Abstract
Purpose of Review
Many studies have examined the effects of adjuvant bisphosphonates on long-term breast cancer outcomes. However, results have been inconsistent. Here, we review the evidence for their role in early breast cancer.
Recent Findings
In a recent meta-analysis, no significant decreases in recurrence or breast cancer mortality were observed in the overall population. In postmenopausal women, statistically significant, but modest, reductions in distant recurrence were observed, driven by decreased bone recurrence. This translated to decreased breast cancer mortality. While most individual studies were not performed exclusively in postmenopausal patients and were not adequately powered to detect subgroup effects based on menopausal status, observed effects were highly consistent.
Summary
Adjuvant bisphosphonates in postmenopausal women should be considered in individual cases of high-risk patients, where the absolute benefit justifies associated risks. There is no evidence supporting their routine use in premenopausal women except in selected patients receiving ovarian function suppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Breast cancer commonly metastasizes to bone, resulting in complications such as pain, fractures, spinal cord compression, and hypercalcemia. While the role of bone-targeted treatments is well established for the prevention of skeletal-related events in advanced breast cancer with bone involvement, such treatment is not associated with improvement in either progression-free or overall survival [1,2,3,4]. Many studies have examined whether the use of bisphosphonates in the adjuvant setting may achieve a greater impact on long-term outcomes. As a result of predominantly negative data from individual trials, the role of bisphosphonates in early breast cancer has been limited to the setting of treatment–induced bone loss until results of a recent meta-analysis suggested benefit in post-menopausal women [5••]. However, while some studies have shown that the use of adjuvant bisphosphonates may benefit selected patients, trial populations and outcomes have been heterogeneous, resulting in difficulty in interpreting results. Here, we review the evidence for the use of bisphosphonates as part of the treatment of early stage breast cancer.
Mechanism of Action
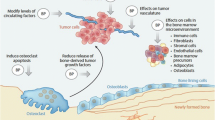
The mechanism of breast cancer dissemination to the bone is not fully understood, but has previously been explained using a “seed and soil” concept [6]. Malignant cells (the “seeds”) spread to the bone (the “soil”), which provides a favorable site for tumor accumulation due to the production of osteolysis-promoting growth factors. Tumor cells also release osteoblast-stimulating growth factors, resulting in the production of nuclear factor-κB ligand (RANKL) and osteoclast activation. The consequent osteolysis leads to the release of tumor-promoting growth factors, further contributing to this destructive cycle. Bisphosphonates, including non-nitrogen containing compounds such as clodronate and aminobisphosphonates such as pamidronate, ibandronate, and zoledronic acid inhibit these growth factors and can induce osteoclast apoptosis, thus decreasing osteoclastic bone resorption. This effect likely results in a more hostile bone microenvironment for cancer cell dissemination.
Menopausal Status in Trials of Bisphosphonates in Early Breast Cancer
The majority of trials of bisphosphonates in early stage breast cancer were performed in patients unselected for menopausal status. Many studies have examined the effect of adjuvant bisphosphonates in subgroups of postmenopausal women either prospectively or retrospectively. However, studies have used varying definitions of menopause, which complicates interpretation of these data. Key studies are summarized in Table 1.
