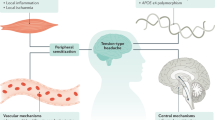
Abstract
Purpose of Review
Description of headache dates back thousands of years, and to date, tension-type headache (TTH) remains the most common form of headache. We will review the history and current understanding of the pathophysiology of TTH and discuss the recommended clinical evaluation and management for this syndrome.
Recent Findings
Despite being the most prevalent headache disorder, TTH pathophysiology remains poorly understood. Patients with TTH tend to have muscles that are harder, more tender to palpation, and may have more frequent trigger points of tenderness than patients without headache. However, cause and effect of these muscular findings are unclear. Studies support both peripheral and central mechanisms contributing to the pain of TTH. Diagnosis is based on clinical presentation, while the focus of evaluation is to rule out possible secondary causes of headache. Treatment options have remained similar over the course of the past decade, with some additional studies supportive of both pharmacological and non-pharmacological options.
Summary
An approach to TTH has been outlined including historical context, evolution over time, and the best evidence regarding our current understanding of the complex pathophysiology and treatment of this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and Historical Background
Descriptions of headache date back to the time of Aristotle [1], and tension-type headache (TTH) is considered to the be the most common form experienced. As the name implies, TTH was historically attributed to emotional conflict/anxiety precipitating sustained contraction of skeletal muscles in the head and neck with resultant head pain [2, 3]. Sustained muscle contraction from physical factors such as prolonged maintenance of a fixed position of the head/neck was also considered a possible precipitating factor [4]. In the 1950s, there was a shift in focus to consider both the peripheral and central nervous system contributions to pain. Travell and Rinzler defined “trigger areas” as hypersensitive regions stimulated in the periphery but overwhelming the central nervous system, leading to pain in regions outside the initial local stimulus (referred pain). They hypothesized that although myofascial pain may initially be precipitated by local processes, there was another mechanism contributing to sustained pain in the absence of ongoing peripheral stimulus [5]. However, it was not until after publication of the first “Classification of Headache” with consensus definitions of “muscle-contraction headache” and other headache types in 1962 that researchers in headache medicine were able to explore the pathophysiology of head pain further [6].
In the first “Classification of Headache,” muscle contraction headache was defined as an “ache or sensation of tightness, pressure or constriction, widely varied in intensity, frequency and duration, associated with sustained contraction of skeletal muscles, usually as part of the individual’s reaction during life stress”[6]. In the early 1960s, treatment remained focused on identifying and subsequently eliminating emotional and physical factors contributing to tension [4]. Given sustained muscle contraction as a defining feature, Langemark and Olesen conducted a blinded, controlled study that demonstrated increased pericranial muscle tenderness to manual palpation in patients with muscle contraction headache [7]. Their study and multiple studies that followed emphasized the origin of TTH in peripheral pericranial myofascial nociception [8–14]. In 1988, new classification and diagnostic criteria were published by the International Headache Society, adopting the term tension-type headache (TTH) with division based on frequency (episodic and chronic forms) as well as the presence or absence of an associated disorder of the pericranial muscles. This was defined as increased tenderness of pericranial muscles by manual palpation/pressure algometer or increased EMG pericranial muscle activity [15].
Over time, the long-accepted concept of sustained muscle contraction as a defining feature of TTH was called into question after multiple EMG studies yielded conflicting results. Some studies showed that pericranial EMG activity was higher in both TTH and migraine headache patients [16], while others, including a large meta-analysis of studies looking at frontal EMG activity, showed no difference between TTH patients and controls [17].
In 2004, the International Classification of Headache Disorders second edition retained the distinction of with or without pericranial tenderness but removed criteria of increased EMG activity of pericranial muscles. At the same time, diagnosis of TTH was further divided into infrequent episodic, frequent episodic, and chronic forms [18]. This has proven important for continued efforts at advancing our understanding of this disorder, including the underlying mechanisms behind progression from episodic to chronic TTH. More recent clinical criteria have been published in the 3rd edition of the International Classification of Headache Disorders and are discussed later in this paper.
Epidemiology
TTH is the most common headache disorder, with a reported global annual prevalence of 26–38% [19•, 20, 21] or nearly 2 billion people with TTH [19•]. In fact, TTH was the third most prevalent disorder when hundreds of disorders were assessed by the Global Burden of Disease study in 2016 [19•]. Lifetime prevalence has been reported as high as 78% with a slight female predominance (male to female ratio of 4:5) [22]. The peak age of TTH is 30–39 years old [19•, 23] with prevalence decreasing with increasing age [22, 24]. The annual prevalence of chronic TTH has been most consistently reported as 2–3% [23, 25–28]. Interestingly, prevalence rates of TTH are highly variable across continents, and while this might be the result of study methodological differences, it may also point to cultural differences or undiscovered environmental and genetic factors [20].
Given observation that first degree relatives of patients with chronic TTH have more than three times the risk of chronic TTH compared to the general population, genetic influences have been considered [29]. The first twin study demonstrated no significant difference in concordance rates between monozygotic and dizygotic twins in episodic TTH; however, the sample size was too small to adequately assess for a genetic effect in chronic TTH [28]. A larger twin study attempted to account for and exclude possible co-occurrence of migraine and demonstrated higher concordance rates in monozygotic compared to dizygotic twins for frequent (but not infrequent) episodic TTH headache. Data on chronic TTH was too limited to draw any conclusions [30]. Additional studies looking at the possible genetic influence on TTH have focused on the genes themselves. One study identified a serotonin transporter polymorphism genotype occurring at a higher frequency in patients with chronic TTH [31], and another study identified a catechol-o-methyltransferase polymorphism associated with chronic TTH and lower widespread pressure pain thresholds in women [32].
Other risk factors identified for the development of TTH include poor self-rated health, inability to relax after work, and decreased sleep [24]. Multiple comorbid conditions have been associated with TTH, including other types of pain and mood disorders. TTH is reported in over 80% of patients with migraine [22]. Anxiety and depression are more prevalent in patients with TTH compared to controls [33]. Neck pain and low back pain also occur more frequently in patients with TTH, though the pathophysiologic relationship of these disorders remains unclear [34, 35].
