Abstract
Purpose of Review
This chapter focuses on limb apraxia, a cognitive-motor disorder of learned skilled movement, and the nature of the spatiotemporal errors that disrupt movement sequences.
Recent Findings
A cognitive model that attempts to reconcile conceptual and preparatory aspects of the motor program with perceptual and kinematic features will be discussed. An update on the localization of the praxis network will be provided. In addition, a long-held view that limb apraxia does not have ecological relevance will be disputed in the context of studies that have shown that limb apraxia (i) is one of the most important predictors of increased caregiver burden and (ii) is associated with impaired activities of daily living in post-stroke patients. This review summarizes current screening tools and the few randomized clinical controlled treatment studies to date.
Summary
Limb apraxia is underdiagnosed and very few therapeutic options are available. Cognitive process models should be used to inform future controlled multi-modal treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Praxis is defined as the ability to perform goal-directed or learned skilled movements. Goal-directed upper limb movements include tool use (transitive) gestures, like using a hammer, and meaningful communicative non-tool (intransitive) gestures, like waving good-bye. Limb apraxia is an acquired cognitive-motor disorder characterized by a breakdown in the spatial and temporal organization of arm and hand movements. Cognitive models propose a division into a praxis production system that controls the motor plans for learned skilled movements and a praxis conceptual system that controls knowledge of object-related action plans. A disorder of the praxis production system is called ideomotor apraxia, whereas a disorder of the praxis conceptual system is called conceptual or ideational apraxia.
There is confusion about the term “apraxia” as it has been applied to a number of disorders that are not cognitive-motor disorders of the upper limb. These include dressing apraxia (disorder of dressing), ocular apraxia (disordered eye movement), and constructional apraxia (disorder of drawing ability). In addition, some investigators have proposed other limb apraxia subtypes, including limb-kinetic apraxia, which is not a cognitive-motor disorder per se, but rather a breakdown in the motor action plan with a “clumsy” movement dissociable from disordered learned skilled movements, often present in Parkinson’s disease. Apraxia has also been used to describe oral motor disorders like buccofacial (oral) apraxia and apraxia of speech. Because these two disorders are often associated with an elemental motor problem involving the lips, face, tongue, or oral pharynx and frequently co-occur with aphasia, these apraxia subtypes cannot easily be disassociated from sensorimotor or language deficits.
Limb apraxia, which will be the focus of this article, is operationally defined as a disorder of learned skilled movements that cannot be explained by sensorimotor or comprehension deficits. Thus, the action errors in limb apraxia, using this narrow definition, must be evaluated independent of an elemental motor deficit such as weakness, deafferentation, abnormalities of posture and tone, ataxia, or lack of understanding. Limb apraxia (dyspraxia in developmental disorders) can occur in a variety of acquired neurological conditions including stroke, Alzheimer’s disease, and other neurodegenerative disorders (e.g., Parkinson’s disease and corticobasal degeneration), and in many developmental disorders such as autism spectrum disorder and specific language disorder. Limb apraxia may also follow traumatic brain injury (TBI) at any age.
The present discussion will be limited to limb apraxia, emphasizing the praxis production system with some reference to the praxis conceptual system within a cognitive-process model. We focus primarily on post-stroke limb apraxia for three main reasons. First, limb apraxia is a common disorder in unilateral stroke, with estimates that as many as 63% of left-hemispheric stroke patients are affected [38, 102]. Second, lesion localization in stroke provides a reductionist model to evaluate the critical brain regions involved in the production of learned skilled movements (praxis production network) and in the action-semantic knowledge (praxis conceptual network) necessary for selecting appropriate limb movements. Finally, there is a wider literature on limb apraxia following stroke compared to other acquired neurological disorders like Alzheimer’s disease, corticobasal degeneration, or TBI. It is beyond the scope of this review to detail limb apraxia in these other important neurological conditions, or to fully examine the history or conceptual-theoretical underpinnings of limb apraxia. This article will include a summary of controversies and consensus regarding current cognitive models, as well as the evaluation and treatment of limb apraxia.
Anatomy and Localization of Limb Apraxia
The Praxis Network
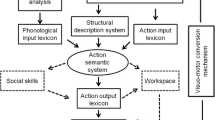
Current models of limb apraxia propose that visuokinesthetic engrams or representations of learned skilled movement (i.e., conceptual motor plans) rely on the left inferior parietal lobe, while the computations guiding these goal-directed movements (i.e., concrete motor plans) are facilitated by left prefrontal motor cortices [17, 18, 28••, 51, 63, 74, 84, 85] (see Fig. 1). This localization can explain goal-directed right limb movements or the praxis production component. For movements to be performed with the left arm and hand, these left hemisphere regions must communicate with contralateral right premotor areas in order to activate descending motor pathways on the left side [37, 51].
Cognitive-motor apraxia types. Type 1: impaired gesture-to-command, gesture imitation preserved: impairments to command reflect a disruption in the motor planning network and may be associated with an inability to access the motor plans from memory. Deficits can exist in the lexical and/or non-lexical route. Lesions may be small in size and disconnect posterior from anterior regions within left frontal-parietal circuits. Lexical and non-lexical routes may be impaired in lesions that extend to callosal fibers that disconnect the right hemisphere. Type 2: impaired gesture-to-command and gesture imitation: impairments of gesture imitation may reflect an inability to implement, execute, or control learned skilled movements. Deficits on gesture imitation may be associated with degraded non-representational movements, as the patient may have a more basic disorder of motor programming. Deficits can exist in the lexical and/or non-lexical route. Type 3: impaired gesture-to-command, impaired gesture recognition-discrimination: lesions are more likely to be large middle cerebral artery (MCA) territory and posterior in location including the inferior parietal lobe (Brodmann’s area—BA 39, 40) with extension to periventricular white matter (PVWM) and/or to ventral temporal regions. There may be dissociations with lexical and non-lexical deficits seen with lesions to both of these regions whereas lesions restricted to BA 39/40 may affect the lexical but spare the non-lexical route. Type 4: impaired gesture-to-command, intact gesture recognition-discrimination: lesions will be more likely to involve anterior frontal sites within the MCA territory (perirolandic)
Converging evidence from lesion, neuroimaging, and neurophysiological studies show that the supramarginal gyrus (SMG, Brodmann’s area 40), supplementary motor area (SMA, medial portion of Brodmann’s area 6), and their white matter connections are the critical components of the parietal-frontal praxis production network [6, 14••, 19, 28••, 33, 34, 45, 58, 100]. Patients with limb apraxia may have deficits at the level of motor planning (frontal) or sensorimotor integration (parietal) [7]. A functional MRI study using repetition suppression to study hand gestures in healthy participants found that performance of a novel action, collapsed across specific actions, showed greater left than right-hemispheric activation within a predominant parietal-frontal circuit involving inferior frontal gyrus and the inferior parietal cortex (SMG) [46]. A recent review by Buxbaum and Randerath [13••, 14••] provides a comprehensive discussion of the importance of the left inferior parietal cortex in limb apraxia.
Lesions, Locations, and Models of Apraxia
There is some empirical evidence that lesions to the left inferior parietal cortex produce disordered gesture production, discrimination, and recognition, whereas isolated left frontal lesions are more likely to yield specific production deficits (i.e., without errors in gesture discrimination and recognition) [53, 81]. These distinct patterns of deficits by lesion location have been incorporated into cognitive models of apraxia that propose at least partially separable modules of praxis production, gesture comprehension, and gesture discrimination. Conceptual aspects of tool use and arm-hand gestures are also at least partially dissociable functions [69], with less clarity regarding functional localization.
