Abstract
Purpose of the Review
This review paper is a comprehensive look at the cardiovascular disease (CVD) risk that is associated with the use of androgen deprivation therapy in prostate cancer. It summarizes when certain cancer therapies are indicated and should guide physicians in identifying patients at increased risk for CVD during prostate cancer therapy.
Recent Findings
GnRH agonist use and maximal androgen blockade (MAB) are associated with increased CVD. This association is not observed in patients on GnRH antagonists. One example is the novel agent abiraterone, which is associated with hypertension whose mechanisms are likely driven by mineralocorticoid excess.
Summary
Incidence of cardiovascular disease events is greatest when using MAB, especially in patients with pre-existing CVD. There is significant confounding that exists given patients with more aggressive cancers tend to be older and have more co-existing CVD. Given the lower CVD event rates with GnRH antagonists, future studies and strategies should focus on high-risk cancer patients with co-existing CVD receiving antagonists over agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common cancer diagnosed in men with early detection being largely attributed to the widespread use of prostate-screening antigen testing [1]. Men diagnosed with local or regional disease have 5-year survival rates approaching 100% with appropriate therapies [2]. The favorable prognosis shifts attention to the potential side effects or downstream consequences of the medications used to treat prostate cancer since they may impact overall health status and quality of life more than the prostate cancer itself. Given that androgen deprivation therapy (ADT) is the mainstay of therapy for locally advanced and metastatic prostate cancer, this review explores the association between ADT use and cardiovascular disease (CVD) (Table 1).
The Role of Androgen Deprivation Drugs in Prostate Cancer
The androgenic pathway is important in stimulating growth of both normal prostatic epithelial cells and prostate carcinoma [3]. The majority of androgens are created in the testes, with a smaller amount emanating from the adrenal glands. By lowering circulating androgen levels, or by preventing their entry within prostate cancer cells, prostate cancer growth is inhibited. Androgen deprivation therapy (ADT), androgen suppression therapy, and hormonal therapy are phrases interchangeably used to reference therapy aimed at lowering androgen levels (Fig. 1).
Endocrine axis of androgen production and the associated targets of androgen deprivation therapy (ADT). Androgen synthesis starts in the hypothalamus and anterior pituitary with the production of gonadotropin-releasing hormone (GnRH). This is the site of central inhibition with GnRH agonists and antagonists. Luteinizing hormone (LH) is then converted to testosterone in the testes. When testosterone activates its corresponding receptor, it leads to prostate cancer cell proliferation. Anti-androgens block the activation of this receptor. Testosterone is also produced in the adrenal glands. Abiraterone works to inhibit the CYP17 enzyme which results in an excess of aldosterone and cortisol by products which are then theorized to drive the observed side effect profile 31,38
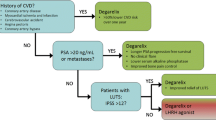
ADT is utilized in both the curative and palliative setting. To help determine a therapeutic approach, and whether systemic therapy such as ADT should be considered, we risk stratify our patients using levels of prostate-specific antigen (PSA) at diagnosis, Gleason score, and tumor stage. Utilizing these clinical findings, patients are classified as having low, intermediate, or high-risk disease. In the low-risk population, treatment options most commonly include definitive radiation vs radical prostatectomy vs active surveillance. Commonly, those with intermediate to high-risk disease are at least considered for ADT in the neo-adjuvant setting, concurrently with definitive radiation or sometimes in the adjuvant space when combined with salvage radiation.
ADT can be achieved using either surgical orchiectomy, resulting in immediate castration, or medical orchiectomy, which is the more common approach, largely driven by patient preference. Gonadotropin-releasing hormone (GnRH) agonists and antagonists act centrally to downregulate testosterone production [4]. Commonly utilized GnRH agonists include leuprolide, goserelin, and triptorelin. The initiation of GnRH agonists causes a brief and immediate testosterone level surge that can stimulate growth of cancerous cells causing a painful flare syndrome in patients with metastatic disease. Symptoms can range from bone pain, compression of a nerve root, spinal cord compression, or blockage of one or both ureters. Given this possibility, patients with symptomatic, bony, or high-volume disease are provided an anti-androgen (AA) such as bicalutamide before initiation of a GnRH agonist. Degarelix is a GnRH antagonist, which can lower circulating testosterone more quickly than GnRH agonists without an associated testosterone flare. The choice of GnRH agonist vs antagonist is largely left to the discretion of the provider and their comfort in managing these medications, as they are equally effective in treating those with advanced prostate cancer [5]. Neither agent is felt to offer a greater impact than the other on disease-specific outcomes. The major benefit of the GnRH antagonist is one that does not require a bicalutamide run-in to mitigate the risk of a painful flare syndrome.