Given the high prevalence, the societal burden of TTH does not go unnoticed. In one study of patients with episodic TTH, lost workdays were reported in 8.3% of patients, with reduced effectiveness at work, home, or school in 43.6% of patients [23]. In chronic TTH, the individual impact is higher, with 11.8% of patients reporting lost workdays, missing an annual average of 27.4 days each [23]. In the Global Burden of Disease study, TTH burden was calculated by considering prevalence, average time with headache, and suspected severity of disability from disease and then reported as disability-adjusted-life-years. TTH resulted in 7.2 million disability-adjusted-life-years globally in 2016 [19•]. Even with these staggering estimates of disability, it seems difficult to accurately quantify the total burden of TTH. It is thought to be under-recognized in clinic and may co-occur with multiple other pain disorders, each sometimes disabling in their own right.
Pathophysiology
In an early study inducing TTH by sustained tooth clenching, increased pericranial tenderness and a lower pain threshold were observed in patients who developed TTH compared to patients who did not develop headache [36]. Additional studies have shown pericranial muscular tenderness in patients with TTH not only during an attack, but also interictally [7–14]. This myofascial tenderness has been demonstrated to increase as the severity and frequency of headache increases [9, 36]. Consistent with this, decreased threshold for detecting pressure pain has been seen in chronic TTH, but not episodic TTH [8, 11, 37].
Interestingly, it seems that this heightened pain sensitivity may occur as a consequence of chronic pain rather than as a risk factor for headache chronification. One population-based study of patients with both TTH and normal baseline pain detection thresholds followed up with the patients 12 years later, to again examine them for tenderness and pain sensitivity. Patients who had developed chronic TTH over the course of the study demonstrated decreased pain detection thresholds in follow-up, whereas patients who developed frequent episodic TTH demonstrated increased pericranial muscle tenderness, but no change in their pain detection threshold [38••].
An effort to understand this increased tenderness and lowered pain threshold has led to observations that support not only the role of peripheral nociception but a possible role of central modulation, with studies of chronic TTH patients demonstrating decreased pressure pain thresholds at sites distant from the pericranial region [8, 11, 14, 37, 39]. Over the years, there has been interest in examining TTH from a molecular perspective as well. This interest is not only in examining the neuropeptides that play a role in any type of head pain, but differentiating the neuropeptides involved in TTH versus migraine headache.
Peripheral Mechanisms
Pericranial muscle tenderness by manual palpation is peripherally transmitted by thinly myelinated A-delta and unmyelinated C-fibers. Normally, thick myelinated fibers such as A-alpha and A-beta mechanosensitive fibers carry only innocuous stimuli. It has been suggested that in select cases of chronic pain, local soft tissue injury or inflammation leads to an abnormal sensitivity of not only the A-delta and C-fibers but also the low-threshold A-beta mechanosensitive fibers [40]. With sensitization of these afferent sensory fibers, the response to peripheral noxious stimuli may become exaggerated (hyperalgesia) or even be triggered by innocuous stimuli (allodynia).
Consistent with this concept, patients with chronic TTH have been shown to not only have increased sensitivity to pain, but in some cases demonstrate a pain response to a typically non-painful level of pressure stimulus. This may support that spinal dorsal horns are receiving noxious input not only from high-threshold mechanosensitive (HTM) neurons, but also from low-threshold mechanosensitive (LTM) neurons, which are typically responsive only to innocuous stimuli [12]. This would correspond with peripheral sensitization or decreased pain threshold of myofascial nociceptive fibers as a result of sustained sensory input. Interestingly, muscle “hardness” has also been correlated with pericranial muscle tenderness and is increased in patients with chronic TTH compared to controls, both during and between headaches [10, 41].
In addition to overall increased tenderness and hardness of the muscles, the presence of increased “myofascial trigger points” in both episodic and chronic TTH patients compared to controls is well documented [42–49]. Myofascial trigger areas are defined as firm areas of muscle with hypersensitivity to pressure, causing referred pain in a characteristic pattern [5]. The number of trigger points in patients with TTH may be increased with increased headache duration, frequency, and severity, although data are conflicting [43–45, 50, 51]. One study demonstrated that widespread pressure hypersensitivity correlated with the number of trigger points identified in patients with TTH, independent of headache frequency [52].
Multiple head/neck trigger areas have been identified as causing pain in a distribution which could reflect the pain of TTH. Schmidt-Hansen and colleagues were able to reproduce characteristic pain patterns and pain sensitivity in healthy volunteers by infusing hypertonic saline into head/neck muscles. These pain patterns were similar to mappings of habitual pain in TTH patients they had seen in the clinic [53]. Though evidence for generalized increased pericranial EMG activity in TTH has not been compelling, one study looked at spontaneous needle EMG activity in just the tender trigger point areas and found that the activity was increased compared to neighboring areas of muscle [17, 54]. Perhaps it is sustained activity at these trigger points that contributes to peripheral sensitization of myofascial nociception in TTH. However, there is an incomplete understanding of the cause-effect relationship and a lack of consistent response to treatment [55–59]. It has been suggested that longitudinal studies investigating the role of development of active trigger points and the effect on the evolution of TTH would be useful in future studies [60, 61].
The possibility of muscle ischemia (possibly as a reflexive cycle of sustained muscle contraction and spasm) with subsequent local inflammation has also been proposed as contributing to muscle pathology and subsequent peripheral sensitization. However, in a study on patients with chronic TTH that examined blood flow to the temporalis during isometric work, there was no correlation between pain and blood flow [62]. Another study that attempted to estimate in vivo blood flow and lactate in tender muscles of patients with chronic TTH before and after exercise showed that there was altered blood flow to the tender muscle, but no evidence of ischemia (lactate was unchanged). The alteration in blood flow was attributed to the increased excitability of the central nervous system and associated increased sympathetic outflow with vasoconstriction [62, 63].
Central Mechanisms
It has been proposed that the reduced pain threshold and tenderness experienced by chronic TTH patients may be related not only to peripheral sensitization at the level of the myofascial nociceptors, but also to sensitization of second order neurons at the level of the spinal trigeminal nucleus and dorsal horn, or even sensitization of supraspinal structures such as the somatosensory cortex, thalamus, limbic system, or motor cortex [64••]. Influence of the central nervous system would seem to be supported by studies that have shown that patients with chronic TTH are hypersensitive not only to stimuli applied to pericranial muscles, but also to regions outside of the head and neck (such as a finger) [11].
