Abstract
Purpose of Review
Advanced mammographic imaging modalities have been implemented in clinical practices throughout the USA. The most notable and widely used has been the three-dimensional derivative of digital mammography, known as digital breast tomosynthesis (DBT). In this article, we review the screening and diagnostic applications of DBT, along with its limitations. We also briefly address several supplemental breast imaging modalities.
Recent Findings
The accumulating evidence from both small and large-scale trials has shown a significant reduction in recall rates and slight increase in cancer detection rates when using DBT. However, the incremental increase in cancers detected remains less than that achieved with several supplemental imaging modalities, including whole-breast ultrasound, MRI, and MBI (molecular breast imaging). Other modalities, such as CEM (contrast-enhanced mammography) and CET (contrast-enhanced tomography), are also being investigated.
Summary
Numerous studies have confirmed the added value of DBT and its increased cancer detection rate in both the screening and diagnostic settings. However, the superior sensitivity of supplemental imaging modalities renders them essential, especially in high-risk patients, and potentially those with dense breasts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the second leading cause of death among women in the USA (https://www.cancer.net/cancer-types/breast-cancer/statistics). Multiple randomized controlled trials and observational studies have shown that breast screening reduces the mortality rates of breast cancer by 30% or more [1,2,3,4,5]. Screening of dense breasts remains a major challenge despite the implementation of full-field digital mammography (FFDM) screening programs since 2005 [6]. Up to 15–30% of cancers are not identified at standard screening [7], and an even higher percentage of cancers are undetected in women younger than 50 years and in women with dense breasts [8, 9]. Because FFDM is a two-dimensional (2D) rendering of the three-dimensional breast, the resultant overlapping of fibroglandular densities may either mimic a tumor (false positive) or may mask one (false negative). The reduced sensitivity of 2D mammography with its associated false-positive recalls and negative biopsies, as well as its inherent radiation, have raised criticism [10, 11]. Whether breast density increases the risk of breast cancer by its masking effect or whether it is a primary risk factor in itself [12,13,14], overcoming the challenge of imaging dense breasts is a main factor in developing imaging technologies to improve diagnostic performance. The evolution of a three-dimensional derivative of digital mammography, known as digital breast tomosynthesis (DBT) has been a major advance in addressing this issue.
Tomosynthesis Technique
Digital breast tomosynthesis is a technique where image acquisition occurs as an x-ray tube moves in an arc over a limited angle (15–50°), creating multiple low dose projection images of the stationary compressed breast. These images are subsequently reconstructed into thin slices, usually 1 mm in thickness, allowing the radiologist to scroll through the breast to evaluate each plane without superimposition of adjacent structures [15, 16].
Historical Overview
Tomosynthesis as a concept was initially established in the 1930s [17]. Miller et al. again investigated tomosynthesis in the 1970s [18]; however, due to the high radiation doses required, tomographic techniques were not implemented clinically at that time. With the development of digital detectors in the 1990s, the interest in tomosynthesis resurfaced. In 1997, Nikalson et al. introduced digital breast tomosynthesis, which in a reader study of mastectomy specimens, revealed improved depiction of lesions over 2D mammograms [19]. As interest grew for this new technology, many different small reader studies were performed in the 2000s, supporting its potential as a breast imaging modality with variable reports of decreased recall rates and/or increased lesion detection [20,21,22,23].
In 2011, the Food and Drug Administration (FDA) approved DBT for its use in clinical practice. Since then, multiple studies have been performed to assess its value in both the diagnostic and screening settings.
DBT in the Screening Setting
Once DBT was approved by the FDA, it was immediately incorporated in the clinical setting at many breast centers in the USA; first experience publications started to appear in the literature by 2013. However, in Europe, large prospective studies were done, looking at the value of DBT in the screening population. The three largest trials were the Oslo trial, the STORM (Screening with Tomosynthesis OR standard Mammography) trial, and the Malmö Breast Tomosynthesis Screening trial [24••, 25•, 26•]. The Oslo trial and the STORM trial compared 2D FFDM alone with 2D FFDM combined with DBT. They found that interpreting mammography using standard 2D FFDM in combination with DBT increased their breast cancer detection rates, and reduced their false-positive rates. Cancer detection rates in the Oslo trial were 6.1 per 1000 examinations for mammography alone, and 8.0 per 1000 examinations for mammography plus tomosynthesis (27% increase) [24••]. In the STORM trial, cancer detection rates were 5.3 cancers per 1000 screens for 2D only, and 8.1 cancers per 1000 screens for integrated 2D and 3D screening. The incremental cancer detection rate attributable for integrated 2D and 3D mammography was 2.7 cancers per 1000 screens [25•].
