Abstract
Purpose of Review
The glymphatic system is a relatively new concept that has been associated with regulation of cerebrospinal fluid (CSF), as well as brain waste clearance. Novel techniques to study glymphatic dysfunction have in turn prompted a reassessment of brain physiology and underlying elements of neurological disease. This review incorporates a contemporary imaging perspective focused on understanding the regulation of CSF flow, thus expanding the putative clinical relevance of this system and the relationships between CSF flow and glymphatic function.
Recent Findings
MR imaging studies, especially those that employ intrathecal gadolinium contrast, have identified potentially new pathways regulating CSF production, absorption, and clearance. These studies, when viewed in the context of more historical anatomic descriptors of CSF production and absorption, provide a more robust description of CSF physiology and waste clearance.
Summary
CSF production and resorption are under-investigated and could be related to various pathophysiologic processes in neurodegeneration. Anatomically based clinical exemplars of CSF clearance are discussed. Future studies should focus on linking glymphatic functionality with neurological disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The suggestion of a glymphatic system in 2012 has resulted in a reinvigorated discussion regarding the regulation of cerebrospinal fluid (CSF) flow, production, and clearance [1]. Glymphatic flow, as assessed in animal models, appears organized and modulated via functional states during which this system is most active (i.e., sleep) [2]. This organized CSF flow occurs through perivascular spaces and within the brain parenchymal interstitium and is regulated by aquaporin 4 channels. Multiple animal models have demonstrated fluid flow from subarachnoid, to periarterial space, where it subsequently enters the interstitial compartment as regulated by the aquaporin 4 channel. Along the efferent end of this system, aquaporin 4 channels allow for passage of fluid from the interstitial space to the perivenular space from which point the fluid again travels to the subarachnoid compartment.
Animal studies have provided key insights into the putative clinical significance of this system with regard to brain health. Perhaps most importantly, this fluid circulation has been intimately associated with clearance of brain waste products, such as amyloid beta plaques. Just as intriguing is the confirmation that this circuit is highly correlated to the sleep cycle [2]. Such findings have prompted new perspectives on the pathophysiology of neurodegenerative disease as well as potential therapeutic approaches, and have offered compelling new hypotheses regarding the beneficial role of sleep and brain health.
An increasing need to develop methods that can reliably detect the presence, functionality, and perturbations of the glymphatic circuit in humans is necessary. Contemporary methods used in animal models that assess glymphatic study are essentially impossible to replicate in humans, and the application of MR imaging methods seems to be the most promising tool for which to interrogate this system. A handful of important studies involving the administration of intrathecal contrast have largely confirmed the presence of the glymphatic system in humans and have offered tantalizing clues to its dysfunction in neurologic disease [3, 4]. Importantly, other MR imaging studies, which do not rely on intrathecal contrast, are beginning to emerge [5,6,7]. These alternative methods offer promising strategies of defining human glymphatic flow without the burden of an invasive procedure, or uncertainties regarding the long-term effect of brain gadolinium deposition.
While the flow of fluid from the subarachnoid space, to the interstitium, and subsequently back to the subarachnoid space, is relatively well understood in animal models and increasingly characterized in humans, CSF flow before and after these regions has received substantially less attention. The choroid plexus, responsible for the production of CSF, has been largely ignored in this discussion. Furthermore, although absorption of CSF is known to occur via both the arachnoid granulations and the cranial nerves and spinal nerve roots, the characterization of this shared absorptive functionality and anatomical attributes of these components appears unaddressed in contemporary studies.
In this review, we focus on two components of CSF flow: the production and absorption of CSF. Improved understanding of the vascular supply and potential responsiveness to choroid plexus stimuli offers insights to the functionality of the glymphatic system, and brain health. Similarly, a reassessment of the anatomy, and relative contributions of the arachnoid granulations, cranial nerves, and spinal nerves in clearance of CSF, can enhance our overall understanding of the glymphatic system in human health and interpret how alterations to this absorption can relate to disease. We further discuss evidence of the existence of a human lymphatic circulation within the dura and review how the parasagittal dural space may help to appropriately distribute CSF and its associated constituents as it leaves the central CNS.