Imaging studies also seem to support central mechanisms for TTH. One functional MRI study of patients with TTH demonstrated decreased regional synchronization of neuronal activity in multiple cortical and subcortical areas that contribute to pain processing [65]. In a separate study looking at structural rather than functional changes, Chen and colleagues showed altered gray matter density in select areas of pain processing in patients with episodic TTH compared to healthy controls [66]. Interestingly, these changes, which included reduced gray matter density in the primary somatosensory cortex and increased gray matter density in the bilateral anterior cingulate cortex and anterior insula, seemed to be dynamic and reversible, present during headache attacks but absent during headache-free intervals [66]. A follow-up study showed that while episodic TTH was associated with increased gray matter volume in multiple areas including the anterior cingulate cortex, chronic TTH had a decrease of gray matter volume in the bilateral insula and anterior cingulate cortex [67]. When studies have tried to compare these patterns of gray matter volume change in different headache disorders, it seems that the regions affected in patients with TTH are different than the regions affected in patients with migraine or medication-overuse headache [67, 68].
It is notable that these imaging studies have suggested a role of the anterior cingulate cortex and insula, both areas known to contribute to cognitive and affective processing of sensory input [67, 68]. Though reaction to “life stress” is no longer included in the definition of TTH, stress or mental tension remains one of the most commonly recognized precipitating factors for this disorder [69–71]. Furthermore, it has been shown that cognitive stress can increase muscle pain in patients with TTH compared to controls [72]. Some have suggested that certain psychological comorbidities such as depression and different coping strategies for pain may make some individuals more vulnerable to increased pain sensitivity [64••]. However, the pathophysiologic mechanism by which stress can act as a trigger for TTH and the full role of the limbic system has not yet been elucidated.
Finally, there has also been evidence pointing to altered modulation of pain by the central nervous system in TTH, with reduced anti-nociceptive activity from supraspinal structures. In typical pain modulation, A-delta and C-fibers trigger supraspinal structures referred to as diffuse noxious inhibitory controls (DNICs), which exert their effect on the spinal trigeminal nucleus and dorsal horn by inhibiting pain in regions outside of the territory of the nociceptive afferent neurons. DNIC function can be assessed via threshold and amplitude response of the nociceptive flexion reflex, a polysynaptic spinal withdrawal reflex that is inhibited when DNICs are activated. Patients with chronic TTH not only appear to have a reduced threshold for this pain reflex, but instead of inhibition, they demonstrate facilitation of this reflex in response to pain, indicative of DNIC dysfunction [39]. Dysfunction of DNICs has been observed in other chronic pain disorders, and the underlying explanation for this descending inhibitory pain pathway dysfunction is still being studied [73].
Molecular Mechanisms
Multiple molecules including nitric oxide, calcitonin gene-related peptide (CGRP), substance P, neuropeptide Y, vasoactive intestinal peptide (VIP), bradykinin, serotonin, and other molecules involved in pain processing have been investigated in patients with TTH. Nitric oxide has been most extensively studied, with potential effects both peripherally and centrally [74, 75]. Nitric oxide has been shown to be able to induce headache in patients with chronic TTH [76]. Furthermore, inhibition of nitric oxide synthase (NOS) with subsequent decreased levels of nitric oxide has been shown to reduce headache intensity and muscle hardness in patients with chronic TTH [74, 77]. Ashina has hypothesized that this anti-nociceptive effect is likely due to reduced central sensitization at the level of the trigeminal spinal nucleus but acknowledges that NOS inhibitors exert effect on endothelial NOS as well and may have direct anti-nociceptive effects in myofascial tissues [78].
Serotonin is also known to be involved in anti-nociceptive pathways at both peripheral and central levels, and given its role in other primary headache disorders, there have been several efforts to investigate its possible role in TTH. However, published studies have shown largely normal peripheral serotonin metabolism in patients with chronic TTH [79, 80]. CGRP is another molecule of interest in TTH, based on its role in migraine and other primary headache disorders. CGRP levels in cerebrospinal fluid and plasma are normal in patients with TTH and seem to be unrelated to headache state and pericranial muscle tenderness [81, 82]. Ashina and colleagues have also studied plasma levels of substance P, neuropeptide Y, and VIP in patients with chronic TTH. No significant differences were found in blood drawn from cranial or peripheral vessels in patients and controls, and there was also no relation to headache state [83].
Given the observed pericranial muscle tenderness associated with TTH, inflammatory mediators and metabolites have also been examined in the specific areas of muscle tenderness in patients with TTH. There were no significant observed differences in concentrations of multiple molecules (prostaglandin E2, adenosine 5′-triphosphate, glutamate and bradykinin) at rest or after static exercise [84]. Mork and colleagues created an experimental human model to assess myofascial pain via intramuscular infusion of a combination of inflammatory mediators (bradykinin, serotonin, histamine, prostaglandin E2). Interestingly, patients with episodic TTH demonstrated increased pain in response to infusion of both inflammatory mediators and placebo (isotonic saline, but with similar mechanical stimulation) compared to controls [85]. The authors felt that the enhanced response to chemical stimuli was consistent with sensitization of peripheral nociceptors with additional possible influence by central mechanisms.
Diagnosis and Differential Diagnosis
TTH is a clinical diagnosis based on headache frequency, duration, and characteristics, as outlined by the International Classification of Headache Disorders third edition (ICHD-3) and included in Table 1 [86]. TTH may present similarly to many other types of headache given its non-specific features. Therefore, the differential diagnosis is broad, including (but not limited to) other primary and secondary headache disorders as outlined in Table 2. In our clinical experience, migraine headache and medication-overuse headache are two of the most frequent diagnoses encountered in clinic. Migraine has many overlapping diagnostic criteria with TTH, with potential differentiating factors including possible presence of aura (not present in all patients with migraine); aggravation by routine physical activity; and more frequent accompanying symptoms including nausea, vomiting, photophobia, phonophobia, and osmophobia. Osmophobia appears to be more specific for migraine than photophobia or phonophobia alone and, therefore, can be a distinguishing feature when present [87]. Importantly, the diagnostic criteria for chronic migraine include the presence of possible tension-type-like headaches as long as the majority of headache days have features consistent with migraine [86]. If the headache diagnosis in a patient with frequent headache remains unclear based upon initial history, then implementation of a headache diary to better track and characterize headaches should be considered.