This segregation into a dual model of praxis networks is somewhat analogous to anterior and posterior language zones, with the former associated with production or expressive abilities and the latter with receptive skills or auditory comprehension. A dorsal-ventral visual processing model has been used to explain how praxis production and recognition-discrimination functions are at least partially distinct from a semantic praxis system [21, 52, 53, 55, 68, 70, 81]. It remains controversial whether action semantics represents a distinct capacity or is inseparably integrated into more generalized knowledge of associated objects. One recent study suggested specificity of action recognition within the lateral occipitotemporal cortex [55]. There also continues to be debate about the degree of laterality of praxis functions, although current consensus is that these are largely lateralized within a left-hemispheric network in most individuals regardless of handedness [2, 5, 29, 48, 60, 65, 66•, 79]. Króliczak and colleagues [61•] found left-lateralized activation of SMG for action planning in both healthy right- and left-handers.
Because the anatomical regions constituting the praxis network are located in close proximity to core language and primary motor regions, many patients with limb apraxia have a concomitant acquired language disorder (i.e., aphasia) and/or right-sided sensorimotor deficit (i.e., contralateral hemiparesis and hemisensory loss) secondary to unilateral left-hemispheric stroke [4, 11, 30, 43, 71••]. Such co-occurrences can confound the assessment process, as the presence of apraxia must be independent of potential contributions of language or motor impairment. Despite evidence that apraxia and aphasia are commonly associated, there are reports of patients with limb apraxia without aphasia, suggesting that these cognitive operations are at least partially dissociable [41, 59, 72, 86].
While typically resulting from sizable cortical lesions, white matter damage, such as a small lesion to the left periventricular white matter [58], can also cause limb apraxia. These small lesions generally occur at convergence areas within the praxis network. In addition, “disconnection apraxia” or “callosal apraxia” has been documented in patients with lesions restricted primarily to the anterior corpus callosum. These patients show difficulty with pantomiming gestures-to-command with the left arm and hand, but they have preservation of these movements when elicited with the right upper extremity [32, 36, 51]. Other patients with callosal injury may selectively make spatial-temporal errors in response to verbal commands (verbal callosal disconnection apraxia) or with imitation and actual tool use (callosal ideomotor apraxia) [15•]. In callosal conceptual apraxia, patients produce content errors, such as incorrectly selecting tools and the objects associated with them, with the left hand but not the right [3, 51].
Dissociations Within Apraxia
One study attempted to localize conceptual apraxia as a disorder within two domains: associative tool knowledge and mechanical tool knowledge [52]. The former group may make content errors when gesturing a tool action (e.g., pounding with hammer) or show deficits in other forms of tool knowledge like tool-object association (e.g., hammer-nail), while the latter may no longer understand the mechanical advantage of tool use (i.e., impaired problem-solving in fashioning an appropriate tool for a desired function). This study included four groups: unilateral left-hemispheric stroke patients with or without apraxia, a right-hemispheric stroke patient control group, and a matched group of healthy controls. Results showed that the left-hemispheric group with apraxia was most impaired on tests of both associative and mechanical conceptual apraxia. These data provide support for the view that conceptual apraxia is more distributed within the left hemisphere relative to the right, although lesion analysis did not localize to any specific region.
In another study, conceptual apraxia was found in the early phase of Alzheimer’s disease and, therefore, was considered as a potential early clinical marker of this neurodegenerative disorder [70]. In order to evaluate the praxis conceptual network in matched controls compared to patients with Alzheimer’s disease, the patients were divided into four groups based on the absence/presence of ideomotor limb apraxia and a lexical-semantic deficit. Results showed that each of the four patient groups differed from controls on at least one measure of conceptual apraxia. In addition, there were some Alzheimer’s patients with conceptual apraxia and preserved lexical functions and others with conceptual apraxia but without ideomotor apraxia, suggesting that both language and praxis production networks are at least partially independent of the praxis conceptual system.
In a recent study of callosal ideomotor apraxia in Alzheimer’s disease, Cimino-Knight, Gonzalez-Rothi, He, and Heilman [15•] found that the patient group had ideomotor and conceptual apraxia of both right and left hands, with the right-hand deficit greater than the left. The authors concluded that these results support a hemispheric disconnection in Alzheimer’s disease. Although limb apraxia is not commonly evaluated in dementia patients, Soulsby and colleagues [95] suggest that ideomotor apraxia could be used as a staging tool for Alzheimer’s disease.
The Heilman-Rothi praxis model can be used to understand these concepts and to reconcile conceptual and motor preparatory aspects of praxis with perceptual and kinematic features in order to ground abstract concepts such as action and object imagery [28••, 51, 68]. Clark and colleagues [16] had stroke patients with apraxia produce the “slicing” action required to cut a loaf of bread to study the kinematics of limb movements. Contextual cues were introduced in a graded fashion to measure effects on spatiotemporal features of the elicited movements. The task was performed with the ipsilesional limb and resulted in impaired spatiotemporal coordination across all cueing conditions, although there was improvement in movement amplitude and speed when an actual loaf of bread was provided as a cue. These results are partially consistent with Haaland and colleagues, who found amplitude and movement trajectory impairments in movements performed with the ipsilesional limb [42, 44, 50, 66•]. In addition, a recent study found a breakdown in visuomotor integration in apraxia, yet not for multisensory integration, providing some evidence for a motor prediction defect in limb apraxia [67•]. These results support the hypothesis that divided visual streams subserve different functions: The disrupted dorsal (occipito-parietal) pathway that should compute action representations of objects (determining “how”) to guide movement operates independently of the ventral (occipitotemporal) pathway’s support for object recognition and general semantic processing (determining “what”) [35].
The Role of Affordances
There have been recent efforts to explain apraxia within the context of affordances [25••, 73•]. Affordances are defined as the meaningful relationship between features of an observed object and the observer’s action network. Thus, affordance integrates cognitive, perceptual, and motor control functions so that these are unified, with consideration of the environment and relevant objects, when identifying and selecting potential motor action plans.
The contributions of affordance mechanisms have been used to explain the movement deficits in limb apraxia. One interesting notion is the separation of stable affordances within the ventro-dorsal stream from variable affordances within the dorso-dorsal stream. These representations are not completely dichotomous. There is some evidence that offline linguistic tasks recruit stable rather than variable affordances [10], whereas tool-object knowledge may be dependent on both [78]. Deficits in limb apraxia may derive from the inability to utilize the flexible features of affordances.
In another study, left-hemispheric stroke patients with apraxia were found to be unable to use knowledge-based (memory-association condition) or higher-order (visual-spatial cue condition) information to learn a grasping task [25••]. This finding was interpreted as evidence for a disruption in integrating the visible and known object properties associated with ventral stream function with the dorsal stream’s role in visual affordances. Evans and colleagues [26•] also used transcranial direct current stimulation (tDCS) to inhibit activity of the left inferior parietal lobe in healthy individuals to approximate the function of patients with apraxia, which impaired perceptual decisions about object manipulation. Based on these results, Evans and colleagues postulate that disruption to “ventro-dorsal” processes can predict the difficulty that some apraxic patients have in learning to manipulate novel objects.
In a study of lesion localization in left-hemispheric stroke patients with apraxia, one unexpected site of lesion location included the left ventral temporal cortex with extension into the temporoparietal junction. This subgroup of patients had a more severe type of limb apraxia. Unfortunately, conceptual apraxia was not evaluated. Nevertheless, the authors postulated that patients with this lesion profile, who had severely degraded gesture production, might have lost the ability of the motor programs to access the stored visual representations of the movements [28••].