As new discoveries of the afferent and efferent flow of the glymphatic system continue, novel relationships will almost certainly emerge. The second portion of this review describes how evidence of one such new relationship, that of the vascular space and the subarachnoid space, is increasingly recognized as communicative and codependent. Finally, we discuss clinical exemplars of spinal cerebrospinal outflow and their associated lymphatic drainage.
CSF Production and Drainage, Afferent, and Efferent Flow
Choroid Plexus
The choroid plexus is traditionally thought to supply the vast majority of cerebrospinal fluid (CSF). While most choroid plexus tissue resides within the lateral ventricles, there exists choroid plexus throughout the ventricular system, roughly in proportion to the overall size of the ventricular components. The choroid plexus is composed of epithelial cells and choroid stroma. The epithelial layer is derived from the ependymal lining of the ventricles and is aligned along the basal lamina. This continuous layer of cells secretes CSF along the opposite pole of the cell via villous projections. Movement of substances and fluid is restricted by tight junctions connecting the epithelial layer which constitutes the blood-CSF barrier [8, 9]. On the internal side of the basal lamina resides the choroid stroma. This is a densely vascularized bed of tissue which is supplied by large fenestrated capillaries which allow for robust fluid exchange. This region is associated with blood flow approximately 4 to 7 times greater than brain tissue in non-human primates and canines respectively [10], whereas, in humans, it measures to be roughly equivalent to gray matter, but with the range of 30–70 mL/100 g/min [8].
Vascular Supply
The lateral ventricle choroid plexus receives its predominant blood supply from the anterior choroidal arteries, as well as the medial and lateral posterior choroidal arteries. The anterior choroidal artery arises from the distal internal cerebral artery, superior to the origin of the posterior communicating artery. The medial and lateral posterior choroidal arteries usually arise from the P2 portion of the posterior cerebral artery. In some individuals, the medial posterior choroidal artery arises from the P1 segment of the posterior cerebral artery [8, 11].
CSF Production
CSF production occurs in all four ventricles as well as in other foramen, including the foramen of Luschka and the Foramen of Magendie. The majority of CSF production occurs in the lateral ventricles, roughly in proportion to their larger size. Human choroid CSF production is on the order of approximately 20 mL/h [12], though this is known to respond to changes in the brain microenvironment and inflammatory stimuli [13]. Although incompletely understood, there are known biofeedback mechanisms relating neurologic stressors to choroid plexus CSF signaling molecule production. The choroid plexus has been shown to secrete various compounds, such as growth factors, which are involved in maintaining the health of the brain parenchyma [9]. For example, TGF-β, a well-known regulatory protein involved in immune regulation and homeostasis, is produced by the choroid plexus and is expressed in times of ischemic stress [14, 15]. Some have posited that TGF-β production in the choroid plexus is regulated in order to optimize calcium homeostasis and affect expression of proto-oncogenes central to regulating cell death even when ischemia is spatially remote from the choroid plexus [16, 17]). It is reasonable therefore, that choroid plexus CSF production itself is also subject to biochemical feedback related to the physiologic status of the brain and spinal cord. As such, in the context of the glymphatic system, it would be logical that the choroid plexus would respond to increased concentrations of waste products or opportune time points of the sleep-wake cycle by increasing CSF production in order to optimize efficient waste clearance [8].
While the choroid plexus is traditionally thought to produce the vast majority of the CSF fluid within the subarachnoid space, there is an increasing debate as to whether fluid may arise from alternative sources. Murine knockout models involving the aquaporin 1 and aquaporin 4 genes support a theory that a substantial portion of CSF production and absorption occurs as a result of efflux and influx at the level of the brain parenchymal capillaries and interstitial fluid [18].
Arachnoid Granulations
The traditional model of CSF flow dictates that following production from the choroid plexus, CSF circulates around the brain and spinal cord and is subsequently resorbed by the arachnoid granulations which in turn facilitate clearances via the dural venous sinuses. Arachnoid structures are characterized by their size: larger structures, visible to the naked eye, are referred to as arachnoid granulations, and those requiring microscopic visualization are termed arachnoid villi. Histologically, as characterized by Wolpow in 1972, two distinct architectural types of arachnoid granulations were revealed by gapless serial sectioning. The smaller of the two types are covered by a dural sheath, with an associated external subdural space surrounding each granulation. The larger of the two subtypes of arachnoid granulations contain fusion points between the arachnoid granulation body and the investing dura, with focal obliteration of the associated subdural space. A portion of these larger granulations is noted to extend into the venous sinuses without a dural covering [19].