The most compelling evidence for bisphosphonate therapy in the adjuvant setting is provided by the Early Breast Cancer Trialists Collaborative Group (EBCTCG) meta-analysis, which pooled patient-level data from randomized trials comparing bisphosphonates to control arms [5••]. Data from 26 studies comprising 18,766 participants were pooled. In this cohort were 11,767 post-menopausal women. Primary outcomes of eligible studies included recurrence, distant recurrence, and breast cancer mortality. The analysis also included pre-specified primary subgroup comparisons based on menopausal status. However, definitions of menopause varied between individual studies, and data from some trials were unknown and therefore assumed based on age (<45 years premenopausal; 45–54 years perimenopausal; ≥55 years postmenopausal). In the overall population, reductions in recurrence were non-significant (Recurrence Risk (RR) 0.94, 95% Confidence Interval (95% CI) 0.87–1.01; p = 0.08). Distant recurrence (RR 0.92, 95% CI 0.85–0.99; p = 0.03), and breast cancer mortality (RR 0.91, 95% CI 0.83–0.99; p = 0.04) were modestly reduced, likely driven by decreased bone recurrence (0.83, 95% CI 0.73–0.94; p = 0·004). In a subgroup analysis of postmenopausal women, reductions in recurrence (RR 0.86, 95% CI 0.78–0.94; p = 0.002), distant recurrence (0.82, 0.74–0.92; p = 0.0003), bone recurrence (0.72, 0.60–0.86; p = 0.0002), and breast cancer mortality (RR 0.82, 95% CI 0.73–0.93; p = 0.002) were all highly significant. Recurrence outside the bone was not significantly reduced (RR 0.90; 95% CI 0.79–1.0; p = 0.10). In further sub-analyses performed in the postmenopausal subgroup, only the choice of agent had a significant effect on bone recurrence (p = 0.01). Compared to clodronate, ibandronate, or zoledronic acid, there appeared to be less benefit with pamidronate. Significant subgroup differences by agent were not observed for distant recurrence or breast cancer mortality. No differences were observed based on estrogen receptor (ER) status, nodal status, tumor grade, dose-intensity, receipt of chemotherapy, or treatment duration.
Trials of Clodronate
In the EBCTCG meta-analysis, studies of clodronate in the post-menopausal subgroup were associated with decreased breast cancer mortality (RR 0.77; 95%CI 0.64–0.93; p = 0·005) [5••], whereas results of individual studies have been mixed [7,8,9,10]. In two studies, clodronate was associated with improved overall survival (OS), although this was a secondary endpoint [7, 8]. In the first study, patients were randomly assigned to receive daily clodronate for 2 years or standard follow-up [7]. Use of clodronate led to significantly decreased mortality (death at median follow-up (8.5 years) 20.4 versus 40.7%; p = 0.049). The primary endpoint, the frequency and number of new bone and visceral metastases, was not different between groups. However, the study was not performed exclusively in postmenopausal patients (63% postmenopausal in clodronate arm; 61% in control arm), and subgroup analyses based on menopausal status were not performed. In another study not restricted based on menopausal status, clodronate met its primary endpoint of reducing bone recurrence (Hazard Ratio (HR) = 0.69, p = 0.04). OS was also improved (HR 0.77, p = 0.048), although due to it being a secondary endpoint, this was not considered a definitive result. No reduction in visceral metastases was observed [8].
Conversely, two different studies with clodronate found no disease-free survival (DFS) benefit and no reduction in bone metastases [9,10,11]. A small Finnish trial of 3 years of adjuvant clodronate did not reduce bone recurrence or improve OS. Furthermore, 10-year DFS was significantly worse in the clodronate group (45 versus 58%, p = 0.01) [10, 11]. The large NSABP (National Surgical Adjuvant Breast and Bowel Project) B34 trial, which randomized 3311 women with stage I–III breast cancer to oral clodronate or placebo for 3 years, also yielded negative results. DFS did not differ between groups (HR 0.91, 95% CI 0.78–1.07), although secondary endpoints of recurrence-free interval (HR 0.75, 95% CI 0.57–0.99, p = 0.045), bone metastases-free interval (HR 0.62, 95% CI 0.40–0.95, p = 0.027), and non-bone metastases free interval (HR 0.63, 95% CI 0.43–0.91, p = 0.014) were significantly improved in women 50 years of age or older [9].