Right Hemisphere Contributions
There is conflicting evidence regarding the contributions of the right hemisphere in mediating movement sequences, especially intransitive (meaningful non-tool) movements [30, 47, 79]. Some have suggested that the left hemisphere is dominant for preparing and programming of movements [45], and for learning to select the limb movement associated with the action of a specific object [87]. It may be that the right hemisphere contributes to some of the perceptual and conceptual aspects of the motor programs required to produce meaningful gestures, whereas the visuokinesthetic engrams or representations of learned skilled movement (i.e., conceptual motor plans) and the computations that activate the motor programs (i.e., concrete motor plans) depend primarily on the left cerebral hemisphere.
Subcortical Contributions
Subcortical networks have also been examined within the context of learned skilled movements [1, 62, 77]. In one study, unilateral left-hemispheric cortical stroke patients were compared to a subcortical group [48]. The cortical patients presented with deficits in the production of transitive (tool) and intransitive (non-tool) gestures-to-command and to imitation. They also had impaired gesture discrimination. In contrast, the subcortical group had intact gesture imitation and discrimination with mild production/executive deficits for transitive (tool) gestures. Qualitative analysis showed additional error types, including postural errors, which were distinct to the subcortical group and not observed in the cortical group. Although this study was limited to a small sample, the results do suggest that cortical and subcortical structures have different roles in praxis.
Assessment of Limb Apraxia
There are many tests of limb apraxia. In general, limb apraxia is classified by the nature of the errors made by the patient and the means by which these errors are elicited (see Table 1 for error types). In the clinical setting, limb apraxia is most commonly examined by having a patient pantomime-to-command a series of gestures (see Table 2 for examples of gestures). In general, patients are most impaired in their performance on a gestures-to-command task, improve with gesture imitation, and may show no difficulty when manipulating and using the actual tool. Because of the finding that many patients with apraxia can actually accurately use tools, some experts have advocated that limb apraxia does not have any ecological implication or real-world effect. Despite this past controversy, there have been multiple empiric studies that have shown the real-world effects of limb apraxia. For example, many patients with limb apraxia have difficulty eating a meal [31] or performing common activities of daily living (ADLs) such as brushing teeth, toileting, or bathing [49, 90]. As a result, limb apraxia can increase caregiver burden [96], and stroke patients with limb apraxia are less likely to return to work compared to patients without apraxia [88]. Limb apraxia can also have a negative impact on the quality of communicative gestures and, therefore, can confound a patient’s language disorder [11, 22, 32].
The best way to evaluate limb apraxia would include a comprehensive neurological examination and the elicitation of specific limb movements that reflect different components of the dual praxis model [28••, 84]. Tests of the praxis production system include (1) gestures-to-command, (2) gestures-to-imitation, and (3) recognition-discrimination of gestures. In addition to the means by which gestures are elicited, the exam should include representational (meaningful) and non-representational (meaningless) movements. Meaningful movements should be further sub-divided into transitive (tool actions) and intransitive (non-tool) limb movements, and non-meaningful movements should include single static hand postures and sequential limb movements [4, 27••, 28••, 84]. The Florida Apraxia Battery (FAB) is one example of a comprehensive test of the praxis production system that is relevant for research into neural mechanism based on cognitive models, but it is time-consuming and difficult to integrate into the clinical setting [76, 83,84,85]. One other assessment tool, the Naturalistic Action Test, describes in detail how to administer and score single test items included in subtests of limb apraxia [89]. This clinical tool, which also targets the praxis production system, was administered to healthy controls, and patients with left-hemispheric stroke, right-hemispheric stroke, and TBI. Unfortunately, cut-off scores were not provided, but test scores can be related to these different patient groups.
Additional tasks that evaluate the conceptual knowledge of action semantics should be included for a complete evaluation of the components of the praxis network. Specific movement errors, such as a content error, may be diagnostic of conceptual apraxia (see Table 2). A patient with conceptual apraxia may not be able to correctly select or match the correct action associated with the use of a specific tool. This tool-object action knowledge could be demonstrated by content errors, such as a patient performing an action of stirring a cup of coffee when asked to pantomime drinking from a glass [82, 84]. Alternately, conceptual apraxia could be demonstrated when a patient is unable to match a tool to the associated object (tool-object association knowledge). This deficit can be examined by giving a patient a block of wood with a partially driven nail with instructions to point to the correct tool used to complete that task from an array of related/unrelated objects. The Florida Action Recall Test (FLART) is a brief screening test of conceptual apraxia, which was developed and validated in a group of patients with Alzheimer’s disease compared to age and education-matched controls [91]. In this test, the subject is shown 45 different line drawings of actions or scenes. The subject is instructed to imagine the proper tool to apply to each pictured object or scene, and then to pantomime the target gesture. Results showed that the FLART seemed to be a sensitive measure of conceptual apraxia in early Alzheimer’s disease. This test is brief and easy to administer, although one limitation is the small sample size on which it was validated.
The type of comprehensive assessment, discussed above, is time-consuming and is usually not feasible or practical in the clinical setting. Assessment methods used clinically or reported in the literature vary widely; differences in task demands may have an impact on the interpretation of the nature of the deficits. Some empiric studies have examined gesture-to-command without gesture imitation, or vice versa. Gesture-recognition and gesture-discrimination tasks are not included in many studies of apraxia. In addition, some widely used praxis tests include a combination of limb, buccofacial, and axial movements [54, 57], making it difficult to evaluate limb praxis in isolation. There are several recent brief standardized apraxia clinical screening tests that can be used to evaluate limb apraxia, including the Cologne apraxia screening (KAS-R) [101••], the Test Battery for the Assessment of Upper Limb Apraxia (TULIA) [98], and the Apraxia Screen of TULIA (AST) [97]. Each one of these clinical tools has strengths and weaknesses. A comprehensive review of many apraxia-screening tests is provided in an excellent recent review of the scientific literature, which is highly recommended to the reader [24••]. The CAS and the TULIA do not include a test of actual tool use and can be supplemented with the Actual Tool Use Subtest, which includes seven tool tasks [20]. Each test can be administered in 10–15 min.
A critically important consideration in praxis testing relates to the limb evaluated. Patients with a hemiparesis or other disorder of elemental motor control, sensory loss, or movement are difficult to evaluate, as are patients with a severe auditory comprehension problem. Given the localization of limb apraxia, many left-hemispheric stroke patients have right-sided weakness and language problems (i.e., aphasia). In order to control for these factors, the left limb is usually examined, and patients with a profound auditory comprehension problem cannot be tested. In unilateral stroke patients, it is critical that the unaffected ipsilesional limb is used when assessing upper limb movements in the evaluation of limb apraxia. In contrast, either limb can be tested in patients with early Alzheimer’s disease, as sensorimotor systems are typically spared in this neurodegenerative disorder. In other neurodegenerative disease, like corticobasal degeneration or Parkinson’s disease, the upper limb with more preserved functions should be assessed. Similar concerns are warranted when evaluating a TBI patient for limb apraxia.