AA monotherapy has been compared with medical or surgical castration in multiple studies, with a subsequent meta-analysis suggesting a trend toward a shorter overall survival of AA treatment compared with ADT, though it did not reach statistical significance [6]. Therefore, AA monotherapy with first-generation agents such as bicalutamide is rarely used alone as first-line therapy for patients with advanced disease. When used in treatment of prostate cancer, it is generally added to a GnRH analog for combined or maximal androgen blockade (MAB). More commonly, second-generation AA, like enzalutamide, is used as monotherapy following biochemical progression on ADT, also known as castrate-resistant prostate cancer (CRPC).
Hoping to improve upon outcomes in the first-line setting for high-risk, hormone-sensitive prostate cancer (HSPC) than what was currently realized with ADT or MAB, studies have investigated combining novel agents to ADT. Abiraterone is an irreversible inhibitor of CYP-17 that subsequently blocks endogenous androgen synthesis in the adrenal glands. When added to ADT, abiraterone improves progression free, but most importantly, overall survival compared with ADT alone [7, 8]
Of note, enzalutamide and abiraterone have not been compared head to head, so there is no concrete rationale on how to sequence these agents. Some providers will utilize abiraterone before enzalutamide if a patient has previously seen bicalutamide. Other providers prefer enzalutamide upfront to minimize the medications their patients require, since abiraterone is always paired with low-dose prednisone.
The Association of Androgen Deprivation Treatment with Cardiovascular Disease
GnRH Therapy: Agonists
GnRH is a decapeptide that is produced by the hypothalamus and regulates testosterone levels through its effects on LH release by the pituitary gland. Continuous stimulation of the GnRH receptors causes desensitization of the receptors resulting in testosterone suppression [9]. Synthetic GnRH agonists achieve castration levels of circulating testosterone without the physical and psychological discomforts associated with surgical orchiectomy. This also causes a number of changes to traditional cardiovascular risk factors and subsequent cardiovascular events, which have been studied extensively with somewhat mixed results.
Men with loco-regional prostate cancer have a favorable cancer prognosis; therefore, long- and short-term treatment consequences from oncologic therapy become more important because it may affect overall health more than the cancer itself [2]. The first study to highlight the potential adverse CVD effects followed 73,196 older men with loco-regional prostate cancer for 10 years using the Surveillance, Epidemiology and End Results (SEER) Medicare database. CVD outcomes were compared in patients receiving GnRH agonists or bilateral orchiectomy. After adjusting for patient and tumor characteristics, use of GnRH agonists was associated with a significantly increased risk for developing diabetes (hazard ratio [HR] 1.44, 95% confidence interval [CI] 1.34 to 1.55), coronary heart disease (HR 1.16, 95% CI 1.10 to 1.21), myocardial infarction (HR 1.11, 95% CI 1.01 to 1.21), or sudden cardiac death (HR 1.16, 95% CI 1.05 to 1.27). Increased risk was seen as early as 1–4 months into therapy and remained elevated in patients with longer courses of therapy, even after ADT completion [2]. In patients undergoing orchiectomy, there was an increased risk of diabetes but not CHD, MI, or SCD, although, only 6.9% of the study population underwent orchiectomy so it may be underpowered for these endpoints.
This was further evaluated in an observational population-based study conducted using the Prostate Cancer Database Sweden 3.0. A total of 6,556 men received GnRH agonists while 3,330 men underwent orchiectomy. Men treated with orchiectomy were older, had more metastatic disease, higher serum PSA levels, greater incidence of pre-existing CVD, and lower education levels compared with men who received GnRH agonists [10]. After multivariate adjustments, risk of CVD was similar in men treated with GnRH agonists compared with orchiectomy (HR 1.02, 95% CI 0.96–1.09); however, CVD events occurred more often in patients with diabetes (HR 1.50, 95% CI 1.32–1.71) or pre-existing CVD (HR 2.03, 95% CI 1.90–2.17) who were treated with GnRH agonists compared with orchiectomy [10].