The Malmö trial uses a different approach, assessing the performance of single view DBT as a stand-alone in comparison to 2D FFDM. The authors plan to accrue 15,000 women, but an exploratory analysis of the first 7500 women showed an increase in detection rate of 43%, with 6.3 cancers per 1000 screens detected with 2D FFDM, and 8.9 cancers per 1000 screens with single view DBT. The recall rate increased from 2.6% with 2D-FFDM to 3.8% with DBT (still well below US standards), which the authors attributed to an increase in detection of stellate distortions, some of which were mammographically occult invasive cancers, while others were radial scars or post-operative scars. However, they noted a downward trend of the recall rate over the first 1.5 years of the trial, implying improvement with increased experience [26•].
The first large retrospective analysis in the USA was published by Freidewald et al. in 2014, and compared 2D FFDM with 2D FFDM and DBT at 13 academic and nonacademic medical centers, totaling 454,850 patients. Reported model-adjusted recall rates were reduced by 16 per 1000 screens with digital mammography plus tomosynthesis, and the reported incremental cancer detection rate increased by 1.2 per 1000 women screened [27•]. This was slightly lower than the European population-based screening trials of DBT, which revealed an incremental increase in cancer detection of 2–3 additional cancers per 1000 women screened. Of note, both Friedewald et al. and Skaane et al. reported that the 40–41% increase in cancer detection using DBT was for invasive cancers, with no significant increase in the detection of ductal carcinoma in situ (DCIS) [24••, 27•].
Multiple additional studies have since been published, with the two main parameters assessed being recall rates and cancer detection rates. The majority of small and large-scale trials have shown a significant reduction in recall rates when DBT was added to 2D FFDM, ranging from 15 to 37% (Table 1) [24••, 25•, 27•, 28,29,30,31,32,33,34,35, 36•, 37]. Asymmetry was the most common mammographic finding associated with a reduction in recall rates, due to decreased summation artifact from superimposition of tissues [30, 33]. In addition, when patients were recalled, a greater percentage underwent ultrasound alone (without additional mammographic views), due to the improved evaluation of lesion margins with DBT [33]. The majority of studies have shown an increase in cancer detection rate with the addition of DBT, ranging from 20 to 54% [24••, 25•, 27•, 29, 31, 32, 34–35, 36•, 37]. Although three studies reported no statistically significant difference in cancer detection [28, 30, 33], they all noted improved recall rates and the importance of decreasing anxiety and cost associated with screening recalls.
Some groups further assessed the impact of DBT in screening by stratifying their analysis into subgroups to address which, if any, breast density and age groups were more affected. A study by Haas et al. revealed that the addition of tomosynthesis reduced recall rates for all breast density and patient age groups, though the greatest reductions were for those younger than 50 years and those with dense breasts [28]. McDonald et al. noted a more pronounced reduction in recall rates in women younger than 50 years. In their study, DBT showed a reduction in recalls of 24.1% in women younger than 50 years versus a reduction of 17.9% in women 50 years or older. In the same study, recall rate reduction for dense breasts was 17.2% versus 24.1% in non-dense breasts [34].
A recent analysis by Rafferty et al. also addressed the screening performance of FFDM alone versus FFDM in combination with DBT as a function of breast density [37]. The primary analysis compared the performance of DBT among dense (BI-RADS C and D) versus non-dense breasts (BI-RADS A and B). The addition of DBT caused a reduction in recall rates and an increase in cancer detection rates for both groups, though this was slightly more pronounced in the dense breast group. However, the exploratory subgroup analysis revealed that the improvements were greatest for heterogeneously dense breasts (BI-RADS C) and scattered fibroglandular densities (BI-RADS B). Differences were not statistically significant for the almost entirely fatty (BI-RADS A) and extremely dense breasts (BI-RADS D).
Skaane et al. also noted the most marked improvement in lesion detection in the BI-RADS B and C breasts, i.e., those with scattered fibroglandular densities or heterogeneously dense parenchyma [24••].