Scoring methods are not standardized. In many clinical tests of apraxia, individual gestures are scored as apraxic or normal; others may use a score of 0 (perfect) to 3 (completely degraded) to characterize the degree of movement anomalies in each gesture assessed. Although this determination is based on an analysis of movement errors, an error analysis is often not included. There are a number of systems that have been developed to examine the nature of errors in limb apraxia. It is important to identify error types that are characteristic of limb apraxia. One common error type is called a body-part-as-tool (BPT; also known as body-part-as-object) gesture (see Table 1). In one example of this error type, the patient will make their hand into a fist and pound the fist into an imaginary wall, when pantomiming how to use a hammer. In another example of a BPT error, when a patient is instructed to pantomime how to cut with scissors, the patient might make a cutting movement using the pointer and middle fingers (using the fingers as the tool) to show this intransitive non-tool gesture-to-command. Historically, Goodglass and Kaplan [41] were the first to describe body part as object (tool) errors. They found that when pantomiming to command, left-hemispheric stroke patients commonly produced BPT and suggested that this error type may be pathognomonic of limb apraxia [80]. Rothi and colleagues [82] developed a qualitative approach to analyze errors in limb apraxia based on American sign language and broadly classified errors into categories including content errors, temporal errors, and spatial errors (see Table 1).
In summary, a clear understanding of the nature of movements required to perform specific action sequences, and a careful analysis of the errors made by an individual patient, can assist the clinician in the determination of whether limb apraxia (ideomotor and/or conceptual) exists. Furthermore, a comprehensive understanding of limb movements including static postures and non-representational movement sequences can help to evaluate behavioral dissociations that may assist in the development of treatment protocols to facilitate functional independence. In a broader context, the clinician should evaluate language function, attention, visuospatial processing, and memory, as these neural systems may be intact (or impaired) in individuals with apraxia. Although apraxia is a common cognitive disorder, it is likely that limb apraxia is underdiagnosed, as there is no widely used standardized test for limb apraxia. In addition, because limb apraxia must exclude sensorimotor deficits, movement disorders, or the inability to understand the task, many patients at risk for limb apraxia cannot be adequately evaluated and treated. Many patients with limb apraxia seem to be unaware of their deficits (anosognosia for limb apraxia).
Treatment of Limb Apraxia
In the past decade, some novel approaches have been developed to treat patients with limb apraxia. The evidence is limited but encouraging because several studies have found that rehabilitation of limb apraxia improves daily living in patients with stroke [92, 93], and in corticobasal degeneration [56]. Specific interventions have included behavioral training programs consisting of gesture-production exercises [93], isolated finger movements [56], treating ipsilesional motor dexterity [94], and transcranial stimulation [8•, 9•, 75]. In contrast to these encouraging results, several case reports or small case series have found that direct treatment of limb apraxia may not generalize [39, 40, 64].
Although completely restoring normal function in the natural environment for patients with limb apraxia due to stroke or neurodegenerative disease is unlikely, treatment of limb apraxia is still extremely important and best managed within the context of multidisciplinary rehabilitation. As the world population ages, more people will be diagnosed with stroke and neurodegenerative syndromes. These individuals are at risk for limb apraxia, which is expected to increase in prevalence in the next 10 to 20 years. The negative impact of limb apraxia on daily activities is likely to increase patients’ need for home services or a supervised setting. Thus, clinical practitioners with aged patients should be familiar with basic management and treatment options.
Compensatory management of these individuals should include removing tools that could be dangerously misused (e.g., guns, power tools), replacing tool tasks with tasks performed without tools when possible (e.g., finger foods instead of utensils at meals), and educating and instructing caregivers and family members (e.g., teaching them to limit tool access, provide cues, and have patients perform familiar overlearned tasks). In contrast, treatment of limb apraxia attempts to improve the deficit itself rather than simply adapting others or the environment. Maher and Ochipa [96] and van Heughten [99] reviewed the literature on treatment of apraxia, and both concluded that direct treatment of abnormal gestures could improve performance.
Two recent articles, which are highly recommended to the reader, review current models and recent treatment studies of limb apraxia [12, 24••]. Dovern and colleagues [24••] surveyed the literature from January 1965 through April 2011 and found three studies that used a randomized controlled trials (RCTs) approach to treat limb apraxia [23, 92, 93]. In left-hemispheric stroke patients, Smania and colleagues [93] compared gesture training in seven subjects (experimental group) to conventional therapy for aphasia in six (control group). Each group had 35 training sessions of 50 min duration. This proof-of-concept study was followed by a second, larger RCT study in 45 left-hemispheric apraxic stroke patients. The experimental task included three different degrees of difficulty, starting with the easiest, and trained three different types of gestures (transitive gestures, intransitive meaningful gestures, intransitive meaningless gestures). A phased approach was used to improve gesture production and gesture imitation across tasks, followed by a period of reducing these cues to facilitate independence. Therapeutic effectiveness was evaluated by a test of real object use, a test of gesture imitation for meaningful and meaningless intransitive gestures, and a gesture-recognition test. The experimental group improved on all measures compared to the control group and showed improved ADLs. A subgroup of 17 patients in the experimental group was followed up after 2 months; a positive effect on ADLs and on apraxia testing was reported. The third RCT study used an approach called strategy training, previously developed by van Heugten [99], to teach patients strategies to help compensate for apraxic deficits in daily life [23]. The control group received standard occupational therapy (OT), whereas the experimental group received OT plus strategy training over 8 weeks. The experimental group showed improvement of ADLs compared to the control group at the end of the treatment phase, although these differences did not persist when the study patients were re-assessed at 5 months.
Conclusions
Limb apraxia is defined as a disorder of learned skilled movement affecting the upper extremities that is characterized by deficits in the performance of meaningful-representational learned skilled movements. By definition, the disorder cannot be accounted for by primary motor or sensory dysfunction, lack of comprehension, or inattention. Patients with limb apraxia have deficits in the control or programming of the spatial-temporal organization and sequencing of goal-directed movements and can have difficulty manipulating and using tools including cutting with a scissors or making a cup of coffee. Afflicted patients are often impaired in their ability to successfully perform some activities of daily living, such as eating a meal or attending to personal hygiene.
Two praxis systems have been identified including a production system (action plan and production) and a conceptual system (action knowledge). Dysfunction of the former produces ideomotor apraxia (e.g., difficulty using scissors), and dysfunction of the latter induces conceptual or ideational apraxia (e.g., difficulty making a cup of coffee). Current anatomic models of limb apraxia propose that the “visuokinesthetic engrams,” or representations of learned skilled movement, depend on the left inferior parietal lobe, and the computations that guide goal-directed movements are localized within frontal cortical regions.
It is important to evaluate patients for this debilitating disorder. Unfortunately, very few treatments have been systematically studied in large numbers of patients with limb apraxia. Clinicians, scientists, and educators must continue to work together to develop validated assessment tools and treatment programs for patients with limb apraxia and related cognitive disorders and motor deficits. This overview of limb apraxia is intended to help clinicians identify and educate patients and caregivers about this debilitating problem and to facilitate the development of better treatments that could benefit many people in the future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Agostoni E, Coletti A, Orlando G, Tredici G. Apraxia in deep cerebral lesions. J Neurol Neurosurg Psychiatry. 1983;46:804–8.
Alexander MP, Baker E, Naeser MA, Kaplan E, Palumbo C. Neuropsychological and neuroanatomical dimensions of ideomotor apraxia. Brain. 1992;115:87–107.
Ashendorf L, Swenson R, Libon DJ, The Boston process approach to neuropsychological assessment: a practitioner’s guide: Oxford University Press; 2013.
Barrett AM, Dore LS, Hansell KA, Heilman KM. Speaking while gesturing: the relationship between speech and limb praxis. Neurology. 2002;58:499–500.
Barrett AM, Foundas AL Apraxia. In: Rizzo M, P. Eslinger P, editors. Principles and Practice of Behavioral Neurology and Neuropsychology. W.B. Saunders Company, 2004.
Basso A, Luzzatti C, Spinnler H. Is ideomotor apraxia the outcome of damage to well-defined regions of the left hemisphere? Neuropsychological study of CAT correlation. J Neurol Neurosurg Psychiatry. 1980;43:118–26.