Trials of Aminobisphosphonates
Zoledronic acid is a nitrogen-containing, intravenously (IV) administered bisphosphonate. In the EBCTCG meta-analysis, the addition of more than 2 years of zoledronic acid decreased the risk of bone recurrence (RR 0.73, 95%CI 0.53–1.00; p = 0.05) in post-menopausal women, but was not associated with reductions in breast cancer mortality (RR 0.88; 95% CI 0.69–1.11). Individual studies of postmenopausal women have also shown reductions in bone recurrence and improvements in DFS. The Adjuvant Zoledronic Acid to Reduce Recurrence (AZURE) study, a multicentre, phase 3 randomized control trial, assessed the effect of zoledronic acid for 5 years in combination with (neo-) adjuvant chemotherapy and/or endocrine therapy. The primary outcome was DFS, and invasive disease-free survival (IDFS) was identified as a key secondary outcome. While bone recurrence was decreased overall (HR 0.81, 95% CI 0.68–0.97; p = 0·022), DFS, IDFS, and OS did not differ significantly in the overall study population. However, improvement in IDFS was observed in post-menopausal women (HR 0.77, 95% CI 0.63–0.96). Of interest, menopause was defined prospectively as more than 5 years since last menstruation [12•]. In the ZO-FAST trial, the effect of upfront versus delayed zoledronic acid (initiated for fracture or substantial bone loss) on bone mineral density (BMD) was assessed in postmenopausal, European women receiving letrozole [13]. In this trial, the primary aim was to explore early versus late treatment with zoledronic acid to prevent treatment-induced bone loss. Breast cancer-specific outcomes such as disease recurrence and survival were secondary endpoints. Early zoledronic acid was associated with improved DFS (HR = 0.66; p = 0.04) and reduced local (0.9 versus 2.3%) and distant (5.5 versus 7.7%) recurrences. OS was not significantly different between groups (HR = 0.69; 95% CI = 0.42–1.14; p = 0.15). In exploratory analyses of women with established postmenopausal status (>5-years postmenopausal or >60 years of age), thus excluding women who became menopausal following the diagnosis of breast cancer but before randomization, early zoledronic acid was associated with a trend for improved DFS (HR = 0.63; p = 0.052) and improved OS (HR = 0.50; p = 0.02). Similar results were observed in the US-based sister study, Z-FAST [14], but no effect on recurrence was observed in the E-ZO-FAST trial conducted in the rest of the world [15]. Similarly, the GAIN study, which examined use of ibandronate for 2 years in patients with early-stage node-positive breast cancer, did not show any DFS benefit [16].
Denosumab
The role of non-bisphosphonate bone-targeted therapy has also been examined. Denosumab, a subcutaneously administered anti-RANK ligand antibody, was studied in a prospective, randomized phase 3 trial, ABCSG-18 [17•, 18]. In this multicenter study, 3420 postmenopausal women with early hormone receptor-positive breast cancer receiving treatment with aromatase inhibitors were randomly assigned to receive denosumab 60 mg or placebo every 6 months. Postmenopausal status was defined as aged 60 years or older, aged less than 60 with postmenopausal-range levels of follicle-stimulating hormone and estradiol or previous bilateral oophorectomy. While the primary endpoint was time to first clinical fracture, secondary endpoints included DFS and OS. This trial was stopped early due to a substantially large effect of bone fracture reduction with denosumab. As such, statistical power for secondary analyses was reduced. Although recurrence occurred infrequently after a median follow-up of 4 years (164/1711 in denosumab group; 199/1709 in placebo group), a borderline significant decrease in DFS was observed (HR 0.82, p = 0.051). Despite the underpowered analysis, the magnitude of breast cancer risk reduction with denosumab was similar to that observed with bisphosphonates in the EBCTCG meta-analysis (18 versus 14% respectively). At this time, OS data are still immature. The ongoing D-CARE study has enrolled over 4500 high-risk early breast cancer patients and randomized them to receive denosumab or placebo for 5 years. Unlike the ABCSG-18 trial, the dosing of denosumab was higher in D-CARE. A de-escalating regimen was utilized with participants receiving 120 mg subcutaneously once, monthly for 6 months, followed by the same dose every 3 months for the next 4.5 years. Results of the D-CARE study are expected in late 2017 or early 2018.
