Abstract
Purpose of Review
To provide an overview of the molecular pathways and recent genetic risk loci associated with restless legs syndrome/Willis-Ekbom disease (RLS/WED) and describe the most recent treatment guidelines.
Recent Findings
Diagnostic criteria for RLS/WED now include a fifth criterion to differentiate from RLS/WED mimics. Our understanding of disease pathophysiology has improved, specifically regarding iron regulation in the brain and the role of other pathways such as opioid signaling and brain and spinal cord circuitry may play. Finally, several genetic risk loci have been described, including MEIS1 which is currently considered to be the strongest genetic risk factor for RLS/WED. Treatment guidelines now suggest α2δ ligands such as gabapentin enacarbil should be used as first-line treatment.
Summary
The current literature focuses on disease pathways as well as the development of animal models based on genetic risk factors for RLS/WED. Updated treatment guidelines expand on first-line treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restless legs syndrome (RLS) is a common movement disorder of wake and sleep. This condition was first characterized by Sir Thomas Willis, who described a patient with difficulty sleeping due to limb discomfort. Karl Axel Ekbom coined the term “restless legs syndrome” and described all the clinical symptoms we now associate with RLS [1]. Throughout the years, there has been some controversy as to the correct terminology to use when referring to this disease. The term RLS is widely recognized not only by the medical community but also in popular media, which has unfortunately led to trivialization of the disease. There is a growing movement to adopt the term Willis-Ekbom disease (WED), which would eliminate incorrect descriptors and acknowledge the historical aspects of this disease. The International Restless Legs Syndrome Study Group suggests the amalgamated form (RLS/WED), which we will use in the current review.
In recent years, several studies have been published focusing on the different pathways that influence this disease. Genome-wide association studies and animal models have also contributed to this understanding and better characterization of susceptible population subsets. Herein, we review the epidemiology and clinical characteristics of RLS/WED, recent studies pertaining to RLS/WED pathophysiology, and provide an overview of the current therapeutic choices, highlighting recent and ongoing clinical trials.
Diagnostic Criteria
RLS/WED diagnosis is based on clinical assessment. The key diagnostic feature is an irresistible urge to move the legs, usually—but not always—in response to an unpleasant sensation. Unpleasant sensations without the urge to move are not sufficient for diagnosis. This urge begins or worsens during periods of inactivity and is at least partially relieved by movement. It is important to note that individuals with severe RLS/WED may not experience any symptom relief with movement but typically had at least some relief in the past, when their symptoms were milder. Interestingly, it is the degree of alertness produced by movement, rather than the intensity of movement, that seems to correlate with symptom reduction [2]. Finally, the urge to move the legs, at least initially, only occurs or worsens at night. In 1995, the International Restless Legs Study Group (IRLSSG) first proposed a set of formal clinical criteria which were revised in 2014, adding a fifth criterion to specify the need to exclude RLS/WED mimics (Box 1) [3]. This consensus group also introduced specifiers for the clinical course and clinical significance of RLS/WED [2].
Box 1. International Restless Legs Syndrome Study Group Consensus Criteria
(Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria - history, rationale, description, and significance. Sleep Med 2014;15:860–73, with permission from Elsevier)[2]
Patient must meet all of the following: | |
1. An urge to move the legs usually but not always accompanied by, or felt to be caused by, uncomfortable and unpleasant sensations in the legs. | |
2. The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity such as lying down or sitting. | |
3. The urge to move the legs and any accompanying unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues. | |
4. The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are worse in the evening or night than during the day. | |
5. The occurrence of the above features is not solely accounted for as symptoms primary to another medical or a behavioral condition (e.g. myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping). | |
Clinical course of RLS/WED: | |
•Chronic-persistent RLS/WED: symptoms when not treated would occur on average at least twice weekly for the past year. | |
•Intermittent RLS/WED: symptoms when not treated would occur on average less than twice/week for the past year, with at least five lifetime events. |
Ancillary testing plays a secondary role in the evaluation of RLS/WED. Polysomnography is not clinically useful to evaluate for sensory symptoms that happen during wakefulness. However, polysomnography can quantify periodic limb movements of sleep (PLMS), which may serve as an indirect index of RLS/WED severity. PLMS are characterized by extension of the toe with partial flexion of ankle, knee, and hip, typically involving both legs. PLMS occur in about 90% of patients with RLS/WED but are not part of the essential criteria [4, 5] as they can occur with other medical conditions or secondary to several drugs [6, 7]. Periodic limb movements occurring during wakefulness have a higher sensitivity for RLS/WED. These movements can be measured with the multiple suggested immobilization test (m-SIT). The m-SIT has been validated to evaluate the severity of motor and subjective symptoms of RLS/WED while the patient is awake [8].