An arachnoid granulation is composed of a neck at its base, a core centrally, and an apical cap. The internal core is made up of channels surrounded by collagen with occasional macrophages which may serve a cleaning function. The cap of the arachnoid granulation may be attached to the endothelium over a region of approximately 200 μm in diameter. The remainder of the core is separated from the endothelium by subdural space and a fibrous cupola. When viewed from an endoluminal approach with electron microscopy, the endothelium overlying appears continuous with small perforating venous channels present at the apex of some of the arachnoid granulations [20].
Development of Arachnoid Granulations
Much remains to be delineated regarding the development, and degeneration, of arachnoid villi and granulations during early life, advanced age, and pathological states. In general, the arachnoid structures are thought to be small in youth, without penetration of the dural wall [21]. Human studies have demonstrated that at 26 weeks, there is a dural depression in the venous wall. These continue to increase in number and complexity from 39 weeks of development and onward [22]. In the elderly, the arachnoid villi progressively degenerate and occlude as the arachnoid membrane thickens, as there is concurrent decreased CSF production in the choroid plexus [23, 24].
Arachnoid CSF Clearance
In vitro animal studies have yielded important insight into the anatomic and functional relationships that dictate arachnoid CSF fluid clearance. A valve-like mechanism appears central to the functioning arachnoid villous. In one non-human primate study, tubes within the arachnoid villous labyrinth were demonstrated to be patent when the arachnoid villus was fixed at normal physiologic pressures; however, when flow of fixation fluid was reversed, the arachnoid tubules became effaced [25].
The histologic mechanism of fluid flow from the subarachnoid space, through the arachnoid villi and into the venous sinuses, is a matter of continuing study. Of particular interest is the mechanism by which fluid and solutes are able to traverse the apical cap of the arachnoid villous and subsequently move through the endothelial membrane. Multiple studies point to a pressure-dependent mechanism of clearance. In sheep and dog studies, intracellular endothelial clefts have been observed when increased pressure was applied to the sinus and CSF [26]. A cynomolgus monkey study demonstrated that when tissue was fixed under normal or increased pressure, large intracellular vacuoles were present which had openings to both the CSF and venous blood [27]. Such studies demonstrate potential mechanisms of fluid and solute transfer in animal models though the exact pathway mechanisms remain to be fully understood, and the applicability of animal studies to human physiology is debatable.
In humans, the histologic description of fluid and solute transport from the subarachnoid space to the dural venous sinuses is much more limited. One study applied an in vitro model of human arachnoid granulation cells with varying polarity of perfusion gradients. Human arachnoid granulation cells were grown on a filter membrane with close attention to the cellular polarity relative to perfusion parameters. When the fluid perfusion was set to the basal to apical direction at physiologic pressures, thus mimicking physiologic conditions, a statistically significant greater flow rate was achieved as compared with when perfusion was set to the reverse, non-physiologic orientation. Interestingly, cells perfused in the non-physiologic direction showed large numbers of dead and dying cells. Furthermore, in the setting of physiologic perfusion parameters, large extracellular cisternal spaces were visible between overlapping arachnoid granulation cells and vacuoles within the cytoplasm. These findings raise the possibility that the spaces within and between cells represent pathways for transcellular and paracellular transport of fluid [28].
Lymphatic Clearance Along Cranial Nerves and Spinal Nerve Roots
A proportion of clearance of the CSF is known to occur along the cranial nerves to the cervical lymph nodes. Similarly, CSF is also known to course along the paraspinal nerve roots en route to the paraspinal lymphatics. The relative importance of these clearance mechanisms across various species, at various ages, and in different physiologic and pathologic states largely remains to be further elucidated.
Cribriform Plate
Multiple animal studies have demonstrated that substances injected into the ventricular system or basilar cisterns will subsequently occupy the foramina of the cranial nerves, particularly the olfactory nerves. Extension of these substances was observed in the cranial nerve foramina and the lumen of lymphatic vessels within the nasal cavity. A direct connection between the subarachnoid space and the nasal cavity was demonstrated in rabbits where the dura in the region of the cribriform plate surrounding the olfactory bulbs joins the periosteum [29, 30].