Role in Premenopausal Women
While the EBCTCG meta-analysis presented evidence for the use of adjuvant bisphosphonate therapy in post-menopausal women, bisphosphonates do not have the same effect in the pre-menopausal subgroup [5••]. Compared to controls, bisphosphonates did not significantly decrease bone recurrence (RR 0.92; 95% CI 0.75–1.12; p = 0.42) or breast cancer mortality RR 1.00; 95% CI 0.86–1.15; p = 0.96). This effect was reflected in data from individual trials including AZURE, where the benefit of zoledronic acid on IDFS observed in postmenopausal women was not seen in those that were pre or perimenopausal (HR 1.03, 95% CI 0.89–1.20). ABSCG-12, a phase 3 multicentre study, randomized premenopausal women with stage I-II breast cancer to monthly goserelin, a gonadotropin-releasing hormone agonist, in combination with 3 years of either tamoxifen or anastrozole. A second randomization assigned patients to zoledronic acid every 6 months for 3 years or no additional therapy. It should be noted that due to use of ovarian function suppression (OFS), data from ABCSG-12 were included in the postmenopausal subgroup of the EBCTCG meta-analysis. However, subsequent studies have shown that estradiol levels may remain above postmenopausal thresholds in premenopausal women receiving OFS especially in younger women and when combined with aromatase inhibitors [19•]. In the ABCSG-12 trial, 30% of participants had node-positive disease; however, cytotoxic chemotherapy was not administered in the adjuvant setting. A minority of participants (<10%) received neoadjuvant chemotherapy. The study’s primary endpoint was met, as the addition of zoledronic acid improved DFS (HR 0.68, 95% CI 0.51–0.91; p = 0.009). A non-significant effect on mortality was also observed (HR 0.67, 95% CI 0.41–1.07; p = 0.09) [20•]. The magnitude of relative recurrence risk reduction was similar in node-positive (HR 0.67, 95% CI 0.45–0.99) and node-negative patients (HR 0.66, 95% CI 0.43–1.03). In a preplanned subset analysis, zoledronic acid did not significantly reduce DFS in patients who were 40 years of age or less at study entry (HR 0.94, 95% CI 0.57–1.56), whereas this remained significant in those older than 40 (HR 0.58, 95% CI 0.40–0.83). Young age has been associated with a higher frequency of failure to achieve adequate ovarian suppression with monthly gonadotropin-releasing hormone agonists [19•]. Failure to achieve truly menopausal levels of estrogen may explain the lack of effect of zoledronic acid in combination with goserelin in younger participants of the ABCSG-12 trial.
Toxicity
The incidence of bisphosphonate-associated adverse events (AEs) is well documented in many of the outlined studies. While bisphosphonates may increase gastrointestinal symptoms such as diarrhea, focus is often given to more serious AEs such as renal failure and osteonecrosis of the jaw (ONJ). The incidence of ONJ and renal failure in key studies are summarized in Table 2. While increases in these bisphosphonate-related AEs were seen in some studies, no confirmed cases of ONJ or increased renal impairment were observed in studies using lower dose intensity regimens, such as ABCSG-12 and ABCSG-18 [17, 20•].
Patient Preference and Treatment Adherence
Data from the EBCTCG meta-analysis indicates that route of administration does not impact outcomes. In SWOG S0307, a 3-arm randomized trial comparing the effect of oral clodronate, oral ibandronate, and IV zoledronic acid, patients were asked for their preference regarding receipt of IV or oral drug, assuming equal efficacy [21]. Prior to randomization, 76% preferred oral administration, and few switched preferences at time of treatment completion. In the analogous setting of metastatic breast cancer, a trial comparing oral ibandronate to IV zoledronic acid showed that overall adherence was similar with ibandronate (83%) and zoledronic acid (86%) [22].