Clinical severity rating scales have been validated and are routinely used in clinical trials and studies. The International RLS Study Group Rating Scale (IRLS) is currently considered the gold standard for symptom assessment [9]. Nonetheless, it relies on subjective assessment and patient recall and has a strong response to placebo effect [10]. The Johns Hopkins Restless Legs Severity Scale determines the severity of RLS/WED according to time of day of symptom onset and has been well validated. Given this is a three-point scale, usefulness is limited for making fine distinctions in disease severity [11]. Finally, the recently validated Restless Legs Syndrome-6 Scale (RLS-6) is the only scale to specifically rate daytime versus nighttime symptoms [12]. Overall, each scale provides different aspects of RLS/WED severity and has important limitations. A more global scale would be useful to provide a better detailed scoring of disease severity and aid in treatment decisions.
Epidemiological Data
In the adult general population, a prevalence between 5 and 15% has been reported, with most studies conducted in Caucasian populations. Asia and South America have a lower prevalence, with estimates of 1.6–2.0% [13,14,15,16]. Women over age 35 years are twice as likely to have the disease compared with men; this difference is not present in adults younger than 35 years [17]. The mean age of onset is during third to fourth decade. RLS/WED has also been described in children, with a prevalence of 2% [18]. Several conditions increase risk of RLS/WED including iron deficiency anemia [19], migraine [20], kidney disease—particularly those patients undergoing hemodialysis [21]—and Parkinson’s disease patients receiving treatment with dopaminergic agents [22]. A recent study showed that multimorbidity was a strong risk factor for RLS/WED, indicating that cumulative disease burden is a stronger risk factor for RLS/WED than the presence of any specific single disease [23].
Prevalence also increases during pregnancy to about 21%; post-delivery, prevalence rapidly decreases to match that of the general population [24•]. Iron deficiency and elevated estrogen and progesterone levels play an important role in driving this increase in prevalence. One of the main complications of RLS/WED in pregnancy is decreased sleep quality, which can lead to prolonged labor and a higher chance of having a cesarean delivery [25]. RLS/WED also increases the risk of pre-eclampsia and mood disorders [26, 27].
RLS/WED may be a risk factor for cardiovascular mortality. Data from the Nurse’s Health Study showed a significantly higher risk of cardiovascular mortality in women with RLS/WED (adjusted HR 1.43, 95%CI 1.02–2.00). No association was found with all-cause mortality [28]. There are currently no data regarding the association between RLS/WED severity and cardiovascular risk factors. A greater understanding of the pathways involved in RLS/WED may help explain this association.
Pathophysiology
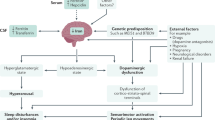
Recent efforts have provided new insights into the pathophysiology of RLS/WED. Involvement of abnormal iron metabolism, multiple neurotransmitters, and the central opiate system have been described (Fig. 1). More recently, neurophysiology studies have described changes in different sensorimotor networks. Finally, with the emergence of new genetic analysis techniques, several genetic variants have been described to be associated with an increased risk of RLS/WED, most of them involved with neural development pathways and iron metabolism.