Radiation Dose and Synthetic 2D Images
Radiation dose from yearly mammograms has been a point of criticism of breast cancer screening. Kopans et al. noted that despite millions of women having undergone mammography since the 1990s, no increased risk of breast cancer from radiation exposure during screening has been observed [15]. In addition, radiation risk to the breast is age related, and by age 40, the breast is mature and relatively resistant to radiation. Thus, the benefits of mammography are felt to outweigh the minimal radiation risks [38]. However, it is agreed that for any radiologic modality to replace digital mammography, it should have a dose that does not exceed that of conventional full-field digital mammography. As each projection of DBT requires only a fraction of the total dose of a 2D mammogram, DBT can be performed at a radiation dose similar or even less than the combined dose for the standard two-view FFDM [15]. However, DBT is often used in combination with 2D FFDM, as two-dimensional images provide an overview, and are used to compare with prior non-DBT studies, and to evaluate calcifications (which may not be perceived as grouped when scattered over several DBT slices). The combination of 2D and DBT doubles the radiation dose, which is equivalent to 1–2 months of annual background radiation in the USA, though remains below the FDA safety limits of 3 mGy/view [39, 40]. This limitation was addressed with the development of synthesized 2D images (s2D). s2D images are two-dimensional mammographic images that are reconstructed from data acquired during tomosynthesis, with the intent of negating the need for a separate 2D-FFDM image acquisition. Several studies have compared mammographic interpretation of DBT with synthetic 2D images versus DBT and 2D-FFDM images.
Houssami, in a recent review, summarized the prospective and retrospective studies comparing s2D/DBT and 2D/DBT breast imaging [41]. He noted that cancer detection rates were not significantly different across the studies (though improved over 2D alone), and that the radiation dose of s2D/DBT was 55–58% of that for 2D/DBT. Although overall similar, some heterogeneity in recall rates was noted, with lower recall rates using s2D reported by Aujero et al. [42] attributed to more experience (having transitioned to s2D/DBT after gaining experience with 2D/DBT). In contrast, Bernardi et al. attributed their increased recall rates with synthesized images to study design (sequential readers recalling without double reading consensus or arbitration), as well as to lack of experience with interpretation of synthetic images, which enhance lesion detail and parenchymal structures [43]. They noted that with further experience and prior s2D images for comparison in the future, recall rates would likely decline.
DBT in the Diagnostic Setting
Although many studies have documented the merits of DBT in the screening population, its value is also appreciated in the diagnostic setting. As noted previously, the ability to accurately assess lesion margins with tomosynthesis has reduced the need for extra views in mammography [33, 44, 45]. Additional images are not completely obviated, as magnification views are necessary to evaluate calcifications and spot compression images may be necessary to evaluate subtle DBT findings including questionable architectural distortion.
The improved lesion characterization of DBT also results in better differentiation of benign from malignant lesions. This improved specificity has resulted in fewer examinations being categorized as BI-RADS 3 (probably benign), which in turn has decreased unnecessary follow-ups [40, 46].
Studies have shown that DBT provides equal accuracy and greater conspicuity in the evaluation of non-calcified breast lesions when compared to spot magnification 2D FFDM views [20, 44, 45, 47,48,49,50]. Two published studies evaluating the role of DBT in assessing calcifications revealed mixed results [51, 52]. 2D FFDM may be superior in detecting calcifications, yet once detected, DBT shows similar accuracy to 2D-FFDM in the evaluation of calcifications.
DBT Limitations
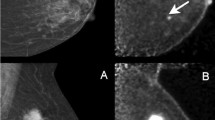
DBT is uniquely sensitive in the detection of subtle architectural distortion (a potential presentation of malignancy), which is in part responsible for its improved cancer detection rates. However, architectural distortion can also be due to non-malignant causes, most notably post-surgical changes or complex sclerosing lesions (i.e., radial scars). As noted previously, Lång et al. attributed their increased recall rate with DBT to the increased sensitivity for radial scars and post-operative scars [26•]. Partyka et al. have suggested that DBT-detected architectural distortion in the absence of a sonographic correlate has a higher likelihood of benignity [53], though others have disagreed. Freer et al., in their study assessing feasibility and accuracy of DBT-guided needle localization, reported that DBT-detected suspicious architectural distortion that is mammographically or sonographically occult has a 47% positive predictive value for malignancy [54]. Thus, at present, most sites agree that any architectural distortion that is visible solely with DBT should undergo DBT-guided biopsy (Fig. 1).
Fifty-year-old female with a history of left breast conservation therapy (lumpectomy and radiation) for invasive ductal carcinoma 9 years ago presents for annual screening mammogram. a 2D FFDM image reveals heterogeneously dense parenchyma, with several rim-calcified cysts (short arrow). b DBT plane reveals architectural distortion (long arrow), which is more apparent on magnification (c). No ultrasound correlate was identified. The patient underwent DBT-guided core biopsy, revealing invasive lobular carcinoma
Another limitation of DBT is the added interpretation time. Several studies have reported an increase in the reading time over 2D FFDM alone ranging from 33 to 50% [24••, 45, 55]. Although DBT does increase the reading time in the screening setting (up to two-fold at initiation), this is partly compensated by the reduction in recalls for diagnostic images, as well as the increase in patients recalled for ultrasound only (not requiring additional mammographic imaging) [33].