Conclusions: Current Perspectives and Future Directions
Based on results of the EBCTCG patient-level meta-analysis, as well as results of individual trials, there is currently no evidence supporting the routine use of adjuvant bisphosphonates in the treatment of premenopausal or peri-menopausal women with breast cancer [5••]. While results of the ABCSG-12 trial suggested that recurrences were decreased with the use of zoledronic acid in premenopausal women receiving OFS, benefit in the premenopausal group was not consistently seen across individual trials or in the pooled analysis. Data from trials of ovarian suppression for early breast cancer have shown that a substantial proportion of women does not achieve adequate ovarian suppression and can have elevated circulating levels of estradiol. If the addition of adjuvant bisphosphonates only provides benefit in women with post-menopausal levels of estrogen as suggested in the EBCTCG analysis, this observation likely explains why inconsistent results were observed in the ABCSG-12. Additionally, since the publication of the SOFT and TEXT trials data, it has become apparent that the addition of ovarian suppression is beneficial only in younger pre-menopausal women and those with higher risk for disease recurrence [23, 24]. Pre-menopausal women with low risk breast cancer and not requiring cytotoxic chemotherapy had an excellent prognosis with tamoxifen alone and did not benefit substantially from ovarian function suppression [23]. This group of patients are quite different from those treated in the ABCSG-12 trial, where higher risk patients did not receive cytotoxic chemotherapy while even the lower risk patients were treated with ovarian suppression. This inconsistency in treatment makes data from the ABCSG-12 trial more difficult to interpret. Consequently, there remains some uncertainty regarding the utility of adjuvant bisphosphonates in pre-menopausal women receiving OFS.
In postmenopausal women, significant reductions in distant recurrence were observed, driven by decreased bone recurrence. This reduction translated to a reduction in breast cancer mortality, suggesting a role for their use in this population. The EBCTCG findings are based on pooling of subgroup data. These methods result in a sub-optimally elevated risk of type 1 (false positive) error, and must be interpreted cautiously. Most individual studies were not performed exclusively in postmenopausal patients, and were not adequately powered to detect subgroup effects based on menopausal status. In addition, definitions of menopause were heterogeneous. The EBCTCG meta-analysis also assumed menopausal status based on patient age when individual patient data were unavailable, further complicating interpretation and clinical application of results. Despite these limitations, the consistency of the effect observed in post-menopausal women suggests that it is likely real. However, these limitations provide some uncertainty about the accuracy of the results. Additionally, it should be noted that the magnitude of the effect observed in the EBCTCG was quite modest, with a less than 20% relative reduction in recurrence risk and breast cancer death. This effect would likely translate to a small absolute effect especially in jurisdictions with breast cancer screening programs where the majority of patients are diagnosed with small, node negative breast cancers.
Finally, results of the EBCTCG meta-analysis are also limited by the variability in primary endpoints of individual studies. While many were designed to detect efficacy endpoints, those aimed at examining the effects of bisphosphonates on treatment-associated bone loss were also included, and are unlikely to have been adequately powered to detect differences in recurrence or survival outcomes. Furthermore, secondary or exploratory efficacy data may not have been collected with the same rigor as those of primary endpoints, potentially leading to increased censoring and bias in time to event outcome analyses. Additionally, in trials where the primary endpoint was avoidance of treatment-related bone loss, a higher risk of contamination of the control group is possible.
Results of large ongoing prospective trials, including SWOG0307 trial (NCT00127205), comparing the use of clodronate to zoledronic acid or ibandronate and SUCCESS (NCT02181101) comparing 5 versus 2 years of zoledronic acid, will provide additional data. Consequently, although we would discourage the routine use of adjuvant bisphosphonates in all postmenopausal women, their use should be considered in individual cases of high-risk patients where the absolute benefit justifies the negative impact of therapy such as toxicity and cost (Figure 1a). Future studies should aim to further delineate subsets of women most likely to benefit from their use. The use of bone-targeted therapy can also be considered in pre-menopausal women receiving OFS (Figure 1b). However, such patients should be counseled about the uncertainties regarding treatment efficacy.
References
Papers of particular interest, published recently, have been highlighted as:• Of importance•• Of major importance
Paterson AH, Powles TJ, Kanis JA, McCloskey E, Hanson J, Ashley S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11(1):59–65.