History, Examination, and Evaluation
Given that the diagnosis of TTH is based on headache characteristics, a detailed history is paramount and should include exploration of possible secondary causes of headache as outlined in Table 2. In inquiring about headache frequency, details surrounding headache onset or change in pattern since onset may elucidate new daily persistent headache or transition from episodic to chronic TTH. Additional evaluation is required for any red flags in the headache history, such as an onset of headache after age 50, rapid rise to peak of pain (thunderclap), progressively worsening headache, systemic symptoms (fever/chills, night sweats), unintentional weight loss, focal neurologic symptoms, and exacerbation by position (lying down or standing up) or Valsalva maneuver. Additional testing is also required if the headaches worsened in the setting of immunodeficiency, pregnancy/post-partum, or with a history of neoplasm or recent trauma to the head/neck or back. Medical comorbidities should be explored, including a history of sleep quality, snoring/apnea, hypertension, and mood disorders, as these may influence the frequency and intensity of headaches and may require their own management. Frequency of acute analgesic use is also an important factor to consider in assessment of TTH, as medication-overuse headache may be concomitantly diagnosed. Medication-overuse headache is defined as taking any combination of pain medicines (triptan, opioid, combination-analgesics, or multiple analgesics) more than 9 days per month. In cases where patients are taking only one simple analgesic (acetaminophen or NSAID) and no other abortive medicines, medication-overuse headache is defined as taking this analgesic more than 14 days per month [86]. When acute analgesics are being overused, pre-existing TTH could be exacerbated, and a subsequent decrease in analgesic use may improve headache.
Detailed neurologic examination should be performed to look for any evidence of focal neurologic deficits as might occur from a space-occupying lesion. This should include a funduscopic examination for papilledema. Examination in the setting of suspected TTH should additionally include manual palpation of the pericranial muscles to assess for tenderness. The ICHD-3 recommends that the muscles be assessed using the index and middle fingers by performing small rotating movements and firm pressure, scoring the local tenderness for each muscle on a scale of 0 to 3. They recommend examining the masseter, temporalis, frontalis, lateral and medial pterygoid muscles, sternocleidomastoid (including insertions on the mastoid process), suboccipital paraspinal muscles, and trapezius and suggest summing the scores [86]. This total tenderness score may be a useful guide to treatment and add credibility to the diagnosis [7, 86]. While palpating pericranial muscles, evaluation for tender points should also be performed. Tender points are characterized by a firm area or taut band with hypersensitivity and referred pain [5]. Assessment of the cervical spine and temporomandibular joint should also be performed to rule out evidence of concomitant joint disease contributing to secondary headache.
Further evaluation should be based upon clinical suspicion for other causes of secondary headache as suggested in Table 2. Many of the differential diagnoses can be eliminated by thorough history, but suspicious symptoms or examination findings may prompt further evaluation. Otherwise, additional testing does not need to be routinely performed in the evaluation of TTH.
Treatment of TTH
In 2010, the European Federation of Neurological Societies published evidence-based guidelines for the treatment of TTH [88•]. Treatment strategies have not significantly changed since that time, though subsequent studies have provided additional support for their recommendations. When considering medical therapy for acute treatment, simple analgesics are recommended and should be limited to prevent medication-overuse headache. Preventative medical therapy should be considered for frequent episodic or chronic TTH, especially in patients having 10 or greater headache days per month [89]. Non-pharmacological treatment should always be considered either alone or as an adjunct to medical management [88•]. In patients who are not responding to treatment, a referral to a headache specialist should be considered [90].
Evidence for Acute Pharmacologic Treatment
Simple analgesics including acetaminophen, aspirin, and NSAIDs have the best evidence and should be considered first-line treatment for acute treatment of TTH. In a systematic review of 23 studies, acetaminophen 1000 mg demonstrated superiority for reducing pain to mild or no pain at 2 h [91]. Aspirin 500–1000 mg demonstrated benefit mainly defined by more participants satisfied with treatment compared with placebo [92]. Ibuprofen 400 mg demonstrated more patients pain free at 2 h [93]. Ketoprofen 25 mg also demonstrated more patients with mild or no pain at 2 h [94]. Naproxen 375 mg demonstrated more pain reduction/resolution compared to placebo in a large randomized double-blinded study [94]. Ketoprofen and naproxen were compared to acetaminophen without evidence of superiority [94, 95]. Diclofenac 12.5–25 mg was similarly assessed in a large randomized double-blinded placebo-controlled study which demonstrated superior pain relief at 3 h [96]. Combination analgesics including caffeine with acetaminophen and aspirin demonstrated superiority compared to acetaminophen and placebo in regard to pain freedom at 2 h [97]. However, combination analgesics are considered a second-line option due to increased risk of medication-overuse headache [88•]. Similarly, opioids should be avoided due to the risk of medicine overuse and potential for misuse [64••].
Evidence for Preventative Pharmacologic Treatment
Amitriptyline was the first recognized preventative treatment option for TTH. In early trials, amitriptyline was compared to citalopram (relatively new selective serotonin reuptake inhibitor at the time) and demonstrated effectiveness of amitriptyline but not citalopram. Amitriptyline reduced the area under the headache curve (duration × intensity) by 30% (p = 0.002), with a significant reduction in headache duration and frequency, as well as reduction in analgesic intake [98]. Potential adverse effects of amitriptyline include possible weight gain, drowsiness/sedation, dry mouth, and constipation. Patients who are prone to hypertension, tachycardia, or acid reflux may notice these symptoms worsen on amitriptyline. Older patients should be monitored for anticholinergic side effects as well as for possible cardiac arrhythmias, with baseline and yearly electrocardiograms recommended [99].
Though amitriptyline is still considered first line as a preventative for TTH, other antidepressants have been studied. In a Cochrane review on the possible use of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for prevention of TTH, these antidepressants were found to demonstrate similar reductions in headache frequency to amitriptyline; however, this reduction was not clearly better than placebo [100]. Amitriptyline was found to reduce analgesic use more efficiently than SSRIs. While participants on SSRIs or SNRIs generally had fewer side effects than those taking amitriptyline, the dropout rate due to side effects was approximately equal [100].