Supplemental Screening Modalities
Whole-Breast Screening Ultrasound
Given the promising improved performance in screening with the addition of DBT, the question arises as to whether additional supplemental imaging is necessary. Multiple studies have shown that whole-breast sonography has an incremental cancer detection rate ranging from 0.8 to 10 per 1000 women when used in the supplemental screening of mammography-negative dense breasts [56,57,58,59]. As yet, no one has directly compared DBT to ultrasound for supplemental screening, though this is currently being evaluated in the ASTOUND prospective multicenter comparative trial [60]. An interim report published in 2016 by Tagliafico et al. revealed that among 3231 mammography-negative screening participants with dense breasts, 24 additional cancers were detected, of which 13 were tomosynthesis-detected versus 23 ultrasound-detected. False-positive recall rates were similar. Although the authors caution that these are only interval results, they report that ultrasound has a better incremental breast cancer detection than tomosynthesis in mammography-negative dense breasts, though suggest that DBT should potentially replace FFDM as the primary screening modality.
Although screening ultrasound in women with dense breasts is very effective in detecting mammographically occult breast cancer, the examination has its limitations. The majority of studies have shown an overall low PPV (positive predictive value) of supplemental ultrasound, with its decreased specificity and decreased PPV3 [40, 58, 61]. Screening ultrasound is also a time-consuming examination, with a handheld ultrasound examination of the breasts requiring an average of 20 min, regardless of whether performed by a radiologist or technologist. Automated breast ultrasound (ABUS) has reduced the time of exam, though research regarding its efficacy as a supplemental screening modality is somewhat limited and requires further assessment [61, 62].
Breast MRI
Supplemental breast MR imaging is most widely used for supplemental screening in high-risk women, most commonly those with greater than 20% lifetime risk of developing breast cancer based on risk-assessment models, BRCA and PTEN genetic mutation carriers or their first-degree untested relatives, and patients with history of chest radiation between the ages of 10–30 years. Although many institutions also include patients with a personal history of breast cancer or pre-malignant breast lesions, patients with a family history of malignancy, and women with dense breasts, its widespread use is limited due to expense, availability, and personal contraindications (including incompatible surgical implants, claustrophobia, contrast allergy, or risk of nephrogenic systemic fibrosis in patients with renal insufficiency that receive gadolinium contrast), as well as high false-positive rates. However, a recent study by Kuhl et al. reported a total supplemental cancer detection rate with MRI of 15.5 per 1000 cases in average risk woman, regardless of breast density [63]. They advocate that MRI replace mammography in screening the average risk woman, given its apparent improved sensitivity for detecting biologically relevant cancers.
Kuhl et al. have also been strong proponents of an abbreviated MRI protocol (AB-MRI) for breast screening [63, 64]. The protocol consists of only one pre- and one post-contrast acquisition, and their derived images (the first post-contrast subtracted [FAST] and maximum-intensity projection [MIP] images). Study acquisition time was reduced from 17 to 3 min, and radiologist reading time was 2.8 s for interpretation of the MIP image (deciding upon presence or absence of significant enhancement) and 28 s for interpretation of the complete abbreviated study, with a reported NPV (negative predictive value) of 99.8 [64]. These values are competitive with batch reading of screening mammograms and are shorter than the time to review DBT images [64]. Further studies to evaluate the performance of AB-MRI are being performed, and although scan and interpretation times in the USA have shortened, they remain longer than those reported by Kuhl. The EA1141 trial is an ongoing prospective multicenter diagnostic accuracy trial sponsored by ECOG-ACRIN in an aim to assess the performance of abbreviated breast MRI and digital breast tomosynthesis in breast cancer screening in women with dense breasts. The trial will assess AB-MRI as both a supplemental screening modality and a stand-alone [65••].
Molecular Breast Imaging
Molecular breast imaging (MBI) is a functional imaging modality that utilizes a short-lived radiotracer (99mTc-sestamibi) to detect cancer. Rhodes et al., in a prospective clinical trial comparing mammography with MBI in patients with dense breasts demonstrated that MBI has a substantially higher cancer detection rate than 2D mammography, with a supplemental cancer detection rate of 8.8 per 1000 woman, when added to FFDM [66•]. Shermis et al. reported a similar 7.7% incremental cancer detection rate when employed in a large, community-based practice [67]. The associated low false-positive rate makes MBI an intriguing candidate for supplemental screening to mammography in dense breasts, allowing the detection of cancer regardless of whether a concordant structural abnormality is identified on DBT or 2D-FFDM. MBI has also shown added value in the supplemental assessment of the dense breast with certain practices entirely substituting it for whole-breast ultrasonography [68, 69]. MBI may also be indicated when a physiologic supplemental examination is needed and there are contraindications to MRI use.