Evidence of a connection between the subarachnoid space and the cervical lymphatics via a perineural course in humans is less well established; however, MRI imaging of the cervical lymph nodes following injection of intrathecal contrast has provided strong evidence to support this route of CSF clearance (Fig. 2) [31]. While some human studies have failed to show direct communication between the space surrounding the olfactory nerves and the lymphatic vessels of the nasal cavity [32], other studies have demonstrated that substances injected into the cerebromedullary cistern filled the perineural space and the nasal cavity lymphatic vessels [33].
Optic Nerve
Contents within the subarachnoid space surrounding the optic nerves have been shown to communicate with the surrounding tissues of the orbit. One study demonstrated communication of the subarachnoid space surrounding the optic nerve with the tissue in the region of optic nerve where it contacts the sclera [34]. Lymphatic vessels in the dura surrounding the bulbar portion of the optic nerve have been observed in humans. Upon injection of india ink particles into the subarachnoid space surrounding the human optic nerve, particles were subsequently noted to occupy the extracellular space of the dura in an area of the lymphatic vessels [35].
Spinal Nerves
Animal literature provides a strong foundation for a mechanism of lymphatic clearance of cerebrospinal fluid in the region of the spinal nerve roots analogous to that occurring at the margins of the cranial compartment. In non-human primates, spinal arachnoid villi have been observed occupying the space between collagen bundles in the dura mater of the spinal nerves. A portion of these arachnoid structures were noted to project into veins associated with the spinal nerve roots [36], closely paralleling the organization of arachnoid granulations associated with the dural venous sinuses. Another study demonstrated a dense network of lymphatic vessels in the non-human primate cervical and upper thoracic spine which drain the CSF. This network was noted to be located predominantly within the epidural spaces surrounding the spinal nerve roots. Drainage of this system was demonstrated to occur via the deep cervical and prevertebral lymph nodes [37].
Dural Lymphatics
Since its initial 2012 description in a murine model, the concept of a glymphatic circulation has prompted a reassessment of CSF circulation and clearance [1]. As the concept of a glymphatic circuit in humans gains acceptance, further questions have arisen regarding the fate of fluid and waste products that have exited the from the interstitial space, to the subarachnoid space via the perivenous space. As clearance of subarachnoid fluid is known to occur via both the arachnoid granulations to the venous sinuses as well as to the cervical lymphatics via the cranial nerves, questions arise as to how and in what proportion the CNS directs fluid from the subarachnoid space to these to outflow tracts.
A potentially important clue to this question surfaced with the discovery that both non-human primates and humans demonstrate histological and imaging evidence of lymphatic vessels adjacent to the dural venous sinuses. These were noted to be particularly closely associated with the superior sagittal sinus. Using putative endothelial markers which are reportedly capable of discriminating lymphatic vessels from venous blood vessels, a branched network of lymphatics was found in the dura mater. This was demonstrated to be most concentrated adjacent to the venous sinuses and more centered along the meningeal layer than the periosteal layer of the dura [38•].
Importantly, the authors of this study went on to provide strong MRI support for this finding by taking advantage of two different gadolinium contrast agents which have different affinity for proteins within the blood pool. Gadofosveset, a blood pool MRI contrast agent that largely stays within the blood pool, has a different distribution when compared with a routine MRI contrast agent such as gadobutrol which is less restricted to the blood pool due to its lack of affinity for blood protein. Areas that demonstrated enhancement in the non-blood pool contrast agent (gadobutrol) but not following the administration of the blood pool agent (gadofosveset) were assessed to reflect contrast within lymphatic vessels. Critical to this observation is that the orientation and location of this putative enhancement of dural lymphatic vessels largely matched the distribution, location, and orientation of the lymphatic vessels as identified on non-human primate and human histologic sections. These were noted to be most concentrated running parallel to the dural venous sinuses, particularly along the superior sagittal sinus [38•].