Beauchamp MS, Martin A. Grounding object concepts in perception and action: evidence from fMRI studies of tools. Cortex. 2007;43:461–8.
• Bianchi M, Cosseddu M, Cotelli M, Manenti R, Brambilla M, Rizzetti MC, et al. Left parietal cortex transcranial direct current stimulation enhances gesture processing in corticobasal syndrome. Eur J Neurol. 2015;22:1317–22 Corticobasal syndrome (CBS) is a neurodegenerative disorder with asymmetric onset of levodopa-resistant parkinsonism, dystonia, myoclonus, and limb apraxia. Transcranial direct current stimulation (tDCS), a non-invasive cortical stimulation procedure, was used to determine whether anodal tDCS over the parietal cortex (PARC) would improve ideomotor upper limb apraxia in CBS patients. Patients with a diagnosis of CBS and upper limb apraxia were studied (N = 14). Each study participant had two sessions of anodal tDCS (left and right PARC) and one session of placebo tDCS. Apraxia was assessed using the De Renzi ideomotor imitation apraxia test. Results showed a significant improvement in the apraxia test scores (post-stimulation versus pre-stimulation) after active anodal stimulation over the left PARC (χ(2) = 17.6, P = 0.0005). No significant effect was found following active anodal stimulation over the right PARC (χ(2) = 7.2, P = 0.07). Post hoc analysis showed selective improvement in the apraxia score of both right (P = 0.03) and left upper limbs (P = 0.01) after active anodal stimulation over the left PARC compared with placebo stimulation. These results suggest that tDCS to the PARC may represent a promising treatment tool to improve functional outcomes in people with a diagnosis of CBS.
• Bolognini N, Convento S, Banco E, Mattioli F, Tesio L, Vallar G, et al. Improving ideomotor limb apraxia by electrical stimulation of the left posterior parietal cortex. Brain. 2015;138:428–39 The authors hypothesized that electrical stimulation of the left posterior parietal cortex would improve limb apraxia. Anodal transcranial direct current stimulation (tDCS) was delivered to the left posterior parietal cortex (PPC), the right motor cortex (M1), and in a sham stimulation condition in a group of left-hemispheric stroke patients (n = 6) compared to healthy controls (n = 6). Subjects were instructed to imitate hand gestures and to perform skilled hand movements with the left hand. Results showed that tDCS to the left PPC reduced the time required to perform skilled movements, and planning, but not execution, times in imitating gestures, in both patients and controls. In patients, the planning time decrease by left PPC tDCS was influenced by lesion size, with a larger parietal lesion associated with a smaller improvement. Left PPC stimulation also ameliorated accuracy in imitating hand gestures in the patient group. In contrast, tDCS to the right M1 diminished execution, but not planning, times in both groups. These results suggest that tDCS may temporarily improve limb apraxia in the left hand of left-brain-damaged patients. Future studies to examine the use of tDCS in the rehabilitation of limb apraxia are warranted.
Borghi AM. Action language comprehension, affordances and goals. In: Coello Y, Bartolo A, editors. Language and Action in Cognitive Neuroscience. Contemporary Topics in Cognitive Neuroscience Series. London; New York, NY: Psychology Press; 2012. p. 125–44.
Borod JC, Fitzpatrick PM, Helm-Estabrooks N, Goodglass H. The relationship between limb apraxia and the spontaneous use of communicative gesture in aphasia. Brain Cogn. 1989;10:121–31.
Buxbaum LJ, Haaland KY, Hallett M, Wheaton L, Heilman KM, Rodriguez A, et al. Treatment of limb apraxia: moving forward to improved action. Am J Phys Med Rehabil. 2008;87:149–61.
•• Buxbaum LJ, Randerath J. Limb apraxia and the left parietal lobe. Handb Clin Neurol. 2018;151:349–63 The authors provide a comprehensive review of the nature of limb apraxia and discuss the network of regions in the left temporal, parietal, and frontal lobes most commonly implicated in stroke patients with this syndrome. In the past few years, there have been advances in understanding the functional neuroanatomy and connectivity of the left-hemisphere “tool use network,” and the patterns of performance that emerge from different lesion locations. This chapter focuses on the left inferior parietal lobe, and its role in tool and body representation, action prediction, and action selection. The authors discuss how these functions relate to the deficits seen in patients with limb apraxia following left parietal stroke. The chapter also provides suggestions for future research directions to facilitate rehabilitation protocols to treat this debilitating disorder.
•• Buxbaum LJ, Shapiro AD, Coslett HB. Critical brain regions for tool related and imitative actions: a componential analysis. Brain. 2014;137:1971–85 Functional neuroimaging studies have shown that bilateral parietal, temporal, and frontal regions are involved in tool-related and pantomimed gesture performance. The potential role of these regions in specific aspects of gestural tasks remains unclear. This study was designed as a prospective study of limb apraxia-related lesions to assess the critical neural substrates of gestures produced in response to viewed tools, imitation of tool-specific gestures demonstrated by the examiner, and imitation of meaningless gestures. To achieve this goal, voxel-based lesion-symptom mapping on data from 71 left hemisphere stroke patients was examined in the context of these two tool-related tasks, and two imitation tasks, enabling pairwise comparisons to highlight commonalities and differences. Lesioned voxels in the left posterior temporal gyrus were significantly associated with lower scores on the posture component for both tool-related gesture tasks. Degraded performance on the kinematic component of all three-gesture tasks was significantly associated with left inferior parietal and frontal lesions. Based on these data, the authors propose a componential neuroanatomical model of action that delineates specific components required for different gestural action tasks. These distinctions advance traditional accounts of apraxia.
• Cimino-Knight AM, Gonzalez Rothi LJ, He Y, Heilman KM. Callosal ideomotor apraxia in Alzheimer’s disease. J Clin Exp Neuropsychol. 2017;39:1–8 Patients with corpus callosum injury have difficulty performing skilled movements with the left upper limb. This is called callosal apraxia and can be demonstrated by spatial-temporal errors primarily in response to verbal commands (verbal callosal disconnection apraxia), with imitation, and when using actual tools (callosal ideomotor apraxia). Some patients with callosal injury may make content errors when selecting and using the incorrect tool with their left upper limb. This type of deficit has been called callosal conceptual apraxia. Patients with Alzheimer’s disease (AD) have anatomic evidence of callosal degeneration, but callosal apraxia in AD has not been previously described. This study was designed to learn whether patients with AD display different forms of callosal apraxia. Study participants included right-handed patients with AD (n = 22) and matched controls (n = 24). Both upper limbs were tested by examining transitive limb movements to command and to imitation. Participants also viewed pictures of an incomplete task and were instructed to pantomime the action needed to complete the task. The AD group had performance deficits consistent with ideomotor and conceptual apraxia of both upper limbs compared to controls. Errors were more pronounced in ideomotor apraxia of the left compared to the right hand, suggesting a hemispheric disconnection.
Clark MA, Merians AS, Kothari A, Poizner H, Macauley B, Rothi LJ, et al. Spatial planning deficits in limb apraxia. Brain. 1994;117:1093–106.
Cubelli R, Marchetti C, Boscolo G, Della Sala S. Cognition in action: testing a model of limb apraxia. Brain Cogn. 2000;44:144–65.
De Renzi E. Apraxia. In: Boller F, Grafman J, editors. Handbook of clinical neurology. Amsterdam: Elsevier; 1990.
De Renzi E, Faglioni P, Lodesani M, Vecchi A. Performance of left brain-damaged patients on imitation of single movements and motor sequences. Frontal and parietal-injured patients compared. Cortex. 1983;19:333–43.