A limitation to MBI is its added radiation dose. Rhodes et al. reported an effective whole body dose of 2.4 mSv. Although higher than the average effective dose from digital mammography (~ 0.5 mSv) and the effective dose from digital mammography combined with DBT (1.2 mSv), it is still below natural background radiation levels (US annual average, 3 mSv) [66•].
Contrast-Enhanced Mammography and Contrast-Enhanced Tomography
Various groups have evaluated the role of contrast-enhanced mammography (CEM) over the years, with increased interest of late due to the adoption of digital mammography. This enhanced examination has the potential to detect vascularization and physiological activity of the breast at a lower cost than MRI. As with MBI, it may be of benefit to women who have a contraindication to MRI, women with dense breasts, or even women of intermediate to high risk of breast cancer. Studies have documented that breast MRI has the highest sensitivity for cancer detection, when compared to MBI, ultrasound, and DBT [70, 71]. However, recent studies have shown that the sensitivity of CEM may approach that of MRI [72, 73]. Some groups have combined CEM with tomosynthesis, to develop contrast-enhanced tomography (CET). A study by Chou et al. reported that CET is superior to non-enhanced breast imaging tools. However, no statistically significant difference in the AUC-value was observed when comparing it to CEM [74].
Neither CEM nor CET has found widespread acceptance. Many opponents disagree with the utilization of IV non-iodinated contrast, with its inherent risks and morbidities (including allergy, anaphylaxis, and potential contrast-induced nephropathy) in the mammography suite.
Conclusion
The ultimate goal of breast cancer mortality rate reduction has led to the implementation of multiple advanced mammographic imaging techniques, the most notable and widely used being digital breast tomosynthesis. Numerous studies have confirmed its added value with increased cancer detection of 1–3 additional cancers per 1000 screens, and in most cases, its decreased recall rates, leading to decreased patient anxiety and diminished cost of screening evaluations. Given these added benefits, DBT is gradually replacing 2D FFDM as the primary screening modality. Despite this incremental improvement in screening, DBT remains less sensitive than other supplemental screening modalities. Multiple studies have shown that MRI has the greatest sensitivity for supplemental cancer detection, though given its high false-positive rate, in addition to cost, often limited availability, and personal contraindications, screening MRI is predominantly reserved for high-risk patients. Supplemental whole-breast ultrasound is the most widely used supplemental screening tool, due to its low cost, absence of radiation, and ease of accessibility. False-positive rates remain high, though proponents argue that with increased experience, these will decrease over time. MBI has a higher sensitivity than ultrasound and higher specificity, but is less widely utilized, in part due to accessibility and radiation dose. With further study and potential decreased radiation, MBI may be a promising supplemental screening modality in patients with dense breasts. CEM and CET are being investigated, though are less likely to gain widespread acceptance, given their reliance on intravenous contrast with its inherent risks and necessary precautions in the mammography suite.
Abbreviations
- 2D-FFDM:
-
Two-dimensional full-field digital mammography
- AB-MRI:
-
Abbreviated MRI
- BRCA gene:
-
Breast cancer susceptibility gene
- CEM:
-
Contrast-enhanced mammography
- CET:
-
Contrast-enhanced tomography
- DBT:
-
Digital breast tomosynthesis
- MBI:
-
Molecular breast imaging
- MRI:
-
Magnetic resonance imaging
- PTEN:
-
Phosphatase and tensin homolog
- PPV3 :
-
Positive predictive value of biopsies performed
- s2D:
-
Synthesized two-dimensional mammography
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tabár L, Vitak B, Chen TH-H, Yen AM-F, Cohen A, Tot T, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260(3):658–63.
Tabar L, Gad A, Holmberg L, Ljungquist U, group KCP, Fagerberg C, et al. Reduction in mortality from breast cancer after mass screening with mammography: randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;325(8433):829–32.
Tabar L, Fagerberg G, Duffy SW, Day NE. The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit. J Epidemiol Community Health. 1989;43(2):107–14.
Tabar L, Fagerberg G, Chen HH, Duffy SW, Smart CR, Gad A, et al. Efficacy of breast cancer screening by age. New results Swedish two-county trial. Cancer. 1995;75(10):2507–17.
Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin N Am. 2004;42(5):793–806.
Pisano ED, Hendrick RE, Yaffe MJ, Baum JK, Acharyya S, Cormack JB, et al. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology. 2008;246(2):376–83.
Duncan K, Needham G, Gilbert FJ, Deans H. Incident round cancers: what lessons can we learn? Clin Radiol. 1998;53(1):29–32.
Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–75.