A growing body of evidence has linked peripheral and brain iron deficiency (BID) and RLS/WED. For a review of peripheral and brain iron metabolism, see [29]. People with iron deficiency anemia are at 5–6 times increased risk of RLS/WED than the general population [19]. Among those with iron deficiency anemia, peripheral iron and hemoglobin levels are similar between those with and without RLS/WED, indicating the presence of some—possibly genetic—predisposing factors [19]. Yet, many people with RLS/WED have normal serum ferritin [30,31,32]. Furthermore, cerebrospinal fluid (CSF) and serum ferritin levels are not strongly correlated, though ferritin levels < 50 ng/mL appear to produce very low CSF ferritin. Because BID—even in the absence of peripheral iron deficiency—ultimately confers the risk of RLS/WED, elucidating the mechanisms of BID with or without peripheral iron deficiency, including iron transport to the brain and brain iron storage and mobilization, will be critical to understanding RLS/WED.
Multiple lines of evidence support BID in RLS/WED. Imaging studies have shown decreased iron concentration in the substantia nigra, red nucleus, thalamus, and striatum [33]. People with RLS/WED have lower CSF ferritin and higher CSF transferrin levels, consistent with an iron-deficient profile, and CSF ferritin levels correlate with the age of onset of RLS/WED [34, 35]. Brain iron mobilization may also be affected. Hepcidin, a protein that regulates iron mobilization in response to iron demand [36, 37], may be decreased in CSF of RLS/WED patients [38••]. Although autopsy studies have shown increased iron levels in CSF, this is not the case for choroid plexus cells or brain parenchyma [39]. These data suggest that brain iron deficiency is due to a problem with brain iron acquisition, mobilization, or storage. In support of this, studies have shown decreased in H-ferritin in substantia nigra, choroid plexus, and microvasculature. H-ferritin, which is highly expressed in neurons, is involved in iron transport; decreases in H-ferritin suggest decreased iron availability to neurons [35].
A formal model of iron transport in the brain needs to be validated. According to the current model, iron enters the brain through microvascular endothelial cells (BMVECs) in the blood-brain barrier or through the choroid plexus and blood-CSF barrier [29]. BMVECs express transferrin receptor 1 (TfR1), which facilitates iron uptake through endocytosis. Iron is reduced to the ferrous state in endosomes and exported to the cytoplasm, possibly via divalent metal-ion transporter 1 (DMT1). Ferrous iron is then transported to interstitial fluid via ferroportin (FPN1) where it may be oxidized by ceruloplasmin and hephaestin. Iron is also transported to the CSF through the choroid plexus via holo-transferrin and ferroportin mechanisms and circulates with iron in the interstitial fluid. Ferrous iron is taken up by astrocytes, oligodendrocytes, and neurons by DMT1. Ferric iron complexes with apo-transferrin circulate in the brain, and ultimately enter neurons through a holo-transferrin-TfR1-dependent mechanism. Astrocyte-secreted hepcidin and ceruloplasmin regulate this process through a feedback mechanism according to the iron status of different neural cells, and this mechanism may be disrupted in patients with RLS/WED [40,41,42].
There is also an interplay between hypoxia pathways and iron metabolism. Iron deficiency activates hypoxemia-inducible factor pathway, which then increases cellular iron, mitochondrial iron, and an increase in mitochondria; cytosolic iron is then diverted to mitochondria, leading to cytosolic iron deficiency [43].
Dopamine dysfunction also plays a critical role in the pathophysiology of RLS/WED. Clinically, RLS/WED appears to involve a decreased dopaminergic signal; dopamine receptor agonists improve symptoms, and dopamine receptor antagonists worsen symptoms. Additionally, iron is a cofactor for tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis. However, the phenotypic manifestations actually reflect decreased post-synaptic dopamine signaling. In fact, evidence points to a prevailing pre-synaptic hyperdopaminergic state in RLS/WED [44,45,46,47]. Prior studies have shown increased tyrosine hydroxylase activity and dopamine synthesis [44, 46], decreased dopamine transporter activity, and decreased dopamine reuptake. It is still unclear which of these changes are the primary or secondary pathologies or compensatory changes [35]. These concepts have been expanded to include the circadian dynamics of dopamine regulation. The model proposes that dopamine signaling and dopamine receptor regulation reach a nadir at night and a peak in the morning. These functions drop below a clinical threshold at night, leading to the nighttime worsening of RLS/WED symptoms [35]. The circadian aspect of dopaminergic activity may explain why dopaminergic agents are effective treatment options in the presence of a hyperdopaminergic state. Post-synaptic downregulation of D2 receptors due to a hyperdopaminergic state results in low dopaminergic signaling when dopamine levels are low in the evening, leading to a relative nighttime dopamine activity deficit [48].