Another important link between the glymphatic system and the region adjacent to the superior sagittal sinus was demonstrated by close MRI analysis over time of this region following the administration of intrathecal gadolinium contrast. The region adjacent to the superior sagittal sinus was imaged 3, 6, 24, and 48 h post-lumbar intrathecal injection of gadolinium contrast. Contrast was noted to concentrate adjacent to the parasagittal dura and pass from the cerebrospinal fluid adjacent to the dura to the dura along the superior sagittal sinus in regions near the entry of cortical veins. This demonstrated that trans-arachnoid molecular passage occurs in this region, and it suggests that the parasagittal dura serves as a bridging link between the human brain and dural lymphatic vessels [39•].
An interesting component of this study was the observation that tracer enhancement below the cribriform plate could only be visualized in 2/18 patients in contrast to the robust enhancement of the parasagittal dural space and the relatively more prominent enhancement of the other cranial nerve outlets at the skull base. The authors speculated that the human olfactory system is proportionally much smaller in size than in rodents and thus molecular and CSF clearance in this region is therefore much less important than in humans [39•].
The anatomic space adjacent to the dural venous sinuses, particularly that adjacent to the superior sagittal sinus, appears to play an important role regarding the fate of fluid and associated waste products at the terminal end of the glymphatic circuit (Fig. 1 a and b). Is this region a clearinghouse for fluid that will be distributed to the venous sinuses and cervical lymphatics in proportions most appropriate to maintain homeostasis? Furthermore, is the dysfunction of this mechanism, perhaps by abnormal buildup of waste products in the elderly and diseased states, responsible for chronic neurodegenerative disease? Further study of this region in animal and human studies is necessary to more clearly understand the potentially profound importance of the appropriate function and pathological degeneration of the parasagittal dural space.
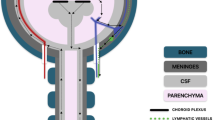
Anatomical points of interest in CSF circulation and glymphatic flow. Important points of interest labeled. a Cross section of the superior sagittal sinus region, showing the superior sagittal sinus, parasagittal dural space (PDS), and arachnoid granulations. b Anatomy of an arachnoid granulation, where subarachnoid flow of CSF reabsorbs into dural venous sinuses. c Cranial nerves, showing location of CSF flow around cranial nerves (i.e., cribriform plate, CNI). d Cerebral aqueduct and 4th ventricle, detailing the path of CSF flow from the third ventricle to the central canal and subarachnoid space. e Axial cross-sectional anatomy of the spinal canal with the relationship of the lymphatic vessels to the thecal sac, epidural space, and spinal nerve roots. f Cervical lymphatic flow, detailing the lymph vessels and nodes. g Choroid plexus, predominantly located in the lateral ventricles, where CSF is produced and secreted in the ventricular system
Anatomically Informed Pathophysiologic Exemplars
Connections Between the Vasculature and the Subarachnoid Space
Although not classily described, there is a growing body of imaging literature that highlights communication between the intravascular space and the subarachnoid space. Initial studies noted enhancement of the perivascular space on T2 FLAIR imaging several hours following intravenous contrast injection [6]. Follow-up studies demonstrated that there was more concentrated contrast enhancement surrounding the vein of Labbe when similar imaging was applied hours following intravenous contrast [40]. These findings demonstrate that communication from the intravenous space and the subarachnoid space does occur and further support that contrast is concentrated along the venous system in an organized manner as it exits the CNS. Critical to our understanding of the overall functioning of the human glymphatic system is a description of this intravenous to subarachnoid communication. As we better understand this link, we will better understand how molecules that exist within the systemic circulation may affect CNS pathologic processes.
Clues to the anatomic sites of communication surfaced when heavily T2-weighted fluid-attenuated inversion recovery sequence obtained at 3 and 24 h post-intravenous injection of contrast demonstrated delayed gadolinium contrast entry into the central nervous system (CNS) via the choroid plexus and the ciliary body of the orbit. Interestingly, there was a significant positive correlation between Fazekas white matter score and signal intensity increase in the perivascular spaces 3 h post-injection [7].
These findings served to further emphasize the important role of the choroid and the facial structures in the regulation of CSF and its associated molecular substances. As gadolinium contrast agents are foreign molecules capable of entering the subarachnoid space in quantities sufficient for MRI detection, this implies that other substances are also capable of taking this same course. Could dysfunction of the choroid or ciliary body provide a door for substances to enter the CSF and lead to downstream CNS disruption?