Day N, Warren R. Mammographic screening and mammographic patterns. Breast Cancer Res. 2000;2(4):247.
Chetlen A, Mack J, Chan T. Breast cancer screening controversies: who, when, why, and how? Clin Imaging. 2016;40(2):279–82.
van den Ende C, Oordt-Speets AM, Vroling H, van Agt HM. Benefits and harms of breast cancer screening with mammography in women aged 40-49 years: a systematic review. Int J Cancer. 2017.
Chiu SY-H, Duffy S, Yen AM-F, Tabár L, Smith RA, Chen H-H. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol Prevent Biomark. 2010;19(5):1219–28.
McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Prevent Biomark. 2006;15(6):1159–69.
Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13(6):223.
Kopans DB. Digital breast tomosynthesis from concept to clinical care. Am J Roentgenol. 2014;202(2):299–308.
Vedantham S, Karellas A, Vijayaraghavan GR, Kopans DB. Digital breast tomosynthesis: state of the art. Radiology. 2015;277(3):663–84.
Des Plantes BZ. Eine neue methode zur differenzierung in der roentgenographie (planigraphie). Acta Radiol. 1932;Original Series, Volume 13(2):182–92.
Miller ER, MoCurry EM, Hruska B. An infinite number of laminagrams from a finite number of radiographs. Radiology. 1971;98(2):249–55.
Niklason LT, Christian BT, Niklason LE, Kopans DB, Castleberry DE, Opsahl-Ong B, et al. Digital tomosynthesis in breast imaging. Radiology. 1997;205(2):399–406.
Poplack SP, Tosteson TD, Kogel CA, Nagy HM. Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography. Am J Roentgenol. 2007;189(3):616–23.
Rafferty EA. Digital mammography: novel applications. Radiol Clin N Am. 2007;45(5):831–43.
Good WF, Abrams GS, Catullo VJ, Chough DM, Ganott MA, Hakim CM, et al. Digital breast tomosynthesis: a pilot observer study. Am J Roentgenol. 2008;190(4):865–9.
Gur D, Abrams GS, Chough DM, Ganott MA, Hakim CM, Perrin RL, et al. Digital breast tomosynthesis: observer performance study. Am J Roentgenol. 2009;193(2):586–91.
•• Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56. This was the first large prospective trial comparing FFDM to FFDM plus DBT in a screening population. They reported an increase in cancer detection rate and decrease in false-positive rate.
• Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–9. This is another early, prospective clinical trial evaluating the addition of DBT to FFDM in a screening population. They reported an incremental cancer detection rate of 2.7 per 1000 screens with the addition of DBT.
• Lång K, Andersson I, Rosso A, Tingberg A, Timberg P, Zackrisson S. Performance of one-view breast tomosynthesis as a stand-alone breast cancer screening modality: results from the Malmö Breast Tomosynthesis Screening Trial, a population-based study. Eur Radiol. 2016;26(1):184–90. A 3 rd , prospective European clinical trial comparing single view DBT to FFDM in a screening population. Although they reported an increased cancer detection rate of 43% with DBT, their recall rate rose from from 3.8 to 2.6%.
• Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. Jama. 2014;311(24):2499–507. First, large, multicenter, retrospective analysis in US looking at FFDM versus FFDM + DBT in the screening population. They reported an incremental cancer detection rate of 1.2 per 1000 screens and a decrease in recall rate of 16/1000 screens when DBT was added to FFDM.
Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269(3):694–700.
Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton Jr R. Implementation of breast tomosynthesis in a routine screening practice: an observational study. Am J Roentgenol. 2013;200(6):1401–8.
Durand MA, Haas BM, Yao X, Geisel JL, Raghu M, Hooley RJ, et al. Early clinical experience with digital breast tomosynthesis for screening mammography. Radiology. 2014;274(1):85–92.
McCarthy AM, Kontos D, Synnestvedt M, Tan KS, Heitjan DF, Schnall M et al. Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program. JNCI J Natl Cancer Inst. 2014;106(11).
Greenberg JS, Javitt MC, Katzen J, Michael S, Holland AE. Clinical performance metrics of 3D digital breast tomosynthesis compared with 2D digital mammography for breast cancer screening in community practice. Am J Roentgenol. 2014;203(3):687–93.
Lourenco AP, Barry-Brooks M, Baird GL, Tuttle A, Mainiero MB. Changes in recall type and patient treatment following implementation of screening digital breast tomosynthesis. Radiology. 2014;274(2):337–42.
McDonald ES, McCarthy AM, Akhtar AL, Synnestvedt MB, Schnall M, Conant EF. Baseline screening mammography: performance of full-field digital mammography versus digital breast tomosynthesis. Am J Roentgenol. 2015;205(5):1143–8.