When looking at the specific type of ADT, a large meta-analysis of six observational studies demonstrated that CVD was associated with GnRH agonists (HR = 1.19, 95%CIs 1.04–1.36; p < 0.001) and GnRH agonist plus oral AA therapy (HR = 1.46, 95%CIs 1.03–2.08; p = 0.04), but not with AA alone or orchiectomy [11]. Contrastingly, Bosco et al., in a similar meta-analysis, showed a 44% increased risk of CVD in patients who underwent orchiectomy and a 22% increased risk in patients undergoing AA monotherapy [12].
The relationship between ADT and cardiovascular death is less clear. Meta-analysis of observational studies suggests ADT was associated with cardiovascular mortality (HR = 1.17, 95%CIs 1.04–1.32; p = 0.01) especially in GnRH and GnRH plus AA therapy groups [11]. However, a large post hoc analysis of 4141 patients from eight randomized controlled trials (RCTs) found no difference (11.0% vs 11.2%; p = 0.41) [13].
Conflicting findings regarding an association between CVD and ADT from prior observational studies could be explained due to differences in study design, patient populations studied, selection bias in men offered ADT, and limited number of cardiovascular events in some studies. Given the incidence of prostate cancer and CVD is directly related to age, it is not surprising to see higher rates of CVD in older men receiving ADT. While large randomized controlled trials do not exist to specifically answer this question, this data does allow the authors of this review to recommend close monitoring of cardiovascular side effects in older patients with prostate cancer as well as patients with pre-existing disease.
GnRH Agonists and Peripheral Vascular Disease
Few studies have examined the effect of ADT on the peripheral vasculature. An observational study of 182,757 American men, age 66 years or older with non-metastatic prostate cancer, received either GnRH agonists (47.85%) or orchiectomy (2.2%). Authors observed an increased risk of incident peripheral arterial disease (PAD) (adjusted HR 1.16; 95% CI, 1.12–1.21) and incident venous thromboembolism (VTE) (adjusted HR 1.10; 95% CI, 1.04–1.15) in the GnRH agonist group. The orchiectomy group had increased PAD (adjusted HR 1.13; 95% CI, 1.02–1.26) and VTE (adjusted HR 1.27; 95% CI, 1.11–1.45) as well. PAD occurred as early as 1–4 months after ADT treatment. Increased VTE was not evident until at least 5 months of therapy [14]. Another Swedish study of 76,600 men had previously found an increased risk of deep vein thrombosis and pulmonary embolism but not PAD [15]. It is theorized that increased testosterone levels are associated with increased levels of antithrombin-3 and suppression of testosterone may induce a hypercoagulable state [14].
GnRH Antagonists
GnRH antagonists were introduced as an alternative to GnRH agonists. Degarelix blocks the GnRH receptor in the anterior pituitary gland and causes a rapid decline in LH, FSH, and subsequent testosterone production. Ninety-six percent of patients achieve castration-level testosterone values ≤ 50 ng/dL by day 3 without associated flare syndrome [16]. Antagonists lead to sustained suppression of both LH and FSH levels [17], whereas agonists suppress LH but there is an eventual rise in FSH levels [9]. These differences in pharmacologic action lead to important differences in both short- and long-term differences in adverse cardiovascular effects.
Safety data from phase III clinical trials on degarelix reveal increased rates of hypercholesterolemia (2%), hypertension (6%), and IHD (4%; 18 of 409). One percent or less of the patients suffered from a stroke, heart failure, and peripheral vascular disease [16]. The incidence of CVD events was further evaluated with data pooled from 1704 subjects in 9 clinical trials. In the general studied population, first-time cardiovascular event rates in the year prior to study entry were similar compared with after starting degarelix treatment (5.5 vs 6.1 per 100 persons/years; p = 0.45) [16]. Event rates before and after degarelix treatment were also similar in the subset of men without pre-existing CVD (5.6 vs 4.3 per 100 persons/year; p = 0.11), but in those with pre-existing CVD there was an overall increase in event rates from 5.3 to 10.5 events per 100 persons/year (p = 0.0013). Interestingly, the event rate increased in the 6-month period prior to initiation of degarelix with no apparent increase after starting treatment [16].