The linkage between the Fazekas score, a measurement of white matter disease, and delayed perivascular enhancement following intravenous contrast administration also implies a relationship between altered glymphatic perivascular flow and CNS pathology in humans.
Alternative Potential Routes of Fungal Spread Through Epidural Lymphatics
A more nuanced understanding of CSF circulation throughout the head, neck, and spine offers a new opportunity to comprehend various types of pathophysiology. One potential example of this is the progression of epidural infectious processes that originate in the spine. In 2013, a spike in spinal fungal abscesses was observed in patients who received epidural injections of supply of methylprednisolone contaminated with fungus.
One component of this outbreak occurred in Tennessee where patients became symptomatic approximately 1 to 4 weeks or later following epidural injections. An interesting manifestation of this outbreak was that approximately 1/8th of these patients presented with stroke [41]. A case series detailed the clinical course of three patients, all in their 8th decade. Each patient had MR imaging manifestations of lumbar epidural infection and had subsequent strokes. Despite robust clinical workup, none had clear evidence of cardiac infection. Notably, all three patients demonstrated stroke manifestations exclusively confined to the posterior circulations, involving the basilar, superior cerebellar, and posterior cerebral artery distributions [42].
A systemic extension of pathogen certainly would be capable of spreading fungal infection to the brain, though the lack of any attribute imaging manifestations to the anterior circulation raises the possibility of an alternative route of spread. As discussed above, there are robust lymphatic networks present throughout the epidural space of the spine. One potential route of spread is via the epidural lymphatic plexus to the cervical spine, with subsequent extension to the cervical posterior arterial circulation and downstream intracranial posterior circulation. Animal studies have shown that components of the CSF circulation vary with age. Could an altered epidural lymphatic flow dynamic in this group of elderly patients predispose them the aforementioned route of fungal spread? As we begin to understand how the glymphatic circulation evolves with age and disease in humans, such descriptions could inform us of additional patterns of CNS disease progression.
Conclusion
Since its initial description in 2012, a substantial amount of animal and human evidence has emerged in support of the critical role that the glymphatic system plays in animal and human physiology. Here, we seek to focus attention on not just the component of that circuit that lies between the periarterial and perivenule spaces but also on the components that precede and conclude this process.
The choroid plexus, responsible for the very production of fluid in this circuit, has a robust vascular supply that is clearly responsive to external stimuli. Despite this important position within the glymphatic system, there is very little modern scientific investigation into the development and signaling biology of the choroid plexus.
At the other end of the glymphatic system, there is now relative consensus regarding the dual contribution of the arachnoid granulations and the cranial/spinal nerves in CSF clearance. Despite this, there is a widespread lack of understanding regarding how these two components interact with each other at various different ages and times of pathologic stress. Importantly, there is even less understanding of how systemic disease processes in the more downstream lymphatic and venous systems may impact appropriate upstream CSF clearance. Novel imaging techniques in humans and in animal models offer substantial promise in our comprehension of these complex and important systems.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. https://doi.org/10.1126/scitranslmed.3003748.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–7. https://doi.org/10.1126/science.1241224.
Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140(10):2691–705. https://doi.org/10.1093/brain/awx191.
Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab. 2019;39(7):1355–68. https://doi.org/10.1177/0271678X18760974.
Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–8. https://doi.org/10.1007/s11604-017-0617-z.
Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based contrast enhancement of the perivascular spaces in the basal ganglia. Magn Reson Med Sci. 2017;16(1):61–5. https://doi.org/10.2463/mrms.mp.2016-0039.
Deike-Hofmann K, Reuter J, Haase R, Paech D, Gnirs R, MTRA, et al. Glymphatic pathway of gadolinium-based contrast agents through the brain overlooked and misinterpreted. Investig Radiol. 2019;54:4. https://doi.org/10.1097/RLI.0000000000000533.
Johnson SE, McKnight CD, Lants SK, Juttukonda MR, Fusco M, Chitale R, et al. Choroid plexus perfusion and intracranial cerebrospinal fluid changes after angiogenesis. J Cereb Blood Flow Metab. 2019;40:1658–71. 271678X19872563. https://doi.org/10.1177/0271678X19872563.