Mirtazapine 15–30 mg daily demonstrated a reduced area under the headache curve of 34% including reduction in headache frequency, duration and intensity when compared to placebo in a randomized double-blind placebo-controlled trial [101]. In a separate study comparing amitriptyline to mirtazapine, these two preventatives seemed comparable in efficacy, with mirtazapine demonstrating better tolerability [102]. The evidence for venlafaxine is low, but it is also generally considered better tolerated than amitriptyline. One randomized double-blind placebo-controlled study demonstrated a decrease in number of headache days after the use of venlafaxine XR 150 mg daily for 12 weeks (p = 0.05) with no significant improvement in secondary efficacy variables [103].
Antiepileptics have also been examined as possible preventatives for TTH, but evidence is limited. Sodium valproate was shown in a single randomized controlled trial of 41 patients with chronic TTH to significantly reduce headache days (from baseline 23.4 days per month to 10.5 days per month vs 22.3 for placebo, p = 0.000), though it was not as effective in the TTH patients as the chronic migraine patients in the same trial [104]. Topiramate has shown some reduction in frequency of headaches in two open-label trials (one in chronic TTH [105] and one in episodic TTH [106]).
Given increased muscle tension and hardness in patients with TTH, medications with muscle relaxing properties have been trialed. Tizanidine has been studied, but results are conflicting and a larger randomized study demonstrated no superiority of tizanidine compared to placebo [107, 108]. Though botulinum toxin has been effective in other headache disorders, there is not good evidence to suggest benefit in TTH [109]. Trigger point injections with lidocaine may be helpful, although data is limited to two small studies, one of which also included concurrent trigeminal nerve and superior cervical ganglion injections [58, 110]. Based on available evidence for pharmacologic prevention of TTH, amitriptyline would be considered the first line, mirtazapine would be considered second line, and venlafaxine or an antiepileptic would then be considered if necessary [88•].
Evidence for Non-pharmacologic Management
Overall, evidence for non-pharmacologic management strategies in TTH is limited, but these are generally considered in all patients, especially in patients who are averse or have contraindications to medication use. It may be that patients do best when both pharmacologic and non-pharmacologic management are combined. One study randomized patients with TTH to receive either an antidepressant (amitriptyline at 50–100 mg daily or nortriptyline at 50–75 mg daily), stress management, a combination of antidepressant plus stress management, or placebo. They found that patients who were treated with a combination of antidepressant and stress management had a better response than either treatment alone [111].
Within the non-pharmacologic treatments, EMG-guided biofeedback may be considered a first-line option based on meta-analysis data which demonstrated decreased headache frequency and less analgesic medication use [112]. In contrast, other behavioral therapies, including stress reduction and cognitive therapy, may result in decreased pain intensity, but high-quality data to support this is lacking [113]. Lifestyle modification focused on stress management, sleep hygiene, healthy diet and regular exercise is often recommended in a comprehensive treatment plan for TTH [64••].
A recent review of physical therapy demonstrated possible improved quality of life in patients with TTH, but the authors warned that results of studies should be taken with caution, given the low level of evidence and high risk of bias [114]. Two recent systematic reviews of acupuncture conclude possible effectiveness for TTH with a trend toward improved headache intensity long term, but largely statistically insignificant results [115, 116]. Alternatively, dry needling of myofascial trigger points, which is similar to acupuncture, demonstrated a statistically significant decrease in headache intensity, frequency, and duration in a randomized controlled trial [117].
Prognosis
There is limited data regarding prognosis of TTH, as few studies have followed patients long term. One longitudinal study followed a cohort of 549 people representative of the Danish population over a 12-year period [118]. They examined 146 patients with TTH, including frequent episodic TTH (they defined as 15–179 headache days per year) and chronic TTH (defined as ≥ 180 headache days per year) and found that 45% of patients improved by follow-up to infrequent TTH (1–14 headache days per year) or no headache days (remission). A subset of patients (16%) had a poor outcome at follow-up (≥ 180 headache days per year). Predictive factors for poor outcome were chronic TTH at baseline, coexisting migraine, not being married, and sleeping difficulty. Predictive factors for remission were absence of chronic TTH at baseline and older age [118].
Conclusion
TTH is the most prevalent headache disorder with significant societal burden. Diagnosis is clinical, based on headache characteristics and frequency, but TTH may present similarly to many other types of headache. Given rather non-specific features, detailed history and examination should be performed to rule out alternative causes of headache. In addition to comprehensive neurologic examination, pericranial muscles should also be palpated for tenderness. Increased pericranial muscle tenderness and a decreased pressure pain threshold have been consistently observed in patients with chronic TTH.
Although the pathophysiology is incompletely understood, evidence supports both peripheral and central mechanisms. TTH may have limited medical therapy options, but available data suggest good outcomes in many patients. Simple analgesics are first-line for acute treatment, and amitriptyline is first-line for preventative treatment. Non-pharmacologic strategies should also always be considered, with most compelling evidence supporting EMG biofeedback.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dalessio, D., Headache mechanisms in handbook of clinical neurology: headaches and cranial neuralgias P.J.a.B. Vinken, G.W., Editor. 1968, American Elsevier Publishing Company, Inc. : New York p. 15–24.
Friedman AP. Migraine and other common headaches. World-wide abstracts of general medicine. 1959;2:10–20.
Kolb, L.C., Psychiatric and psychogenic factors in headache in headache: diagnosis and treatment. 1959, F.A. Davis Co. : Philadelphia p. 259–298.
Blumenthal, L.S., Tension headache in handbook of clinic neurology: headaches and cranial neuralgias, P.J.a.B. Vinken, G.W., Editor. 1968, American Elsevier Publishing Company Inc. : New York p. 157–171.
Travell, J.a.R., S.H. , The myofascial genesis of pain. Postgrad Med, 1952: p. 425–434.
Ad Hoc Committee on Classification of Headache of the National Institute of Neurological Diseases and Blindness, Classification of headache. Arch Neurol, 1962. 6: p. 173–176.