When comparing GnRH antagonists to agonists, pooled data was compared from six phase-III prospective RCTs of 2328 men to see if there was a difference in CVD event rates. In men with preexisting CVD, (30% in each treatment arm) those treated with GnRH antagonists had half the number of cardiac events compared with agonists (HR 0.438, 95% CI 0.260–0.736, p = 0.0018) (18••) for an absolute risk reduction of 8.2% during the first year of treatment with a GnRH antagonist and number needed to treat of 12 to prevent one cardiac event or death (18••). This benefit was not seen in patients without preexisting CVD. These were, however, post hoc analyses of studies not powered to specifically detect differences in CVD events.
Pathophysiology
GnRH agonists and antagonists cause low testosterone levels that affect metabolic activity, leading to weight gain, visceral and subcutaneous adipose tissue changes, increased insulin resistance, and increased low-density lipoprotein (LDL) and triglyceride levels [19]. In a small study of locally advanced non-metastatic prostate cancer, GnRH agonists were associated with a greater than 9% increase in total cholesterol, 7% increase in LDL, and a 26% increase in triglycerides [20]. Furthermore, overall weight increased about 2%, a greater than 9% increase in fat body mass, and a decrease in lean body mass by over 2.5% [20].
The severity of metabolic derangement may differ by the type of ADT used. In LDL receptor knockout mice treated with orchiectomy, GnRH agonist, or degarelix for 4 months, mice treated with degarelix gained less weight, had less visceral fat accumulation, higher HDL, lower LDL, fewer atherosclerotic plaques, and smaller necrotic areas within the plaques [21]. Compared with the other two groups of mice, degarelix-treated mice also had the lowest levels of FSH, which is continuously suppressed by GnRH antagonists, but rises back toward pre-treatment levels with agonists. FSH receptors can be found on adipocytes and likely play a role in lipid metabolism and fat accumulation [22]. FSH also binds to receptors on circulating monocytes, leading to increased osteoclast differentiation. In addition to bone resorption and increased risk of osteoporosis, osteoclasts can resorb calcified regions within atherosclerotic plaques contributing to plaque softening and instability [23]. Therefore, FSH activity (as seen in GnRH agonists but not antagonists) can lead to plaque destabilization, predisposing plaque to acute rupture resulting in CVD events such as an acute myocardial infarction, stroke, or other vascular events. This may explain the increased incidence of CVD events observed with patients receiving GnRH agonists who have known CVD at baseline. The early increase in cardiovascular events within the first year is unlikely to be explained by an increase in risk factors such as fat accumulation and insulin sensitivity, which would be expected to affect long-term cardiovascular risk.
Besides an FSH-mediated destabilization of atherosclerotic plaque, GnRH may play a direct role in plaque stability. A vulnerable plaque has a lipid core covered by a fibrous cap. Inflammatory cells (macrophages and T lymphocytes) are present and play a role in the development and progression of these atherosclerotic plaques [24]. Activated T helper cells produce inflammatory cytokines, interferon and tumor necrosis factor, that will inhibit smooth muscle collagen synthesis and promote macrophage collagenase activity to break down the fibrous cap, increasing the risk of rupture. T lymphocytes have been found to express GnRH receptors. When activated by GnRH agonists (but not antagonists), T lymphocytes lead to proliferation and differentiation into pro-atherogenic cells. In a mouse model with apolipoprotein E-deficient (ApoE−/−) mice with a high-fat diet, both advanced and stable plaques were evaluated after treatment with either degarelix or leuprolide [25]. The authors first confirmed with immunofluorescence the presence of GnRH receptor expressing T lymphocytes in the plaques. After 4 weeks of treatment with ADT, increased areas of necrosis were found in the plaques from those treated with leuprolide, but not in degarelix-treated mice. Plaque necrosis increases the risk of plaque rupture, which can lead to an acute vascular event such as myocardial infarction or stroke.