Neurophysiologic studies suggest a state of increased excitability in cortical neurons, particularly in the motor cortex, and reduced inhibition in spinal cord pathways. A recent review of transcranial magnetic stimulation in RLS/WED highlighted a reduction in short-interval intracortical inhibition as the most consistent finding between studies. These abnormalities show a circadian distribution and can be reversed by dopamine agonist treatment [49]. Several studies also highlight supra-spinal GABA-mediated disinhibition [49, 50] and one study showed spinal cord hyperexcitability [51] in RLS/WED. Furthermore, electroencephalographic spectral analysis of waking-rest conditions indicates a state of hyperarousal [52]. Together, these studies suggest abnormalities in the brain and spinal cord circuitry that may explain PLMS, some symptoms, and hyperarousal in RLS/WED.
Recent data have identified other mechanisms that may tie together brain iron deficiency and dopamine signal dysfunction. Several sources of evidence have implicated glutamatergic signaling in RLS/WED. For example, antagonism of N-methyl-d-aspartate (NMDA) receptors by ketamine and methadone improve RLS/WED symptoms [53••]. Furthermore, ligands for α2δ subunits—located on glutamatergic terminals—inhibit pre-synaptic glutamatergic transmission are also effective treatments for RLS/WED symptoms [53••]. BID alters glutamatergic transmission and leads to cortico-striatal terminal hypersensitivity. This effect is blocked by dopamine receptor agonists and α2δ ligands, suggesting the glutamatergic dysfunction occurs upstream of dopamine dysfunction; however, it is unclear if it causes the hyperdopaminergic state [54].
Alterations of adenosine signaling may also be involved. A1 receptors are involved in sleep homeostasis and inhibit the ascending arousal system. A1 and A2A receptors connect with D1 and D2 receptors, respectively. These complexes—which are highly expressed in the striatum—allow for inhibitory modulation of dopamine signaling such that low adenosine activity increases dopaminergic activity [55]. Moreover, A1 receptors inhibit pre-synaptic glutamate activity. A1 and A2A receptors complex in the cortico-striatal terminals and modulate glutamate release. Low concentrations of adenosine activate A1 receptors to inhibit glutamate release; high concentrations of adenosine activate A2A receptors to increase glutamate release [56]. BID decreases A1 receptors—more so than A2A receptors—resulting in a hypoadenosinergic state [56]. By this mechanism, it is possible that this hypoadenosinergic state, potentially caused by BID, leads to both hyperdopaminergic and hyperglutamatergic states, with increased glutamate activity worsening the hyperdopaminergic state [53••]. The hypoadenosinergic and hyperglutamatergic states may explain the hyperarousal seen in RLS/WED, and the hyperglutamatergic and hyperdopaminergic states may explain the neurophysiologic changes in cortico-striatal-thalamic circuitry, PLMS, and RLS/WED symptoms [53••].
Opioid receptors are also implicated in the pathogenesis of RLS/WED [57]. Opioid withdrawal can provoke RLS [58], and opioid medications can improve RLS/WED symptoms. Post-mortem studies revealed a deficiency of β-endorphin and met-enkephalin in the thalamus—but not the substantia nigra—in patients with RLS/WED symptoms [59]. These data suggest that endogenous opioids are decreased in sensory but not in motor pathways in these patients. Endogenous opioid deficiency may lead to abnormal processing of noxious stimuli, resulting in the characteristic “urge to move” in RLS/WED.