Sharpe RE Jr, Venkataraman S, Phillips J, Dialani V, Fein-Zachary VJ, et al. Increased cancer detection rate and variations in the recall rate resulting from implementation of 3D digital breast tomosynthesis into a population-based screening program. Radiology. 2016;278(3):698–706. https://doi.org/10.1148/radiol.2015142036.
• Conant EF, Beaber EF, Sprague BL, Herschorn SD, Weaver DL, Onega T, et al. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: a cohort study within the PROSPR consortium. Breast Cancer Res Treat. 2016;156(1):109–16. This study addressed whether DBT is associated with improved screening outcomes by evaluating followup data and assessing false negatives. They support DBT screening, noting that in addition to previously documented increased cancer detection and decreased recall rates, there was no difference in false-negative screening exams.
Rafferty EA, Durand MA, Conant EF, Copit DS, Friedewald SM, Plecha DM, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–6.
Mettler FA, Upton AC, Kelsey CA, Ashby RN, Rosenberg RD, Linver MN. Benefits versus risks from mammography: a critical reasessment. Cancer. 1996;77(5):903–9.
Svahn T, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast. 2015;24(2):93–9.
Hooley RJ, Durand MA, Philpotts LE. Advances in digital breast tomosynthesis. Am J Roentgenol. 2017;208(2):256–66.
Houssami N. Evidence on synthesized two-dimensional mammography versus digital mammography when using tomosynthesis (three-dimensional mammography) for population breast cancer screening. Clin Breast Cancer. 2017.
Aujero MP, Gavenonis SC, Benjamin R, Zhang Z, Holt JS. Clinical performance of synthesized two-dimensional mammography combined with tomosynthesis in a large screening population. Radiology. 2017;283(1):70–6. https://doi.org/10.1148/radiol.2017162674.
Bernardi D, Macaskill P, Pellegrini M, Valentini M, Fantò C, Ostillio L, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol. 2016;17(8):1105–13.
Brandt KR, Craig DA, Hoskins TL, Henrichsen TL, Bendel EC, Brandt SR, et al. Can digital breast tomosynthesis replace conventional diagnostic mammography views for screening recalls without calcifications? A comparison study in a simulated clinical setting. Am J Roentgenol. 2013;200(2):291–8.
Zuley ML, Bandos AI, Ganott MA, Sumkin JH, Kelly AE, Catullo VJ, et al. Digital breast tomosynthesis versus supplemental diagnostic mammographic views for evaluation of noncalcified breast lesions. Radiology. 2013;266(1):89–95.
Poplack S. Breast tomosynthesis: clinical evidence. Radiol Clin N Am. 2017;55(3):475–92. https://doi.org/10.1016/j.rcl.2016.12.010.
Hakim CM, Chough DM, Ganott MA, Sumkin JH, Zuley ML, Gur D. Digital breast tomosynthesis in the diagnostic environment: a subjective side-by-side review. Am J Roentgenol. 2010;195(2):W172–W6.
Tagliafico A, Astengo D, Cavagnetto F, Rosasco R, Rescinito G, Monetti F, et al. One-to-one comparison between digital spot compression view and digital breast tomosynthesis. Eur Radiol. 2012;22(3):539–44. https://doi.org/10.1007/s00330-011-2305-1.
Noroozian M, Hadjiiski L, Rahnama-Moghadam S, Klein KA, Jeffries DO, Pinsky RW, et al. Digital breast tomosynthesis is comparable to mammographic spot views for mass characterization. Radiology. 2012;262(1):61–8. https://doi.org/10.1148/radiol.11101763.
Morel JC, Iqbal A, Wasan RK, Peacock C, Evans DR, Rahim R, et al. The accuracy of digital breast tomosynthesis compared with coned compression magnification mammography in the assessment of abnormalities found on mammography. Clin Radiol. 2014;69(11):1112–6. https://doi.org/10.1016/j.crad.2014.06.005.
Spangler ML, Zuley ML, Sumkin JH, Abrams G, Ganott MA, Hakim C, et al. Detection and classification of calcifications on digital breast tomosynthesis and 2D digital mammography: a comparison. Am J Roentgenol. 2011;196(2):320–4. https://doi.org/10.2214/AJR.10.4656.
Kopans D, Gavenonis S, Halpern E, Moore R. Calcifications in the breast and digital breast tomosynthesis. Breast J. 2011;17(6):638–44. https://doi.org/10.1111/j.1524-4741.2011.01152.x.
Partyka L, Lourenco AP, Mainiero MB. Detection of mammographically occult architectural distortion on digital breast tomosynthesis screening: initial clinical experience. Am J Roentgenol. 2014;203(1):216–22.