Anti-androgen Therapies
First generation AA therapies such as bicalutamide, flutamide, and nilutamide are usually used in combination with a GnRH agonist or antagonist to complete MAB when treating prostate cancer. A prospective cohort study on 7637 newly diagnosed prostate cancer patients compared patients receiving GnRH agonist and an anti-androgen with patients who had not received either agent in the course of their therapy. Crude rates of CVD events were higher in the cohort of patients treated with ADT; however, the authors similarly identify that these patients tended to be older and have more advanced tumor characteristics. After multivariate modeling, patients without pre-existing CVD who received ADT were nearly 30% more likely to develop congestive heart failure (CHF) (adjusted HR = 1.27, 95% CI 1.06–1.51). Additionally, patients who had known CVD and underwent ADT had an increased risk of developing arrhythmias (adjusted HR = 1.44, 95% CI 1.02–2.01) and conduction system disorders (adjusted HR = 3.11, 95% CI 1.22–7.91) [1].
It is unclear if the relationship observed between MAB and an increased incidence of CHF is specific to ADT, AA, or the combination of these therapies. A nested case-control study suggests that AA therapy in combination with GnRH agonists was associated with an increase in the risk of hospitalization of CHF (OR 4.33; 95% CI 1.68–11.13) whereas AA monotherapy was not. In sub-analysis, however, flutamide monotherapy was associated with increased risk of CHF hospitalization but not bicalutamide. The authors caution the presence of confounding factors and small sample size as weakness in their study and suggest their findings be used as hypothesis generating [26].
In contrast, second-generation AA medications (e.g., enzalutamide) can be useful as monotherapy for CRPC patients. The phase-III AFFIRM trial had 1199 patients with metastatic CRPC randomized to enzalutamide vs placebo. Survival and adverse event rates were compared. Over a 24-month period, no statistically significant difference in overall adverse cardiac events occurred in the enzalutamide group compared with placebo (6% vs 8%). Incidence of grade 3 (severe) adverse cardiac effects was also similar in both groups (1% vs 2%), but there was a higher incidence of hypertension in the enzalutamide cohort compared with placebo (6.6% vs 3.3%) [27].
When specifically looking at the incidence of atrial fibrillation (AF) and acute coronary syndrome (ACS), the PREVAIL trial authors found that AF occurred at a similar rate in patients on enzalutamide compared with placebo (2% vs 1%). ACS occurred in 7 patients taking enzalutamide monotherapy vs. 4 in the placebo arm (1% vs < 1%). Hypertension was again found to be more common with enzalutamide (13% vs 4%). Importantly, the average observation period was significantly longer for the enzalutamide group compared with the control (17.1 vs 5.4 months). Additionally, the cardiac event rates were low and the study was not powered specifically for these, so we are to draw any definitive conclusions about the cardiovascular risk associated with enzalutamide [28].
Adrenal Gland CYP17 Inhibitors
Abiraterone is a novel agent that is a selective inhibitor of androgen biosynthesis vis-à-vis the adrenal glands that can irreversibly block the CYP17 enzyme in the adrenals. Blockade of this pathway can lead to undetectable levels of testosterone and improved overall survival in patients with prostate cancer [7, 8, 29, 30••]. However, it may be associated with cardiovascular side effects.
Abiraterone irreversibly inhibits the enzyme that produces the precursors of the testosterone and cortisol pathway, but not the aldosterone pathways [31]. This leads to decreased production of testosterone and cortisol. Cortisol acts to provide negative feedback on ACTH, in its absence, ACTH production increases and results in increased production of mineralocorticoid precursors resulting in increased aldosterone production [31, 32] (Fig. 1). The mineralocorticoid precursors can lead to fluid retention, hypertension, as well as hypokalemia. These effects can be prevented by concomitant administration of low doses of prednisone, corticosterone, or dexamethasone [31].
Based on the mechanism of action of abiraterone and the indirect increased production of mineralocorticoid results, it is no surprise that the predominant cardiovascular events seen are new diagnosis of hypertension and worsening of baseline hypertension. In the phase 3 clinical trial of metastatic castration-resistant prostate cancer patients, patients were randomized to abiraterone + prednisone or placebo + prednisone. Safety data revealed similar rates of cardiac disorders (19% vs 16%), respectively; however, mineralocorticoid-related side effects were more commonly seen in the study drug arm (hypertension 22% vs 13%, hypokalemia 17% vs 13%, fluid retention or edema 28% vs 24%), respectively. Most of these events were considered mild grade 1 or 2 adverse events [29]. Additional phase 3 data showed there was an increased incidence of atrial fibrillation and tachycardia events. Hypertension is a common trigger of atrial fibrillation [7].