Opioids also interact with the dopaminergic system. The beneficial effect of opioids appears to be mediated through the dopamine pathway. The symptom improvement with opioid medication is blocked by both dopamine antagonists and the opioid antagonist naloxone, whereas the effect of dopaminergic medication is not affected by naloxone [59]. Furthermore, previous studies have suggested dopamine inhibits opiates, and dopamine antagonists increase opiate peptides [60]. In this way, the hyperdopaminergic state in RLS/WED may lead to a decrease in endogenous opiates. Conversely, endogenous opiate activity may inhibit dopamine release [61, 62], suggesting the presence of a bidirectional inhibitory mechanism. Therefore, it is possible that the development of a hyperdopaminergic state due to BID inhibits endogenous opiate activity, leading to further dopamine release. The relationship between iron and the opiate-dopamine interaction is unclear. While some studies suggest that iron deficiency increases opiate production [60], others show decreased opiates in RLS/WED [59]. Further studies of the interaction between opioid, dopaminergic, and iron pathways are needed to fully understand their roles in RLS/WED pathophysiology.
Genetics
RLS/WED is a genetically complex disorder. Prior studies have shown more than 60% of individuals with RLS/WED have a family history [63]. Although several studies, including twin studies, have indicated a genetic component [64, 65], segregation studies have been inconclusive [66]. Epigenetic factors also play a role and could explain the presence of non-Mendelian features and phenocopies in families with RLS/WED [67]. At least eight major susceptibility loci have been identified through linkage studies, most with an autosomal dominant inheritance pattern [68•]. A recent GWAS meta-analysis confirmed three previously identified risk loci—MEIS1, PTPRD, and TOX3—and three new loci—SEMA6D, SETBP1, and MYT1 [69•]. A prior GWAS study also describes risk loci in BTBD9 and MAP2K5/SKOR1. These genomic regions of interest account for < 10% of heritability [70]. We will highlight two loci of particular interest: MEIS1 and BTBD9.
MEIS1 is involved in the development of the central nervous system and appears to have an important role in brain iron metabolism. A recent meta-analysis confirmed this was the strongest genetic risk factor for RLS/WED [69•]. The most common variant associated with RLS/WED has been associated with reduced expression of MEIS1 in the embryonic ganglionic eminences, which lead to the development of basal ganglia. A recent review presented unpublished data describing evidence of elevated MEIS1 protein in the brain microvasculature in patients with RLS/WED. Furthermore, MEIS1 expression increases with iron deficiency and decreases with iron loading, suggesting a role of MEIS1 in brain iron metabolism [38••]. RLS/WED autopsy cases show MEIS1 variants were associated with an increase in H-ferritin and DMT1 RNA expression in the thalamus, predisposing to the lower iron condition [71]. Blocking MEIS1 expression has been associated with an increase in ferroportin mRNA and transferrin-2 receptor mRNA [72].
BTB/POZ domain-containing protein 9 (BTBD9) function is incompletely understood. A pathogenic polymorphism in BTBD9 was correlated with decreased iron levels [73]. An experimental mouse model of RLS/WED showed BTBD9 knockout had elevated serum iron levels and an increase in 5-hydroxyindoleacetic acid, a metabolite of serotonin, in the striatum. These mice had altered the sensory perception that improved with ropinirole [74]. Drosophila models show a decrease in brain dopamine of 50% and altered sleep consolidation patterns that improved with treatment with pramipexole [75]. Furthermore, inhibition of MEIS1 production increases BTBD9 expression [72].
Most variants of interest are associated with neural development and iron pathways. Functional studies to elucidate the specific molecular mechanisms and connections to other pathways involved in RLS/WED need to be carried out. Large-scale whole-genome sequencing studies will help determine the contribution of rare and structural variants which might explain another part of the heritability of RLS/WED.
Treatment
Treatment is usually started when symptoms interfere with a patient’s quality of life. Mild or infrequent symptoms may not require treatment. The Movement Disorders Society Task Force published a new set of guidelines in 2016 [76••]. The greatest change to the prior practice guidelines was the encouragement to use α2δ ligands as first-line treatment rather than dopamine receptor agonists. Current guidelines recommend starting with monotherapy; combination therapy has been anecdotally effective but randomized clinical trial data are lacking. A summary of pharmacologic treatments is presented in Table 1. Some non-pharmacological treatments have also been reported to be beneficial [76••].