Emerich DF, Vasconcellos AV, Elliott RB, Skinner SJM, Borlongan CV. The choroid plexus: function, pathology and therapeutic potential of its transplantation. Expert Opin Biol Ther. 2004;4:1191–201. https://doi.org/10.1517/14712598.4.8.1191.
Faraci FM, Mayhan WG, Heistad DD. Effect of serotonin on blood flow to the choroid plexus. Brain Res. 1989;478:121–6. https://doi.org/10.1016/0006-8993(89)91483-2.
Wiesmann M, Yousry I, Seelos KC, Yousry TA. Identification and anatomic description of the anterior choroidal artery by use of 3D-TOF source and 3D-CISS MR imaging. AJNR Am J Neuroradiol. 2001;22:305–10.
Guyton AC, Hall JE. Textbook of medical physiology. 11th ed. Philadelphia: Elsevier Saunders; 2006.
Kaur C, Rathnasamy G, Ling EA. The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol. 2016;75:198–213. https://doi.org/10.1093/jnen/nlv030.
Klempt ND, Sirimanne E, Gunn AJ, Klempt M, Singh K, Williams C, et al. Hypoxiaischemia induces transforming growth factor beta 1 mRNA in the infant rat brain. Brain Res Mol Brain Res. 1992;13:93–101. https://doi.org/10.1016/0169-328x(92)90048-g.
Knuckey NW, Finch P, Palm DE, Primiano MJ, Johansom CE, Flanders KC, et al. Differential neuronal and astrocytic expression of transforming growth factor beta isoforms in rat hippocampus following transient forebrain ischemia. Brain Res Mol Brain Res. 1996;40:1–14. https://doi.org/10.1016/0169-328x(96)00016-2.
Chodobski A, Szmydynger-Chodobska J. Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech. 2001;52:65–82. https://doi.org/10.1002/1097-0029(20010101)52:1<65::AID-JEMT9>3.0.CO;2-4.
Prehn JH, Bindokas VP, Marcuccilli CJ, Krajewski S, Reed JC, Miller RJ. Regulation of neuronal Bcl2 protein expression and calcium homeo- stasis by transforming growth factor type beta confers wide-ranging protection on rat hippocampal neurons. Proc Natl Acad Sci U S A. 1994;91:12599–603. https://doi.org/10.1073/pnas.91.26.12599.
Trillo-Contreras JL, Toledo-Aral JJ, Echevarría M, Villadiego J. AQP1 and AQP4 contribution to cerebrospinal fluid homeostasis. Cells. 2019;8(2):197. https://doi.org/10.3390/cells8020197.
Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724–7. https://doi.org/10.3171/jns.1972.37.6.0724.
Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg. 1985;63(6):867–75. https://doi.org/10.3171/jns.1985.63.6.0867.
Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:9. https://doi.org/10.1186/1743-8454-7-9.
Gomez DG, Ehrmann JE, Potts DG, Pavese AM, Gilanian A. The arachnoid granulations of the newborn human an ultrastructural study. Int J Dev Neurosci. 1983;1:139–47. https://doi.org/10.1016/0736-5748(83)90040-0.
Johanson CE, Duncan JA III, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. https://doi.org/10.1186/1743-8454-5-10.
Rubenstein E. Relationship of senescence of cerebrospinal fluid circulatory system to dementia of the aged. Lancet. 1998;24:283–5. https://doi.org/10.1016/S0140-6736(97)09234-9.
Welch K, Friedman V. The cerebrospinal fluid valves. Brain. 1960;83:454–69. https://doi.org/10.1093/brain/83.3.454.
Gomez DG, Potts DG, Deonarine V, Reilly DF. Effect of pressure gradient changes on the morphology of arachnoid villi and granulations of the monkey. Lab Investig. 1973;28:648–57.
Tripathi RC. Tracing the bulk outflow route of cerebrospinal fluid by transmission and scanning electron microscopy. Brain Res. 1974;80:503–6. https://doi.org/10.1016/0006-8993(74)91033-6.