Long-term safety data in a study of 51 patients with concomitant CVD risk factors, similar to above, demonstrated the most frequently reported adverse events were hypertension (16%) and volume overload (18%). There were no reported changes in cardiac left ventricular ejection fraction or major cardiac events reported [33]. A similar slightly larger 87-patient study conducted by Prati et al. showed 30% of patients with pre-existing hypertension had worsening of their blood pressure and 6% of patients with risk factors for CVD developed new onset of hypertension. All patients achieved control of blood pressure with medical therapy [34].
The studies discussed above are in the setting of abiraterone being given in monotherapy in castrate-resistant prostate cancer. When abiraterone and prednisone is added to ADT in metastatic prostate cancer (compared with ADT + double placebo), a similar increase in hypertension (grade 3 and 4) is observed (20% and 0%, respectively) [8]. The incidence of hypertension was higher than in previous trial, which the authors potentially attributed to lower doses of prednisone and longer duration of abiraterone compared with previous trials. The safety data also revealed an increased incidence of atrial fibrillation (8 vs 2 patients) in the abiraterone group [8]. While the overall number is low, similar findings in regard to atrial fibrillation and hypertension were observed in clinical trials comparing ADT alone to ADT and abiraterone in patients with new diagnosis metastatic and non-metastatic prostate cancer (30••).
In conclusion, abiraterone was found to have a manageable cardiovascular side effect profile tolerance rate in patients with prostate cancer. Clinical trial safety data showed no significant change in left ventricular ejection fraction, heart failure symptoms, or cardiovascular-associated death. Hypertension, lower extremity edema, and atrial fibrillation were the most common side effects due to the mineralocorticoid excess.
QT Interval
Both endogenous and exogenous sex hormones have been found to have an effect on the QT interval [16, 35]. Testosterone shortens the action potential, and hypogonadism has been associated with higher prevalence of prolonged QT intervals and potentially increased risk of ventricular tachyarrhythmia. All forms of ADT have similarly been found to prolong the QT interval and the risks and benefits of this effect should be evaluated in each patient [36].
The Role of a Cardiologist in the Management of ADT Associated CVD
With the observed CVD associations seen in patients receiving ADT, monitoring with a cardiologist or primary care physician may lead to better management of CVD side effects. In a joint statement by the American Heart Association, American Cancer Society, American Urological Association, and the American Society for Radiation Oncology, the authors recognize the metabolic effects of ADT and its potential associations with CVD. The effects can be seen as early as 3 months; therefore, after initiations of therapy, high-risk patients warrant close follow-up. While the group did not recommend a pre-treatment evaluation or pre-treatment testing, they did recommend guideline-directed therapy (GDT) to control CVD risk factors such as hypertension, hyperlipidemia, diabetes, and smoking [37]. The QT interval and the presence of other concomitant QT-prolonging drugs, such as antibiotics, should be evaluated in patients receiving ADT and used cautiously in patients with values > 450 ms [36].
Bhatia et al. translated the committee’s recommendations into a paradigm for patients with prostate cancer [38]. They emphasize the importance of GDT to control blood pressure, cholesterol, diabetes, and smoking. They specifically recommend angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) to manage hypertension in prostate cancer patients due to its beneficial impact on overall survival [39]. In patients with abiraterone-induced hypertension, the authors of this review hypothesize using spironolactone given its action on the mineralocorticoid pathway.
Additionally, exercise is known to improve parameters of blood pressure and other metabolic derangements that may exacerbate during cancer therapy with ADT and predispose patients to CVD. A total of 150 min/week of moderate exercise should be encouraged, which has been associated with improved quality of life and physical functioning in prostate cancer patients [40].
Lastly, Bhatia et al’s protocol recommends the use of aspirin for primary and secondary prevention in the high-risk prostate cancer population. In a recent database analysis, Jacobs et al. found that low-dose aspirin started after cancer diagnosis was associated with lower prostate cancer-specific mortality only in the high-risk prostate cancer population (HR = 0.50; 95% CI, 0.27 to 0.92), possibly thought to be due to characteristics of high-grade cancers making them more susceptible to aspirin inhibition [41••].