Langemark, M. and J. Olesen, Pericranial tenderness in tension headache. A blind, controlled study. Cephalalgia, 1987. 7(4): p. 249–55.
Langemark M, et al. Pressure pain thresholds and thermal nociceptive thresholds in chronic tension-type headache. Pain. 1989;38(2):203–10.
Jensen R, et al. Muscle tenderness and pressure pain thresholds in headache A population study. Pain. 1993;52(2):193–9.
Sakai, F., et al., Pericranial muscle hardness in tension-type headache. A non-invasive measurement method and its clinical application. Brain, 1995. 118 ( Pt 2): p. 523–31.
Bendtsen L, Jensen R, Olesen J. Decreased pain detection and tolerance thresholds in chronic tension-type headache. Arch Neurol. 1996;53(4):373–6.
Bendtsen L, Jensen R, Olesen J. Qualitatively altered nociception in chronic myofascial pain. Pain. 1996;65(2–3):259–64.
Lipchik GL, et al. Pericranial muscle tenderness and exteroceptive suppression of temporalis muscle activity: a blind study of chronic tension-type headache. Headache. 1997;37(6):368–76.
Fernandez-de-Las-Penas C, et al. Increased pericranial tenderness, decreased pressure pain threshold, and headache clinical parameters in chronic tension-type headache patients. Clin J Pain. 2007;23(4):346–52.
Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia, 1988. 8 Suppl 7: p. 1–96.
Philips C. Tension headache: theoretical problems. Behav Res Ther. 1978;16(4):249–61.
Wittrock DA. The comparison of individuals with tension-type headache and headache-free controls on frontal EMG levels: a meta-analysis. Headache. 1997;37(7):424–32.
Headache Classification Subcommittee of the International Headache, S., The international classification of headache disorders: 2nd edition. Cephalalgia, 2004. 24 Suppl 1: p. 9–160.
• Collaborators GBDH. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–76. (This study is the most recent population study demonstrating the immense global prevalence and burden of TTH, highlighting the need for ongoing research.)
Sahler K. Epidemiology and cultural differences in tension-type headache. Curr Pain Headache Rep. 2012;16(6):525–32.
Stovner L, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210.
Rasmussen BK, et al. Epidemiology of headache in a general population–a prevalence study. J Clin Epidemiol. 1991;44(11):1147–57.
Schwartz BS, et al. Epidemiology of tension-type headache. JAMA. 1998;279(5):381–3.
Lyngberg AC, et al. Incidence of primary headache: a Danish epidemiologic follow-up study. Am J Epidemiol. 2005;161(11):1066–73.
Castillo, J., et al., Kaplan Award 1998. Epidemiology of chronic daily headache in the general population. Headache, 1999. 39(3): p. 190–6.
Scher AI, et al. Prevalence of frequent headache in a population sample. Headache. 1998;38(7):497–506.
Pascual J, Colas R, Castillo J. Epidemiology of chronic daily headache. Curr Pain Headache Rep. 2001;5(6):529–36.
Ulrich V, Gervil M, Olesen J. The relative influence of environment and genes in episodic tension-type headache. Neurology. 2004;62(11):2065–9.
Ostergaard S, et al. Comparison of first degree relatives and spouses of people with chronic tension headache. BMJ. 1997;314(7087):1092–3.
Russell MB, Saltyte-Benth J, Levi N. Are infrequent episodic, frequent episodic and chronic tension-type headache inherited? A population-based study of 11 199 twin pairs. J Headache Pain. 2006;7(3):119–26.
Park JW, et al. Serotonin transporter polymorphism and harm avoidance personality in chronic tension-type headache. Headache. 2004;44(10):1005–9.
Fernandez-de-Las-Penas C, et al. Catechol-O-Methyltransferase (COMT) rs4680 Val158Met polymorphism is associated with widespread pressure pain sensitivity and depression in women with chronic, but not episodic, tension-type headache. Clin J Pain. 2019;35(4):345–52.
Song, T.J., et al., Anxiety and depression in tension-type headache: a population-based study. PLoS One, 2016. 11(10): p. e0165316.
Ashina S, et al. Prevalence of neck pain in migraine and tension-type headache: a population study. Cephalalgia. 2015;35(3):211–9.
Ashina S, et al. Increased pain sensitivity in migraine and tension-type headache coexistent with low back pain: a cross-sectional population study. Eur J Pain. 2018;22(5):904–14.
Jensen, R. and J. Olesen, Initiating mechanisms of experimentally induced tension-type headache. Cephalalgia, 1996. 16(3): p. 175–82; discussion 138–9.
Schoenen J, et al. Cephalic and extracephalic pressure pain thresholds in chronic tension-type headache. Pain. 1991;47(2):145–9.
•• Buchgreitz L, et al. Increased pain sensitivity is not a risk factor but a consequence of frequent headache: a population-based follow-up study. Pain. 2008;137(3):623–30.. (This was a longitudinal study following TTH patients for 12 years, demonstrating that increased pain sensitivity was a consequence and not merely a risk factor for development of chronic headache.)
Sandrini G, et al. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia. 2006;26(7):782–9.
Woolf CJ, Doubell TP. The pathophysiology of chronic pain–increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994;4(4):525–34.
Ashina M, et al. Muscle hardness in patients with chronic tension-type headache: relation to actual headache state. Pain. 1999;79(2–3):201–5.
Couppe C, et al. Myofascial trigger points are very prevalent in patients with chronic tension-type headache: a double-blinded controlled study. Clin J Pain. 2007;23(1):23–7.
Fernandez-de-las-Penas C, et al. Referred pain from myofascial trigger points in head and neck-shoulder muscles reproduces head pain features in children with chronic tension type headache. J Headache Pain. 2011;12(1):35–43.
Fernandez-de-Las-Penas C, Cuadrado ML, Pareja JA. Myofascial trigger points, neck mobility, and forward head posture in episodic tension-type headache. Headache. 2007;47(5):662–72.
Fernandez de las Penas, C., et al., Referred pain from the trochlear region in tension-type headache: a myofascial trigger point from the superior oblique muscle. Headache, 2005. 45(6): p. 731–7.
Fernandez-de-las-Penas C, et al. Trigger points in the suboccipital muscles and forward head posture in tension-type headache. Headache. 2006;46(3):454–60.