De Renzi E. Methods of limb apraxia examination and their bearing on the interpretation of the disorder. In: Roy EA, editor. Neuropsychological studies of apraxia and related disorders. Amsterdam: North Holland; 1985. p. 45–64.
De Renzi E, Lucchelli F. Ideational apraxia. Brain. 1988;111:1173–85.
Dee HL, Benton AL, Van Allen MW. Apraxia in relation to hemispheric locus of lesion and aphasia. Trans Am Neurol Assoc. 1970;95:147–50.
Donkervoort M, Dekker J, Stehmann-Saris JC, Deelman BG. Efficacy of strategy training in left hemisphere stroke patients with apraxia: a randomized clinical trial. Neuropsychol Rehabil. 2001;11:549–66.
•• Dovern A, Fink GR, Weiss PH. Diagnosis and treatment of upper limb apraxia. J Neurol. 2012;259:1269–83 There were two main goals of this review article. First, the authors provide the clinician with the tools needed to reliably diagnose limb apraxia. This goal was achieved by an analysis of all of the tests developed to diagnose upper limb apraxia in the past 20 years, followed by a systematic analysis of strengths and weaknesses of validated screening tests. The second goal was to review and discuss the few randomized controlled studies that have investigated the efficacy of different apraxia treatments. In addition, this review article provides a comprehensive update on issues related to the ecological or real-world implications of limb apraxia.
•• Evans C, Edwards MG, Taylor LJ, Ietswaart M. Impaired communication between the dorsal and ventral stream: indications from apraxia. Front Hum Neurosci. 2016;1(10):8 This study examined grasping performance in 27 right-handers including left-hemispheric stroke patients with (n = 3) and without (n = 9) limb apraxia, and healthy controls (n = 14). The hypothesis was that disrupted communication between dorsal and ventral pathways leads to limb apraxia because of the patient’s difficulty with successfully utilizing these stored representations. The authors found that the patients with limb apraxia had more difficulty with utilizing memory-associated or visual-spatial cued information compared to the other groups. Individual differences were seen in the patients with limb apraxia; lesion location may explain some of these behavioral differences. These results suggest that disruption to the ventro-dorsal pathways impairs use of familiar objects (i.e., tools) and can lead to difficulty in manipulating new or novel objects.
• Evans C, Edwards MG, Taylor LJ, Ietswaart M. Perceptual decisions regarding object manipulation are selectively impaired in apraxia or when tDCS is applied over the left IPL. Neuropsychologia. 2016;86:153–66 This study evaluated whether apraxia can be understood as due to impaired motor representations or motor imagery necessary for appropriate object-use, imitation, and pantomime. The causal role of the left inferior parietal lobe (IPL) was evaluated by using transcranial direct current stimulation (tDCS). Using a task assessing object-use perception, unilateral stroke patients with apraxia had a selective deficit during perceptual decisions reliant on the integration of visible and known object properties to select the appropriate grasp for object use. Apraxia severity was related to the severity of this deficit. Converging evidence was found using a modified version of the same task that used tDCS to target the left IPL in healthy participants. Inhibitory stimulation over the left IPL reduced performance during perceptual decisions regarding object manipulation; performance was unaffected during functional semantic decisions. Excitatory stimulation of the left IPL did not affect performance in either task. These results suggest that the left inferior parietal lobe is critical for motor imagery, and apraxia may be due in part to the inability to use internal motor representations of object manipulation. The authors propose an additional ventro-dorsal sub-stream within the dorsal and ventral visual pathways model to explain these results.
•• Foundas AL. Apraxia: neural mechanisms and functional recovery. Handb Clin Neurol. 2013;110:335–45 This chapter provides an update on limb apraxia with reference to the two praxis systems including a production system (action plan and production) and a conceptual system (action knowledge). Dysfunction of the former produces ideomotor apraxia (e.g., difficulty using a scissors), and dysfunction of the latter induces ideational apraxia (e.g., difficulty making a cup of coffee). Neural mechanisms, including how to evaluate apraxia, are presented in the context of these two praxis systems. Information about these praxis systems, including the nature of the disordered limb movement, was reviewed in the context of improving diagnosis and treatment of this debilitating cognitive-motor disorder. Unfortunately, very few treatments have been systematically studied in large numbers of patients with limb apraxia. These treatment studies are reviewed in order to help rehabilitation clinicians educate patients and caregivers about this debilitating problem, and to facilitate the development of better treatments that could benefit many people in the future.
•• Foundas AL. Limb Apraxia: A disorder of goal directed actions. In: Chatterjee A, Coslett HB, editors. The Roots of Cognitive Neuroscience: Behavioral Neurology and Neuropsychology. Oxford University Press, 2014 Apraxia is a disturbance in goal-directed behavior defined as a cognitive-motor disorder specific to learned skilled movements (e.g., tool use). The disordered movement can manifest as the imprecise orientation (spatial disturbance) of the object (e.g., toothbrush not oriented correctly to the teeth) or as the incorrect sequencing (temporal disturbance) of an action at a proximal rather than a distal limb joint (e.g., rotating at the shoulder rather than moving at the wrist to brush your teeth). This chapter reviewed several cognitive-process models of limb apraxia including Liepmann’s early model, Geschwind’s disconnection model, De Renzi’s multi-modal model, and the Heilman-Rothi dual-component model of limb apraxia. Lesion localization studies and converging evidence from functional imaging studies are reviewed in order to present an updated anatomical model of limb praxis with an emphasis on the praxis production system.
Foundas AL, Daniels SK, Maher LM, Raymer A, Rothi LJG, Heilman KM. Lesion localization in ideomotor limb apraxia: a relationship to praxis. Neurology. 2002 58:499–500
Foundas AL, Henchey R, Gilmore RL, Fennell EB, Heilman KM. Apraxia during Wada testing. Neurology. 1995a;45:1379–83.
Foundas AL, Macauley BL, Raymer MA, Maher LM, Heilman KM, Gonzalez Rothi LJ. Ecological implications of limb apraxia: evidence from mealtime behavior. J Int Neuropsychol Soc. 1995b;1:62–6.
Foundas AL, Macauley BL, Raymer AM, Maher LM, Heilman KM, Rothi LJ. Gesture laterality in aphasic and apraxic stroke patients. Brain Cogn. 1995c;29:204–13.
Freund HJ. The parietal lobe as a sensorimotor interface: a perspective from clinical and neuroimaging data. Neuroimage. 2001;14:S142–6.
Freund HJ, Hummelsheim H. Lesions of premotor cortex in man. Brain. 1985;108:697–733.
Frey SH. What puts the how in where? Tool use and the divided visual streams hypothesis. Cortex. 2007 Apr;43(3):368–75.
Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:237–94.
Geschwind N, Kaplan E. A human cerebral deconnection syndrome. A preliminary report. Neurology. 1962;12:675–85.
Goldenberg G. Defective imitation of gestures in patients with damage in the left or right hemispheres. J Neurol Neurosurg Psychiatry. 1996;61:176–80.
Goldenberg G, Daumuller M, Hagmann S. Assessment and therapy of complex activities of daily living in apraxia. Neuropsychol Rehabil. 2001;11:147–69.
Goldenberg G, Hagmann S. Therapy of activities of daily living in patients with apraxia. Neuropsychol Rehabil. 1998;8:123–41.
Goodglass H, Kaplan E. Disturbance of gesture and pantomime in aphasia. Brain. 1963;86:703–20.
Haaland KY. Left hemisphere dominance for movement. Clin Neuropsychol. 2006;20:609–22.
Haaland KY, Flaherty D. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn. 1984;3:370–84.