α2δ Ligands
Gabapentin
Gabapentin is used off-label and is effective for the treatment of RLS/WED. Four older studies of gabapentin at doses of 800 mg daily to 1855 mg daily (divided into 2 doses) demonstrated its efficacy over 12 weeks, particularly in patients undergoing hemodialysis [76••]. One crossover study showed a significant decrease in RLS Rating Scale after 6 weeks of treatment compared with placebo (mean ± SE, 9.5 ± 1.35 vs 17.9 ± 1.35; p < 0.0005) [77]. Side effects are dose-dependent. Gabapentin may be beneficial as adjunctive medication, but this has not been formally investigated. Gabapentin can be particularly useful in patients with pain as a primary RLS/WED symptom. A prior study showed gabapentin significantly improved RLS/WED symptoms in hemodialysis patients compared with levodopa [78]. Because gabapentin is renally excreted, lower doses should be used in patients undergoing hemodialysis.
Gabapentin Enacarbil
Gabapentin enacarbil (GEn) is an actively transported, extended-release prodrug of gabapentin. One of its main advantages over gabapentin is its low inter-patient variability and well-sustained plasma levels [79]. GEn is the only calcium channel ligand approved by the FDA for the treatment of RLS/WED. Three randomized, double-blind, placebo-controlled studies which included adults with moderate-to-severe RLS/WED showed improvement in symptoms [80,81,82] and IRLS total score [83]. However, response to GEn may be reduced when used after long-term dopaminergic treatment. Compared with patients taking GEn who were taking dopamine agonists (DA) for the past 5 years, DA-naïve patients receiving GEn had a significantly greater improvement in IRLS total score, Clinical Global Index-severity scale, and the m-SIT [84•].
GEn also improves clinical symptoms and sleep in patients with RLS/WED. A pooled modified intent-to-treat population of adults with RLS/WED showed that GEn at both 600 mg and 1200 mg once daily over 12 weeks significantly improved all IRLS items, including sleep disturbance, daytime tiredness, and RLS/WED severity as well as the impact on daily affairs and mood disturbance [85].
Pregabalin
Pregabalin also binds to α2δ subunit in voltage-gated calcium channels. It is similar to gabapentin but is more rapidly and readily absorbed and has a higher binding affinity for the α2δ subunit [86]. Furthermore, pregabalin is considered a controlled substance because it is considered to have potential for abuse and physiological dependence [87]. Three older randomized controlled trials reported that pregabalin at doses between 150 and 450 mg per day was effective for RLS/WED symptoms over a period ranging from 6 to 52 weeks [76••]. More recently, a randomized, double-blind, placebo-controlled, crossover trial showed that pregabalin was non-inferior to pramipexole for the overall management of RLS/WED symptoms. Additionally, pregabalin increased slow-wave sleep and decreased the number of awakenings [88]. Allen et al. showed greater improvement in the Clinical Global Impression at 12 and 52 weeks with pregabalin 300 mg daily compared with pramipexole 0.25 mg but not 0.5 mg daily. Augmentation was less frequent with pregabalin (2.1%) than pramipexole 0.5 mg (7.7%) but not 0.25 mg daily (5.3%). Another salient aspect was that pregabalin was associated with an increased rate of dizziness, somnolence, weight gain, and suicidal ideation [89]. Safety and efficacy of pregabalin beyond 52 weeks have not been studied.
Dopaminergic Agents
Levodopa efficacy for RLS/WED treatment was first described in 1982. Levodopa is considered efficacious but conveys a high risk of augmentation [76••]. Since then, several dopaminergic agonists have been approved for RLS/WED treatment. Pramipexole has been shown to be effective up to 56 weeks, with doses as low as 0.25 mg [76••, 90]. Ropinirole is also considered efficacious, with most studies following patients for up to 12 months. Several new studies have demonstrated the efficacy of rotigotine transdermal patch for the management of moderate to severe RLS [91, 92]. All patients on dopamine agonists should be monitored for augmentation and impulse control disorders [93].