Grzybowski DM, Holman DW, Katz SE, Lubow M. In vitro model of cerebrospinal fluid outflow through human arachnoid granulations. Invest Ophthalmol Vis Sci. 2006;47:3664–72. https://doi.org/10.1167/iovs.05-0929.
Sokołowski W, Barszcz K, Kupczyńska M, Czubaj N, Skibniewski M, Purzyc H. Lymphatic drainage of cerebrospinal fluid in mammals-are arachnoid granulations the main route of cerebrospinal fluid outflow? Biologia (Bratisl). 2018;73(6):563–8. https://doi.org/10.2478/s11756-018-0074-x.
Mollanji R, Bozanovic-Sosic R, Silver I, Li B, Kim C, Midha R, et al. Intracranial pressure accommodation is impaired by blocking pathways leading to extracranial lym- phatics. Am J Phys. 2001;280:1573–81. https://doi.org/10.1152/ajpregu.2001.280.5.R1573.
Eide PK, Vatnehol SAS, Emblem KE, Ringstad G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep. 2018;8:7194. https://doi.org/10.1038/s41598-018-25666-4.
Löwhagen P, Johansson BB, Nordborg C. The nasal route of cerebrospinal fluid drainage in man. A light-microscope study. Neuropathol Appl Neurobiol. 1994;20(6):543–50. https://doi.org/10.1111/j.1365-2990.1994.tb01008.x.
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:1–13. https://doi.org/10.1186/1743-8454-1-2.
Lüdemann W, von Rautenfeld DB, Samii M, Brinker T. Ultrastructure of the cerebrospinal fluid outflow along the optic nerve into the lymphatic system. Childs Nerv Syst. 2005;21:96–103. https://doi.org/10.1007/s00381-004-1040-1.
Killer HE, Laeng HR, Groscurth P. Lymphatic capillaries in the meninges of the human optic nerve. J Neuroophthalmol. 1999;19:222–8.
Welch K, Pollay M. The spinal arachnoid villi of the monkeys Cercopithecus aethiops sabaeus and Macaca irus. Anat Rec. 1963;145:43–8. https://doi.org/10.1002/ar.1091450107.
Miura M, Kato S, Lüdinghausen MV. Lymphatic drainage of the cerebrospinal fluid from monkey spinal meninges with special reference to the distribution of the epidural lymphatics. Arch Histol Cytol. 1998;61:277–86. https://doi.org/10.1679/aohc.61.277.
• Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6:e29738. https://doi.org/10.7554/eLife.29738This study provides strong histological and imaging evidence for the presence of lymphatic vessels in human and non-human primates meninges.
• Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun. 2020;11(1):354. https://doi.org/10.1038/s41467-019-14195-xThis study provides new evidence that the parasagittal dura space serves as an important staging area for CSF resorption.
Ohashi T, Naganawa S, Ogawa E, Katagiri T, Kuno K. Signal intensity of the cerebrospinal fluid after intravenous administration of gadolinium-based contrast agents: strong contrast enhancement around the vein of Labbe. Magn Reson Med Sci. 2019 Jul 16;18(3):194–9. https://doi.org/10.2463/mrms.mp.2018-0043.
Kainer MA, Reagan DR, Nguyen DB, Wiese AD, Wise ME, Ward J, et al. Fungal infections associated with contaminated methylprednisolone in Tennessee. N Engl J Med. 2012;367(23):2194–203. https://doi.org/10.1056/NEJMoa1212972.
Kleinfeld K, Jones P, Riebau D, Beck A, Paueksakon P, Abel T, et al. Vascular complications of fungal meningitis attributed to injections of contaminated methylprednisolone acetate. JAMA Neurol. 2013;70(9):1173–6. https://doi.org/10.1001/jamaneurol.2013.3586.
Funding
This research is supported by the Natitional Institutes of Health, R01AG062574 to M.J.D., and D.O.C, and Department of Defense W81XWH-19-1-0812 to D.O.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dementia
Rights and permissions
About this article
Cite this article
McKnight, C.D., Rouleau, R.M., Donahue, M.J. et al. The Regulation of Cerebral Spinal Fluid Flow and Its Relevance to the Glymphatic System. Curr Neurol Neurosci Rep 20, 58 (2020). https://doi.org/10.1007/s11910-020-01077-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11910-020-01077-9