Haaland KY, Harrington DL. Hemispheric asymmetry of movement. Curr Opin Neurobiol. 1996;6:796–800.
Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123:2306–13.
Hamilton AF, Grafton ST. Repetition suppression for performed hand gestures revealed by fMRI. Hum Brain Mapp. 2009;30:2898–906.
Hanna-Pladdy B, Daniels SK, Fieselman MA, Thompson K, Vasterling JJ, Heilman KM, et al. Praxis lateralization: errors in right and left hemisphere stroke. Cortex. 2001;37:219–30.
Hanna-Pladdy B, Heilman KM, Foundas AL. Cortical and subcortical contributions to ideomotor apraxia: analysis of task demands and error types. Brain. 2001;124:2513–27.
Hanna-Pladdy B, Heilman KM, Foundas AL. Ecological implications of ideomotor apraxia: evidence from physical activities of daily living. Neurology. 2003;60:487–90.
Harrington DL, Haaland KY. Representations of actions in ideomotor limb apraxia: clues from motor programming and control. In: Rothi LJ, Heilman KM, editors. Apraxia: The Neuropsychology of Action. East Sussex: Psychology Press; 1997. p. 111–47.
Heilman KM, Gonzalez Rothi LJ. Apraxia. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. 4th ed. Oxford: Oxford University Press; 2003. p. 215–35.
Heilman KM, Maher LM, Greenwald ML, Rothi LJ. Conceptual apraxia from lateralized lesions. Neurology. 1997;49:457–64.
Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32:342–6.
Helm-Estabrooks N. Test of oral and limb apraxia (TOLA). Austin: PRO-ED; 1998.
Kable JW, Chatterjee A. Specificity of action representations in the lateral occipitotemporal cortex. J Cogn Neurosci. 2006;18:1498–517.
Kawahira K, Noma T, Iiyama J, Etoh S, Ogata A, Shimodozono M. Improvements in limb kinetic apraxia by repetition of a newly designed facilitation exercise in a patient with corticobasal degeneration. Int J Rehabil Res. 2009;32:178–83.
Kertesz A. The aphasia quotient: the taxonomic approach to measurement of aphasic disability. Can J Neurol Sci. 2004 31:175–84.
Kertesz A, Ferro JM. Lesion size and location in ideomotor apraxia. Brain. 1984;107:921–33.
Kertesz A, Hooper P. Praxis and language: the extent and variety of apraxia in aphasia. Neuropsychologia. 1982;20:275–86.
Kroliczak G, Frey SH. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb Cortex. 2009;19:2396–410.
• Króliczak G, Piper BJ, Frey SH. Specialization of the left supramarginal gyrus for hand-independent praxis representation is not related to hand dominance. Neuropsychologia. 2016;93:501–12 There is considerable evidence that the left hemisphere has a dominant role for language and manual gestures, although the pattern of laterality may be more variable in left-handers compared to right-handers. That is, more left than right-handers have atypical right-hemispheric dominance for language. The authors postulated that if this dependence is linked to a common cerebral organization, then left-handers with atypical language organization would show a similar pattern of organization for manual gestures. To test this hypothesis, the authors used a functional MRI paradigm to evaluate laterality. Consistent with the a priori hypothesis, fMRI data showed that left-handers (5/15) with bilateral, or right-lateralized, language representations in the inferior frontal cortex exhibited a similar atypical pattern in the representations of familiar gestures in the inferior parietal cortex.
Leiguarda R. Limb apraxia: cortical or subcortical. Neuroimage. 2001;14:S137–41.
Liepmann H. Apraxie. Ergebn Ges Med 1920;1:516–43.
Maher LM, Ochipa C. Management and treatment of limb apraxia. In: Rothi LJG, Heilman KM, editors. Apraxia: the neuropsychology of action. Hove, East Sussex: Psychology Press; 1997.
Meador KJ, Loring DW, Lee K, Hughes M, Lee G, Nichols M, et al. Cerebral lateralization: relationship of language and ideomotor praxis. Neurology. 1999;53:2028–31.
• Mutha PK, Stapp LH, Sainburg RL, Haaland KY. Motor adaptation deficits in ideomotor apraxia. J Int Neuropsychol Soc. 2017;23:139–49 This study was designed to examine motor learning in limb apraxia. The hypothesis was that deficits in patients with limb apraxia might arise from degraded internal representations of action plans. This deficit would be associated with an impaired ability to develop new representations through motor learning. Three groups were studied including healthy controls (n = 13) and unilateral stroke patients with (n = 12) and without (n = 11) limb apraxia. The paradigm required subjects to adapt movements in response to changing targets (i.e., using a cursor to reach from a central start position to one of eight targets). Baseline performance was compared to an Adaptation block, followed by an after-effect block identical to baseline. Results showed that the apraxic patients did develop an after-effect similar to the other groups; however, this initial benefit was not retained suggesting a disrupted slow learning process.
• Nobusako S, Ishibashi R, Takamura Y, Oda E, Tanigashira Y, Kouno M, et al. Distortion of visuo-motor temporal integration in apraxia: evidence from delayed visual feedback detection tasks and voxel-based lesion-symptom mapping. Front Neurol. 2018;9:709 It is unclear whether there is a specific deficit in the temporal integration of visuo-motor compared to visuo-tactile and visuo-proprioceptive integration in left-hemispheric stroke patients with limb apraxia. A delayed visual feedback detection task with three different conditions (tactile, passive movement, and active movement) was used to examine these different functions. The behavioral studies showed that the delay detection threshold was extended, and the probability curve for delay detection was less steep in the apraxic patients compared to controls (pseudo-apraxic patients and unaffected patients), but only for the active movement condition. Apraxia severity was correlated with the delay detection threshold and the steepness of the probability curve in the active movement condition. These results showed that apraxic patients had difficulties with visuo-motor temporal integration. Lesion analyses revealed that both apraxia and the distortion of visuo-motor temporal integration were associated with lesions to the left inferior parietal and left inferior frontal regions. Based on these data, the authors proposed that damage to the left inferior fronto-parietal network causes deficits in motor prediction for visuo-motor temporal integration, but not for sensory-sensory (visuo-tactile and visuo-proprioception) temporal integration.
Ochipa C, Rapcsak SZ, Maher LM, Rothi LJ, Bowers D, Heilman KM. Selective deficit of praxis imagery in ideomotor apraxia. Neurology. 1997;49:474–80.
Ochipa C, Rothi LJ, Heilman KM. Ideational apraxia: a deficit in tool selection and use. Ann Neurol. 1989;25:190–3.
Ochipa C, Rothi LJ, Heilman KM. Conceptual apraxia in Alzheimer’s disease. Brain. 1992;115:1061–71.
•• Ortiz KZ, Mantovani-Nagaoka J. Limb apraxia in aphasic patients. Arq Neuropsiquiatr. 2017;75:767–72 This study was designed to evaluate the occurrence of limb apraxia in aphasic left-hemispheric stroke patients and to analyze the nature of these deficits. Two groups were evaluated including: aphasic patients (n = 28) and a group of age and education matched controls (n = 44). Limb apraxia subtests included tasks that evaluated lexical-semantic aspects related to the comprehension/production of gestures and to motor movements. Results showed that the aphasic patients were most impaired on tasks related to the conceptual aspects of gestures. The difficulty with imitation of dynamic gestures suggested that these aphasic patients also had difficulty with gesture planning. These results reinforce the importance of conducting a comprehensive assessment of limb apraxia based on a cognitive process model. In addition, the results support the notion that difficulties with pantomiming gestures to command (content errors) may be a good predictor of disorders at the level of semantics.