Based on the data presented above, the authors of this review theorize that in patients with significant CVD burden, preference be given to GnRH antagonists over agonists when designing MAB. Even though GnRH antagonists are associated with an increased risk of CVD, this opinion is based on relatively lower rates of CVD in patients receiving degarelix compared with agonists. There is, however, no prospective data comparing the two treatment options in patients with pre-existing CVD, risk factors for CVD, or no pre-existing risk factors for CVD. Future prospective, randomized studies should test this strategy and potential pharmacologic treatment strategies, as it may be practice changing.
Conclusion
In conclusion, androgen deprivation therapy is a broad term that includes GnRH agonists, antagonists, anti-androgens, adrenal testosterone inhibitors, and surgical castration. ADT in general has been observed to increase cardiovascular risk factors such as hypertension, hyperlipidemia, diabetes, and obesity, along with an association with cardiovascular events, but no definitive association with cardiovascular mortality. Older patients with pre-existing CVD are at higher risk, although these patients present more often with advanced cancer and get more aggressive therapy, so selection bias cannot be excluded. GnRH agonists may additionally increase risk of CVD in patients with pre-existing CVD through plaque destabilization and exacerbation of metabolic risk factors. Novel agents such as enzalutamide and abiraterone have been associated with low rates of CVD events in phase III clinical trials, with an increased incidence of hypertension and atrial fibrillation.
Presence of CVD side effects, however, should not limit the use of these life-saving therapeutics in the treatment of prostate cancer. The paradigm suggested by Bhatia et al. (ABCDE protocol) focusing on GDT can be used to evaluate and treat risk factors, including aspirin for high-risk prostate cancer, moderate-intensity exercise, and hypertension management with ACE/ARBs or spironolactone in abiraterone-induced hypertension. For patients with pre-existing CVD, we recommend that oncologists consider GnRH antagonists over agonists, as lower event rates were seen in this population, although choice of treatment agents remains an individualized discussion over risks and benefits of managing both cancer and cardiac disease.
The above reviewed clinical trial and real-world data on ADT and its impact on CVD are not without limitations. Randomized controlled trials often exclude patients with significant CVD and may partly explain the low CVD event rates observed, and real-world data can have inherent confounding bias that cannot adequately be controlled for. Given the known impact of ADT on CVD, long-term use of ADT drugs, increased incidence of CVD with age, and the aging population, it is important that future trials are designed to properly define the relationship between these two identities and optimal treatment strategies for patients both affected with CVD and prostate cancer, in order to provide long-term good outcomes for both disease states.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Haque R, UlcickasYood M, Xu X, Cassidy-Bushrow AE, Tsai HT, Keating NL, et al. Cardiovascular disease risk and androgen deprivation therapy in patients with localised prostate cancer: a prospective cohort study. Br J Cancer. 2017;117:1233–40.
Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4448–56.
Huggins C, Clark PJ. Quantitative studies of prostatic secretion : ii. The effect of castration and of estrogen injection on the normal and on the hyperplastic prostate glands of dogs. J Exp Med. 1940;72:747–62.
Morris MJ, Rumble RB, Basch E, Hotte SJ, Loblaw A, Rathkopf D, et al. Optimizing anticancer therapy in metastatic non-castrate prostate cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36:1521–39.
Trachtenberg J, Gittleman M, Steidle C, Barzell W, Friedel W, Pessis D, et al. A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol. 2002;167:1670–4.
Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000;132:566–77.
Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology. 2012;13:983–92.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60.
Lepor H, Shore ND. LHRH agonists for the treatment of prostate cancer: 2012. Reviews in urology. 2012;14:1–12.
Thomsen FB, Sandin F, Garmo H, Lissbrant IF, Ahlgren G, van Hemelrijck M, et al. Gonadotropin-releasing hormone agonists, orchiectomy, and risk of cardiovascular disease: semi-ecologic, nationwide, population-based study. Eur Urol. 2017;72:920–8.
Zhao J, Zhu S, Sun L, Meng F, Zhao L, Zhao Y, et al. Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies. PLoS One. 2014;9:e107516.
Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015;68:386–96.
Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. Jama. 2011;306:2359–66.
Hu JC, Williams SB, O’Malley AJ, Smith MR, Nguyen PL, Keating NL. Androgen-deprivation therapy for nonmetastatic prostate cancer is associated with an increased risk of peripheral arterial disease and venous thromboembolism. Eur Urol. 2012;61:1119–28.
Van Hemelrijck M, Adolfsson J, Garmo H, et al. Risk of thromboembolic diseases in men with prostate cancer: results from the population-based PCBaSe Sweden. The Lancet Oncology. 2010;11:450–8.