Theriault RL, Lipton A, Hortobagyi GN, Leff R, Glück S, Stewart JF, Costello S, Kennedy I, Simeone J, Seaman JJ, Knight RD, Mellars K, Heffernan M, Reitsma DJ, Group ftPABCS (1999) Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. J Clin Oncol 17 (3):846
Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y, Watanabe T, Goessl C, Ohashi Y, Takashima S. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23(15):3314–21. doi:10.1200/jco.2005.05.116.
Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD, Heffernan M, Reitsma DJ, Kennedy I, Allan SG, Mellars K. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med. 1996;335(24):1785–92. doi:10.1056/NEJM199612123352401.
•• Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials (2015). The Lancet 386 (10001):1353–1361. doi:10.1016/s0140-6736(15)60908-4. Meta-analysis providing rationale for use of bone modifying agents in postmenopausal women.
Kakonen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97(3 Suppl):834–9. doi:10.1002/cncr.11132.
Diel IJ, Jaschke A, Solomayer EF, Gollan C, Bastert G, Sohn C, Schuetz F. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow—a long-term follow-up. Ann Oncol. 2008;19(12):2007–11. doi:10.1093/annonc/mdn429.
Powles T, Paterson A, McCloskey E, Schein P, Scheffler B, Tidy A, Ashley S, Smith I, Ottestad L, Kanis J. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006;8(2):R13. doi:10.1186/bcr1384.
Paterson AHG, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, Weir LM, Brufsky AM, Dakhil S, Lad T, Baez-Diaz L, Gralow JR, Robidoux A, Perez EA, Zheng P, Geyer CE, Swain SM, Costantino JP, Mamounas EP, Wolmark N. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and bowel project protocol B-34): a multicentre, placebo-controlled, randomised trial. The Lancet Oncology. 2012;13(7):734–42. doi:10.1016/s1470-2045(12)70226-7.
Saarto T, Blomqvist C, Virkkunen P, Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol. 2001;19(1):10–7.
Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43(7):650–6. doi:10.1080/02841860410032885.
• Coleman R, Cameron D, Dodwell D, Bell R, Wilson C, Rathbone E, Keane M, Gil M, Burkinshaw R, Grieve R, Barrett-Lee P, Ritchie D, Liversedge V, Hinsley S, Marshall H. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. The Lancet Oncology. 2014;15(9):997–1006. doi:10.1016/s1470-2045(14)70302-x. Key trial providing evidence for use of zoledronic acid in postmenopausal women
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, von Minckwitz G, Sleeboom HP, Forbes J, Barrios C, Frassoldati A, Campbell I, Paija O, Martin N, Modi A, Bundred N. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013;24(2):398–405. doi:10.1093/annonc/mds277.
Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, Jin L, Warsi G, Argonza-Aviles E, Hohneker J, Ericson SG, Perez EA. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192–201. doi:10.1002/cncr.26313.
Llombart A, Frassoldati A, Paija O, Sleeboom HP, Jerusalem G, Mebis J, Deleu I, Miller J, Schenk N, Neven P. Immediate Administration of Zoledronic Acid Reduces Aromatase Inhibitor&#×2013; associated bone loss in postmenopausal women with early breast cancer: 12-month analysis of the E-ZO-FAST trial. Clinical Breast Cancer. 2012;12(1):40–8. doi:10.1016/j.clbc.2011.08.002.
von Minckwitz G, Mobus V, Schneeweiss A, Huober J, Thomssen C, Untch M, Jackisch C, Diel IJ, Elling D, Conrad B, Kreienberg R, Muller V, Luck HJ, Bauerfeind I, Clemens M, Schmidt M, Noeding S, Forstbauer H, Barinoff J, Belau A, Nekljudova V, Harbeck N, Loibl S. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol. 2013;31(28):3531–9. doi:10.1200/JCO.2012.47.2167.
• Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, Wette V, Balic M, Haslbauer F, Melbinger E, Bjelic-Radisic V, Artner-Matuschek S, Fitzal F, Marth C, Sevelda P, Mlineritsch B, Steger GG, Manfreda D, Exner R, Egle D, Bergh J, Kainberger F, Talbot S, Warner D, Fesl C, Singer CF. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–43. doi:10.1016/s0140-6736(15)60995-3. Key trial providing evidence for use of adjuvant denosumab
Gnant M, Pfeiler G, Dubsky P, Hubalek M, Greil R, Jakesz R, Wette V, Balic M, Haslbauer F, Melbinger-Zeinitzer E, Bjelic-Radisic V, Artner-Matuschek S, Fitzal F, Marth C, Sevelda P, Mlineritsch B, Steger G, Manfreda D, Exner R, Egle D, Bergh J, Kainberger F, Talbot S, Warner D, Fesl C, Singer C The impact of adjuvant denosumab on disease-free survival: Results from 3,425 postmenopausal patients of the ABCSG-18 trial. In: San Antonio Breast Cancer Symposium, San Antonio, 2015.
• Bellet M, Gray KP, Francis PA, Lang I, Ciruelos E, Lluch A, Climent MA, Catalan G, Avella A, Bohn U, Gonzalez-Martin A, Ferrer R, Catalan R, Azaro A, Rajasekaran A, Morales J, Vazquez J, Fleming GF, Price KN, Regan MM. Twelve-month estrogen levels in premenopausal women with hormone receptor-positive breast cancer receiving adjuvant Triptorelin plus exemestane or tamoxifen in the suppression of ovarian function trial (SOFT): the SOFT-EST Substudy. J Clin Oncol. 2016;34(14):1584–93. doi:10.1200/JCO.2015.61.2259. Study providing evidence that estradiol levels may remain in postmenopausal range in younger premenopausal women receiving OFS and aromatase inhibitors
• Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M, Hubalek M, Pristauz G, Bauernhofer T, Eidtmann H, Eiermann W, Steger G, Kwasny W, Dubsky P, Hochreiner G, Forsthuber E-P, Fesl C, Greil R. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. The Lancet Oncology. 2011;12(7):631–41. doi:10.1016/s1470-2045(11)70122-x. Key trial of zoledronic acid in premenopausal women receiving GnRH agonists
Gralow J, Barlow WE, Paterson AHG, Lew D, Stopeck A, Hayes DF, Hershman DL, Schubert M, Clemons M, Poznak CHV, Dees EC, Ingle JN, Falkson CI, Elias AD, Messino MJ, Margolis JH, Dakhil SR, Chew HK, Livingston RB, Hortobagyi GN (2014) SWOG S0307 phase III trial of bisphosphonates as adjuvant therapy in primary breast cancer: Comparison of toxicities and patient-stated preference for oral versus intravenous delivery. Journal of Clinical Oncology 32 (5 s (suppl; abstr 558))
Barrett-Lee P, Casbard A, Abraham J, Hood K, Coleman R, Simmonds P, Timmins H, Wheatley D, Grieve R, Griffiths G, Murray N. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: a randomised, open label, non-inferiority phase 3 trial. The Lancet Oncology. 2014;15(1):114–22. doi:10.1016/s1470-2045(13)70539-4.
Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, Gomez HL, Tondini C, Burstein HJ, Perez EA, Ciruelos E, Stearns V, Bonnefoi HR, Martino S, Geyer Jr CE, Pinotti G, Puglisi F, Crivellari D, Ruhstaller T, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Bernhard J, Luo W, Ribi K, Viale G, Coates AS, Gelber RD, Goldhirsch A, Francis PA. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–18.
Francis PA, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA, Burstein HJ, Martino S, Davidson NE, Geyer CEJ, Walley BA, Coleman R, Kerbrat P, Buchholz S, Ingle JN, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Colleoni M, Viale G, Coates AS, Goldhirsch A, Gelber RD. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–46. doi:10.1056/NEJMoa1412379.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Josee-Lyne Ethier, Rebecca M. Prince, and Eitan Amir declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Ethier, JL., Prince, R.M. & Amir, E. Bone Modifier Use as Adjuvant Therapy for Early Breast Cancer. Curr Oncol Rep 19, 15 (2017). https://doi.org/10.1007/s11912-017-0577-6
Published:
DOI: https://doi.org/10.1007/s11912-017-0577-6