Papagno C, Della Sala S, Basso A. Ideomotor apraxia without aphasia and aphasia without apraxia: the anatomical support for a double dissociation. J Neurol Neurosurg Psychiatry. 1993;56:286–9.
• Pellicano A, Borghi AM, Binkofski F. Editorial: bridging the theories of affordances and limb apraxia. Front Hum Neurosci. 2017;11:148. https://doi.org/10.3389/fnhum.2017.00148 This editorial discusses the potential use of theories of affordances to understand the mechanisms of limb apraxia. Affordances integrate perceptual and cognitive variables into the action plan. Limb apraxia is a disorder of learned skilled movements that can lead to disruption of tool gestures, imitation of tool use, and can disrupt the actual manipulation of tools. This article discusses how these issues relate to mechanisms underlying affordances and implications for limb apraxia.
Petreska B, Adriani M, Blanke O, Billard AG. Apraxia: a review. Prog Brain Res. 2007;164:61–83.
Pomeroy VM. Stroke rehabilitation. Disabil Rehabil. 2006;28(13–14):813–4.
Power E, Code C, Croot K, Sheard C, & Gonzalez Rothi LJ. Florida Apraxia Battery-Extended and Revised Sydney (FABERS): Design, description, and a healthy control sample. J Clin Exp Neuropsychol 2009, 32:1–19.
Pramstaller PP, Marsden CD. The basal ganglia and apraxia. Brain. 1996;119:319–40.
Randerath J, Li Y, Goldenberg G, Hermsdorfer J. Grasping tools: effects of task and apraxia. Neuropsychologia. 2009;47:497–505.
Rapcsak SZ, Ochipa C, Beeson PM, Rubens AB. Praxis and the right hemisphere. Brain Cogn. 1993;23:181–202.
Raymer AM, Maher LM, Foundas AL, Heilman KM, Rothi LJ. The significance of body part as tool errors in limb apraxia. Brain Cogn. 1997;34:287–92.
Rothi LJ, Heilman KM, Watson RT. Pantomime comprehension and ideomotor apraxia. J Neurol Neurosurg Psychiatry. 1985;48:207–10.
Rothi LJ, Mack L, Verfaellie M, Brown P, Heilman KM. Ideomotor apraxia: error pattern analysis. Aphasiology. 1988;2:381–8.
Rothi LJ, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis. Cogn Neuropsychol. 1991;8:443–58.
Rothi LJ, Raymer AM, Heilman KM. Limb praxis assessment. In: Rothi LJ, Heilman KM, editors. Apraxia: The Neuropsychology of Action. East Sussex: Psychology Press; 1997. p. 61–73.
Rothi LJ, Raymer AM, Ochipa C, Maher, LM, Greenwald ML, & Heilman KM Florida apraxia battery, experimental edition, 1992.
Roy EA. Neuropsychological studies of apraxia and related disorders. New York: Elsevier Science; 1985.
Rushworth MF, Nixon PD, Wade DT, Renowden S, Passingham RE. The left hemisphere and the selection of learned actions. Neuropsychologia. 1998;36:11–24.
Saeki S, Ogata H, Okubo T, Takahashi K, Hoshuyama T. Return to work after stroke. Stroke. 1995;26:399–401.
Schwartz MF, Buxbaum LJ. Naturalistic action. In: Rothi LJ, Heilman KM, editors. Apraxia: the neuropsychology of action. East Sussex: Psychology Press; 1997. p. 269–89.
Schwartz MF, Segal M, Veramonti T, Ferraro M, Buxbaum LJ. The naturalistic action test: a standardised assessment for everyday action impairment. Neuropsychol Rehabil. 2002;12:311–39.
Schwartz RL, Adair JC, Raymer AM, Williamson DJ, Crosson B, Rothi LJ, et al. Conceptual apraxia in probable Alzheimer’s disease as demonstrated by the Florida action recall test. J Int Neuropsychol Soc. 2000;6:265–70.
Smania N, Aglioti SM, Girardi F, Tinazzi M, Fiaschi A, Cosentino A, et al. Rehabilitation of limb apraxia improves daily life activities in patients with stroke. Neurology. 2006;67:2050–2.
Smania N, Girardi F, Domenicali C, Lora E, Aglioti S. The rehabilitation of limb apraxia: a study in left-brain-damaged patients. Arch Phys Med Rehabil. 2000;81:379–88.
Sunderland A. Recovery of ipsilateral dexterity after stroke. Stroke. 2000;31:430–3.
Soulsby WD, El-Ruwie N, Gatla S, Anderson Zimmer K, Najmi S, Chen A, et al. Ideomotor limb apraxia as a staging tool in individuals with Alzheimer’s disease (ILIAD). Ann Clin Psychiatry. 2016;28:255–62.
Sundet K, Finset A, Reinvang I. Neuropsychological predictors in stroke rehabilitation. J Clin Exp Neuropsychol. 1988;10(4):363–79.
Vanbellingen T, Kersten B, Van de Winckel A, Bellion M, Baronti F, Müri R, et al. A new bedside test of gestures in stroke: the apraxia screen of TULIA (AST). J Neurol Neurosurg Psychiatry. 2011;82:389–92.
Vanbellingen T, Kersten B, Van Hemelrijk B, Van de Winckel A, Bertschi M, Muri R, et al. Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). Eur J Neurol. 2009;17:59–66.
van Heugten CM. Apraxia. In: Eslinger PJ, editor. Neuropsychological interventions: clinical research and practice. New York: Guilford; 2002.
Watson RT, Fleet WS, Gonzalez-Rothi L, Heilman KM. Apraxia and the supplementary motor area. Arch Neurol. 1986;43(8):787–92.
•• Wirth K, Held A, Kalbe E, Kessler J, Saliger J, Karbe H, et al. A new diagnostic tool for apraxia in patients with right-hemisphere stroke: the revised Cologne apraxia screening (KAS-R). Fortschr Neurol Psychiatr. 2016;84(10):633–9 Limb apraxia is more commonly seen in patients with left-hemispheric (LH) stroke (up to 63%) compared to patients with a right-hemispheric (RH) stroke (up to 53%). The Cologne apraxia screening (KAS) was developed to diagnose apraxia following LH stroke. The goal of the current study was to modify the KAS for use in patients with RH stroke (KAS-R). The study sample included: RH stroke patients (n = 100), and healthy, matched controls (n = 77). Psychometric analyses led to the exclusion of eight items from the KAS for a final KAS-R, consisting of 12 items. The KAS-R was found to have good internal consistency (α = 0.795), and high sensitivity (79.4%) and specificity (84.4%). Using a cut-off value of ≤ 46 (out of 48) points, 39 of 100 RH stroke patients were diagnosed with limb apraxia. Significant correlations were found between the KAS-R, an imitation test, and expert ratings, indicating high construct validity. These results showed that the KAS-R is a reliable and valid brief diagnostic screening tool for apraxic deficits in both RH and LH stroke patients.
Zwinkels A, Geusgens C, van de Sande P, van Heugten C. Assessment of apraxia: inter-rater reliability of a new apraxia test, association of apraxia and other cognitive deficits and prevalence of apraxia in a rehabilitation setting. Clin Rehabil. 2004;18:819–27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Behavior
Rights and permissions
About this article
Cite this article
Foundas, A.L., Duncan, E.S. Limb Apraxia: a Disorder of Learned Skilled Movement. Curr Neurol Neurosci Rep 19, 82 (2019). https://doi.org/10.1007/s11910-019-0989-9
Published:
DOI: https://doi.org/10.1007/s11910-019-0989-9