Fernandez-de-Las-Penas C, et al. The local and referred pain from myofascial trigger points in the temporalis muscle contributes to pain profile in chronic tension-type headache. Clin J Pain. 2007;23(9):786–92.
Fernandez-de-Las-Penas C, et al. Myofascial trigger points in the suboccipital muscles in episodic tension-type headache. Man Ther. 2006;11(3):225–30.
Fernandez-de-Las-Penas C, et al. Myofascial trigger points and their relationship to headache clinical parameters in chronic tension-type headache. Headache. 2006;46(8):1264–72.
Fernandez-de-Las-Penas C, et al. Referred pain from trapezius muscle trigger points shares similar characteristics with chronic tension type headache. Eur J Pain. 2007;11(4):475–82.
Sohn JH, Choi HC, Jun AY. Differential patterns of muscle modification in women with episodic and chronic tension-type headache revealed using surface electromyographic analysis. J Electromyogr Kinesiol. 2013;23(1):110–7.
Palacios-Cena M, et al. Trigger points are associated with widespread pressure pain sensitivity in people with tension-type headache. Cephalalgia. 2018;38(2):237–45.
Schmidt-Hansen PT, et al. Patterns of experimentally induced pain in pericranial muscles. Cephalalgia. 2006;26(5):568–77.
Hubbard, D.R. and G.M. Berkoff, Myofascial trigger points show spontaneous needle EMG activity. Spine (Phila Pa 1976), 1993. 18(13): p. 1803–7.
Do TP, et al. Myofascial trigger points in migraine and tension-type headache. J Headache Pain. 2018;19(1):84.
Arendt-Nielsen L, et al. Muscle triggers as a possible source of pain in a subgroup of tension-type headache patients? Clin J Pain. 2016;32(8):711–8.
Harden RN, et al. Botulinum toxin a in the treatment of chronic tension-type headache with cervical myofascial trigger points: a randomized, double-blind, placebo-controlled pilot study. Headache. 2009;49(5):732–43.
Karadas O, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1–2):95–101.
Moraska AF, et al. Myofascial trigger point-focused head and neck massage for recurrent tension-type headache: a randomized, placebo-controlled clinical trial. Clin J Pain. 2015;31(2):159–68.
Fernandez-De-Las-Penas C, Arendt-Nielsen L. Improving understanding of trigger points and widespread pressure pain sensitivity in tension-type headache patients: clinical implications. Expert Rev Neurother. 2017;17(9):933–9.
Fernandez-de-Las-Penas C. Myofascial head pain. Curr Pain Headache Rep. 2015;19(7):28.
Langemark M, Jensen K, Olesen J. Temporal muscle blood flow in chronic tension-type headache. Arch Neurol. 1990;47(6):654–8.
Ashina M, et al. In vivo evidence of altered skeletal muscle blood flow in chronic tension-type headache. Brain. 2002;125(Pt 2):320–6.
•• Ashina S, et al. Tension-type headache. Nat Rev Dis Primers. 2021;7(1):24.. (This is a recently published comprehensive review of TTH including integration of multiple proposed pathophysiologic mechanisms contributing to chronification of TTH.)
Wang P, et al. Regional homogeneity abnormalities in patients with tension-type headache: a resting-state fMRI study. Neurosci Bull. 2014;30(6):949–55.
Chen B, et al. Cortical plasticity between the pain and pain-free phases in patients with episodic tension-type headache. J Headache Pain. 2016;17(1):105.
Chen WT, et al. Comparison of gray matter volume between migraine and “strict-criteria” tension-type headache. J Headache Pain. 2018;19(1):4.
Schmidt-Wilcke T, et al. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65(9):1483–6.
Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53(1):65–72.
Ulrich V, et al. A comparison of tension-type headache in migraineurs and in non-migraineurs: a population-based study. Pain. 1996;67(2–3):501–6.
Spierings EL, Ranke AH, Honkoop PC. Precipitating and aggravating factors of migraine versus tension-type headache. Headache. 2001;41(6):554–8.
Leistad RB, et al. Stress-induced pain and muscle activity in patients with migraine and tension-type headache. Cephalalgia. 2006;26(1):64–73.
Rossi P, et al. The contribution of clinical neurophysiology to the comprehension of the tension-type headache mechanisms. Clin Neurophysiol. 2011;122(6):1075–85.
Ashina M, et al. Possible mechanisms of action of nitric oxide synthase inhibitors in chronic tension-type headache. Brain. 1999;122(Pt 9):1629–35.
Ashina M, et al. Possible mechanisms of glyceryl-trinitrate-induced immediate headache in patients with chronic tension-type headache. Cephalalgia. 2000;20(10):919–24.
Ashina M, et al. Nitric oxide-induced headache in patients with chronic tension-type headache. Brain. 2000;123(Pt 9):1830–7.
Ashina M, et al. Effect of inhibition of nitric oxide synthase on chronic tension-type headache: a randomised crossover trial. Lancet. 1999;353(9149):287–9.
Ashina M. Neurobiology of chronic tension-type headache. Cephalalgia. 2004;24(3):161–72.
Bendtsen L, et al. Serotonin metabolism in chronic tension-type headache. Cephalalgia. 1997;17(8):843–8.
Bendtsen L, Mellerup ET. The platelet serotonin transporter in primary headaches. Eur J Neurol. 1998;5(3):277–82.
Bach FW, et al. Effect of sulpiride or paroxetine on cerebrospinal fluid neuropeptide concentrations in patients with chronic tension-type headache. Neuropeptides. 1994;27(2):129–36.
Ashina M, et al. Plasma levels of calcitonin gene-related peptide in chronic tension-type headache. Neurology. 2000;55(9):1335–40.
Ashina M, et al. Plasma levels of substance P, neuropeptide Y and vasoactive intestinal polypeptide in patients with chronic tension-type headache. Pain. 1999;83(3):541–7.
Ashina M, et al. Tender points are not sites of ongoing inflammation -in vivo evidence in patients with chronic tension-type headache. Cephalalgia. 2003;23(2):109–16.
Mork, H., et al., Possible mechanisms of pain perception in patients with episodic tension-type headache. A new experimental model of myofascial pain. Cephalalgia, 2004. 24(6): p. 466–75.
Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition. Cephalalgia, 2018. 38(1): p. 1–211.
Terrin A, et al. A prospective study on osmophobia in migraine versus tension-type headache in a large series of attacks. Cephalalgia. 2020;40(4):337–46.
• Bendtsen L, et al. EFNS guideline on the treatment of tension-type headache - report of an EFNS task force. Eur J Neurol. 2010;17(11):1318–25.. (These are the most recent evidence-based guidelines for treatment of TTH.)
Robbins MS. Diagnosis and management of headache: a review. JAMA. 2021;325(18):1874–85.
Loder E, Rizzoli P. Tension-type headache. BMJ. 2008;336(7635):88–92.
Stephens, G., S. Derry, and R.A. Moore, Paracetamol (acetaminophen) for acute treatment of episodic tension-type headache in adults. Cochrane Database Syst Rev, 2016(6): p. CD011889.
Derry, S., P.J. Wiffen, and R.A. Moore, Aspirin for acute treatment of episodic tension-type headache in adults. Cochrane Database Syst Rev, 2017. 1: p. CD011888.
Derry, S., et al., Ibuprofen for acute treatment of episodic tension-type headache in adults. Cochrane Database Syst Rev, 2015(7): p. CD011474.
Prior, M.J., et al., Efficacy and safety of acetaminophen and naproxen in the treatment of tension-type headache. A randomized, double-blind, placebo-controlled trial. Cephalalgia, 2002. 22(9): p. 740–8.
Veys, L., S. Derry, and R.A. Moore, Ketoprofen for episodic tension-type headache in adults. Cochrane Database Syst Rev, 2016. 9: p. CD012190.
Kubitzek F, et al. Low-dose diclofenac potassium in the treatment of episodic tension-type headache. Eur J Pain. 2003;7(2):155–62.
Diener HC, Gold M, Hagen M. Use of a fixed combination of acetylsalicylic acid, acetaminophen and caffeine compared with acetaminophen alone in episodic tension-type headache: meta-analysis of four randomized, double-blind, placebo-controlled, crossover studies. J Headache Pain. 2014;15:76.
Bendtsen L, Jensen R, Olesen J. A non-selective (amitriptyline), but not a selective (citalopram), serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension-type headache. J Neurol Neurosurg Psychiatry. 1996;61(3):285–90.
Lexicomp Facts and Comparisons, Amitriptyline. 2021, Wolters Kluwer Health, Inc.
Banzi, R., et al., Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev, 2015(5): p. CD011681.
Bendtsen L, Jensen R. Mirtazapine is effective in the prophylactic treatment of chronic tension-type headache. Neurology. 2004;62(10):1706–11.
Martin-Araguz A, Bustamante-Martinez C, de Pedro-Pijoan JM. Treatment of chronic tension type headache with mirtazapine and amitriptyline. Rev Neurol. 2003;37(2):101–5.
Zissis NP, et al. A randomized, double-blind, placebo-controlled study of venlafaxine XR in out-patients with tension-type headache. Cephalalgia. 2007;27(4):315–24.
Yurekli VA, et al. The effect of sodium valproate on chronic daily headache and its subgroups. J Headache Pain. 2008;9(1):37–41.
Lampl C, et al. A prospective, open-label, long-term study of the efficacy and tolerability of topiramate in the prophylaxis of chronic tension-type headache. Cephalalgia. 2006;26(10):1203–8.
Aleksic-Shihabi A, Lojen G, Vukovic V. Prevention of tension type headache with topiramate. Acta Med Croatica. 2008;62(2):145–9.
Fogelholm R, Murros K. Tizanidine in chronic tension-type headache: a placebo controlled double-blind cross-over study. Headache. 1992;32(10):509–13.
Murros K, et al. Modified-release formulation of tizanidine in chronic tension-type headache. Headache. 2000;40(8):633–7.
Ghadiri-Sani, M. and N. Silver, Headache (chronic tension-type). BMJ Clin Evid, 2016. 2016.
Karadas O, Gul HL, Inan LE. Lidocaine injection of pericranial myofascial trigger points in the treatment of frequent episodic tension-type headache. J Headache Pain. 2013;14:44.
Holroyd KA, et al. Management of chronic tension-type headache with tricyclic antidepressant medication, stress management therapy, and their combination: a randomized controlled trial. JAMA. 2001;285(17):2208–15.
Nestoriuc Y, et al. Biofeedback treatment for headache disorders: a comprehensive efficacy review. Appl Psychophysiol Biofeedback. 2008;33(3):125–40.
Gu Q, Hou JC, Fang XM. Mindfulness meditation for primary headache pain: a meta-analysis. Chin Med J (Engl). 2018;131(7):829–38.
Falsiroli Maistrello, L., M. Rafanelli, and A. Turolla, Manual therapy and quality of life in people with headache: systematic review and meta-analysis of randomized controlled trials. Curr Pain Headache Rep, 2019. 23(10): p. 78.
Linde, K., et al., Acupuncture for the prevention of tension-type headache. Cochrane Database Syst Rev, 2016. 4: p. CD007587.
Kolokotsios, S., et al., The effectiveness of acupuncture on headache intensity and frequency in patients with tension-type headache: a systematic review and meta-analysis. Cureus, 2021. 13(4): p. e14237.
Gildir, S., et al., A randomized trial of trigger point dry needling versus sham needling for chronic tension-type headache. Medicine (Baltimore), 2019. 98(8): p. e14520.
Lyngberg AC, et al. Prognosis of migraine and tension-type headache: a population-based follow-up study. Neurology. 2005;65(4):580–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Stephanie Steel declares no conflicts of interest. Mark Whealy receives honoraria as an author for UpToDate. Carrie Robertson receives honoraria as an author for UpToDate and has served on advisory boards for Amgen, Lundbeck, Biohaven, Impel, and Eli-Lilly.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Headache
Rights and permissions
About this article
Cite this article
Steel, S.J., Robertson, C.E. & Whealy, M.A. Current Understanding of the Pathophysiology and Approach to Tension-Type Headache. Curr Neurol Neurosci Rep 21, 56 (2021). https://doi.org/10.1007/s11910-021-01138-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11910-021-01138-7