadaver pictures. This study can be helpful in facilitating preoperative planning for the head and neck surgeon.
Shahinian H, Dornier C, Fisch U. Parapharyngeal space tumors: the infratemporal fossa approach. Skull Base Surg. 1995;5(2):73–81.
Poletti AM, et al. Surgical management of parapharyngeal space tumors: the role of cervical and lateral skull base approaches. Ear Nose Throat J. 2016;95(12):E1–e6.
Jungehuelsing M, et al. Modifications of the midline mandibulotomy for access to the parapharyngeal space. Laryngoscope. 2010;120(8):1557–62.
Kolokythas A, et al. Mandibular osteotomies for access to select parapharyngeal space neoplasms. Head Neck. 2009;31(1):102–10.
Benet A, Plata Bello J, El-Sayed I. Combined endonasal-transcervical approach to a metastatic parapharyngeal space papillary thyroid carcinoma. Cureus. 2015;7(7):e285.
• Duek I, et al. Minimally invasive surgery for resection of parapharyngeal space tumors. J Neurol Surg B Skull Base. 2018;79(3):250–6. This article reviews 11 patients who underwent surgery for parapharyngeal space masses using a transcervical endoscopic, transoral robotic, or combined endoscopic-robotic approach. This study provides a thorough explanation of how the new technologies (endoscopes and robot) result in improved visualizaation, safe vascular control, and complete tumor removal without spillage.
Pilolli F, et al. Parapharyngeal space tumours: video-assisted minimally invasive transcervical approach. Acta Otorhinolaryngol Ital. 2016;36(4):259–64.
Li SY, Hsu CH, Chen MK. Minimally invasive endoscope-assisted trans-oral excision of huge parapharyngeal space tumors. Auris Nasus Larynx. 2015;42(2):179–82.
Chen Z, et al. Excision of tumors in the parapharyngeal space using an endoscopically assisted transoral approach: a case series and literature review. J Int Med Res. 2019;47(3):1103–13.
Dallan I, et al. Endoscopic-assisted transoral-transpharyngeal approach to parapharyngeal space and infratemporal fossa: focus on feasibility and lessons learned. Eur Arch Otorhinolaryngol. 2016;273(11):3965–72.
Sun X, et al. A comparative analysis of endoscopic-assisted transoral and transnasal approaches to Parapharyngeal space: a cadaveric study. J Neurol Surg B Skull Base. 2018;79(3):229–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Head and Neck Cancers
Rights and permissions
About this article
Cite this article
Strohl, M.P., El-Sayed, I.H. Contemporary Management of Parapharyngeal Tumors. Curr Oncol Rep 21, 103 (2019). https://doi.org/10.1007/s11912-019-0853-8
Published:
DOI: https://doi.org/10.1007/s11912-019-0853-8