Freer PE, Niell B, Rafferty EA. Preoperative tomosynthesis-guided needle localization of mammographically and sonographically occult breast lesions. Radiology. 2015;275(2):377–83. https://doi.org/10.1148/radiol.14140515.
Wallis MG, Moa E, Zanca F, Leifland K, Danielsson M. Two-view and single-view tomosynthesis versus full-field digital mammography: high-resolution X-ray imaging observer study. Radiology. 2012;262(3):788–96.
Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. Am J Roentgenol. 2003;181(1):177–82.
Corsetti V, Houssami N, Ferrari A, Ghirardi M, Bellarosa S, Angelini O, et al. Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur J Cancer. 2008;44(4):539–44.
Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–63.
Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265(1):59–69.
Tagliafico AS, Calabrese M, Mariscotti G, Durando M, Tosto S, Monetti F, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial. J Clin Oncol. 2016;34(16):1882–8. https://doi.org/10.1200/jco.2015.63.4147.
Geisel J, Raghu M, Hooley R, editors. The role of ultrasound in breast cancer screening: the case for and against ultrasound. Seminars in Ultrasound, CT and MRI; 2018, Elsevier.
Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. Am J Roentgenol. 2015;204(2):234–40. https://doi.org/10.2214/AJR.13.12072.
Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–70. https://doi.org/10.1148/radiol.2016161444.
Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers R-D, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–10.
•• Kuhl CK. Abbreviated breast MRI for screening women with dense breast: the EA1141 trial. Br J Radiol. 2017;90. https://doi.org/10.1259/bjr.20170441. Important ongoing multicenter ECOG-ACRIN trial that will compare abbreviated MRI and DBT. Results could change the role of breast MRI in screening.
• Rhodes DJ, Hruska CB, Conners AL, Tortorelli CL, Maxwell RW, Jones KN, et al. JOURNAL CLUB: molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts. Am J Roentgenol. 2015;204(2):241–51. https://doi.org/10.2214/AJR.14.13357. This study explores the role of MBI in supplemental breast screening, and reports a supplemental cancer detection rate of 8.8 per 1000 woman when MBI is added to FFDM, with minimal decrease in specificity. Data suggests that MBI could play an important role in supplemental imaging of the dense breast.
Shermis RB, Wilson KD, Doyle MT, Martin TS, Merryman D, Kudrolli H, et al. Supplemental breast Cancer screening with molecular breast imaging for women with dense breast tissue. Am J Roentgenol. 2016;207(2):450–7. https://doi.org/10.2214/AJR.15.15924.
Shermis RB, Redfern RE, Burns J, Kudrolli H. Molecular breast imaging in breast cancer screening and problem solving. Radiographics. 2017;37(5):1309–606. https://doi.org/10.1148/rg.2017160204.
Brem RF. Invited commentary on “molecular breast imaging in breast cancer screening and problem solving”. Radiographics. 2017;37(5):1328–9. https://doi.org/10.1148/rg.2017170174.
Berg WA. Current status of supplemental screening in dense breasts. J Clin Oncol. 2016;34(16):1840–3.
Covington MF, Pizzitola VJ, Lorans R, Pockaj BA, Northfelt DW, Appleton CM, et al. The future of contrast-enhanced mammography. Am J Roentgenol. 2018;2017:1–9. https://doi.org/10.2214/AJR.17.18749.
Lee-Felker SA, Tekchandani L, Thomas M, Gupta E, Andrews-Tang D, Roth A, et al. Newly diagnosed breast cancer: comparison of contrast-enhanced spectral mammography and breast MR imaging in the evaluation of extent of disease. Radiology. 2017;285(2):389–400.
Fallenberg EM, Schmitzberger FF, Amer H, Ingold-Heppner B, Balleyguier C, Diekmann F, et al. Contrast-enhanced spectral mammography vs. mammography and MRI—clinical performance in a multi-reader evaluation. Eur Radiol. 2017;27(7):2752–64. https://doi.org/10.1007/s00330-016-4650-6.
Chou C-P, Lewin JM, Chiang C-L, Hung B-H, Yang T-L, Huang J-S, et al. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis—comparison to contrast-enhanced breast MRI. Eur J Radiol. 2015;84(12):2501–8. https://doi.org/10.1016/j.ejrad.2015.09.019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Arwa A. Alzaghal and Pamela J. DiPiro declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Breast Cancer
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Alzaghal, A.A., DiPiro, P.J. Applications of Advanced Breast Imaging Modalities. Curr Oncol Rep 20, 57 (2018). https://doi.org/10.1007/s11912-018-0700-3
Published:
DOI: https://doi.org/10.1007/s11912-018-0700-3