Smith MR, Klotz L, van der Meulen E, Colli E, Tanko LB. Gonadotropin-releasing hormone blockers and cardiovascular disease risk: analysis of prospective clinical trials of degarelix. J Urol. 2011;186:1835–42.
Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–8.
Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565–73 Trial showing fewer CVD events in patients with pre-existing CVD who received an antagonist instead of an agonist. This study may change selection of cancer therapy protocols in patients with prostate cancer and CVD.
Collier A, Ghosh S, McGlynn B, Hollins G. Prostate cancer, androgen deprivation therapy, obesity, the metabolic syndrome, type 2 diabetes, and cardiovascular disease: a review. Am J Clin Oncol. 2012;35:504–9.
Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–5.
Hopmans SN, Duivenvoorden WC, Werstuck GH, Klotz L, Pinthus JH. GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model. Urol Oncol. 2014;32:1126–34.
Liu XM, Chan HC, Ding GL, Cai J, Song Y, Wang TT, et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell. 2015;14:409–20.
Crawford ED, Schally AV, Pinthus JH, Block NL, Rick FG, Garnick MB, et al. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol Oncol. 2017;35:183–91.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Knutsson A, Hsiung S, Celik S, Rattik S, Mattisson IY, Wigren M, et al. Treatment with a GnRH receptor agonist, but not the GnRH receptor antagonist degarelix, induces atherosclerotic plaque instability in ApoE(−/−) mice. Sci Rep. 2016;6:26220.
Martin-Merino E, Johansson S, Morris T, Garcia Rodriguez LA. Androgen deprivation therapy and the risk of coronary heart disease and heart failure in patients with prostate cancer: a nested case-control study in UK primary care. Drug Saf. 2011;34:1061–77.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509–21.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51 Trial shows favorable outcomes with the addition of a novel drug with minimal CVD side effects.
Rehman Y, Rosenberg JE. Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug design, development and therapy. 2012;6:13–8.
O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–25.
Verzoni E, Grassi P, Ratta R, Niger M, de Braud F, Valdagni R, et al. Safety of long-term exposure to abiraterone acetate in patients with castration-resistant prostate cancer and concomitant cardiovascular risk factors. Therapeutic advances in medical oncology. 2016;8:323–30.
Prati V, Ruatta F, Gernone A, Aversa C, Galizia D, Torino S, et al. Prospective evaluation of the cardiovascular safety profile of abiraterone acetate (AA) in mCRPC patients (pts). J Clin Oncol. 2016;34:e16534.
Sedlak T, Shufelt C, Iribarren C, Merz CN. Sex hormones and the QT interval: a review. J Women’s Health. 2012;21:933–41.
Garnick MB, Pratt CM, Campion M, Shipley J. The effect of hormonal therapy for prostate cancer on the electrocardiographic QT interval: phase 3 results following treatment with leuprolide and goserelin, alone or with bicalutamide, and the GnRH antagonist abarelix. J Clin Oncol. 2004;22:4578.
Levine GN, D’Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–40.
Bhatia N, Santos M, Jones LW, Beckman JA, Penson DF, Morgans AK, et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation. 2016;133:537–41.
Mc Menamin ÚC, Murray LJ, Cantwell MM, Hughes CM. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: a systematic review. Cancer Causes Control. 2012;23:221–30.
Harrison MR, Jones LW. Exercise as treatment for androgen deprivation therapy-associated physical dysfunction: ready for prime time? Eur Urol. 2014;65:873–4.
Jacobs EJ, Newton CC, Stevens VL, Campbell PT, Freedland SJ, Gapstur SM. Daily aspirin use and prostate cancer-specific mortality in a large cohort of men with nonmetastatic prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:3716–22 Study highlights benefits of aspirin for the prostate cancer population.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Megha Agarwal, Timothy Canan, Greg Glover, Nidhi Thareja, Andre Akhondi, and Joshua Rosenberg declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Agarwal, M., Canan, T., Glover, G. et al. Cardiovascular Effects of Androgen Deprivation Therapy in Prostate Cancer. Curr Oncol Rep 21, 91 (2019). https://doi.org/10.1007/s11912-019-0841-z
Published:
DOI: https://doi.org/10.1007/s11912-019-0841-z