One of the main complications of dopaminergic treatment is augmentation. This phenomenon is characterized by an increase in symptoms intensity, sometimes even more than before starting treatment, symptoms occurring earlier in the day, and symptoms spread to other body regions. One should suspect augmentation in a patient whose symptoms have been stable and suddenly requires frequent increases in medication dose. It is important to differentiate augmentation from other causes of acute worsening of RLS/WED, such as new medications (antihistamine, dopamine receptor antagonist, antidepressants), sleep deprivation, and new or worsening iron deficiency [94].
Augmentation has been observed with all doses of levodopa, developing in 73% of patients. Pramipexole has the highest rate among dopamine agonists, with a risk of 7% per year [90], whereas the incidence with ropinirole is 4% at 26 weeks [95]. Rotigotine seems to have a decreased incidence compared with oral dopaminergic agonists which might be due to masking of symptoms in the setting of continued exposure over 24 h [96].
Although the mechanism of augmentation is not completely understood, downregulation of inhibitory D3 receptors and upregulation of excitatory D1 receptors seem to be involved. High dopamine concentrations target excitatory (D1) receptors and can maintain locomotor-like activity in the isolated spinal cord, which may trigger augmentation. Long-term treatment with D3 receptor agonists also leads to upregulation of excitatory D1 receptors in the spinal cord [97].
Management of augmentation depends on symptom severity. If symptoms are mild, earlier dosing or transition to a longer acting dopamine agonist should be considered. With moderate to severe symptoms, the dopamine agonist should be tapered or stopped. Cross-titration with another class of medication may also be used to manage symptoms [94]. In cases which it is unclear if worsening of symptoms is due to augmentation, tapering or stopping the dopaminergic therapy to re-establish a baseline symptom severity may be helpful; however, benefits of this approach should be weighed against the severity of symptom burden to the patient.
Benzodiazepines
Benzodiazepines, particularly clonazepam, have anecdotally been used for the treatment of RLS/WED. A systematic review by the American Academy of Sleep Medicine states benzodiazepines can be used as co-adjuvant therapy [98]. Benzodiazepines may reduce PLMS and arousals [99]. There are no randomized placebo-controlled clinical trials that assess the effectiveness of benzodiazepines for RLS/WED [100]. One randomized open-label trial showed that clonazepam, compared with nortriptyline, improved quality of life from RLS/WED in middle-aged women [101]. There are additional risks of dependence and tolerance, sedation, drowsiness, and falling.
Opioids
Opioids have long been recognized as effective for symptoms of RLS/WED. However, due to the increased concern about overuse and addiction, their use can be controversial. As a result, opioid therapy or opioid use is favored only for severe, treatment-resistant RLS/WED and in patients who experience augmentation. Respiratory depression is a major concern, particularly in those with pre-existing respiratory compromise. Patients should also be closely monitored for signs of dependence.
A recent study showed oxycodone-naloxone up-titrated to 40/20 mg twice daily was efficacious in treatment-resistant RLS/WED. Oxycodone on its own is also likely efficacious in those with daily symptoms, although no recent studies have been conducted [102, 103]. Methadone is a long-acting μ-opioid receptor agonist and NMDA antagonist, and its beneficial effects may work through both mechanisms. Two open-label studies showed that methadone was effective for refractory RLS/WED, without evidence of augmentation. Additionally, methadone has decreased abuse potential compared with other narcotics [104]. Given how effective opioids can be in RLS/WED, further studies are needed to examine their utility and appropriate use.
Iron Supplementation
Iron deficiency needs to be identified since iron supplementation may improve or eliminate symptoms. A recent meta-analysis concluded both intravenous and oral supplementation to be beneficial and associated with improvement in the IRLS scale [105]. Oral iron supplementation may be beneficial in patients with serum ferritin ≤ 75 μg/L and should be given with ascorbic acid (vitamin C) to enhance absorption [106]. Oral iron uptake may be decreased if serum ferritin is not low enough, in which case intravenous supplementation should be considered. Of the available formulations, iron dextran, ferric carboxymaltose, and iron isomaltoside are more readily transported to the brain [38••]. Ferric carboxymaltose is the only formulation to have sufficient evidence to support its efficacy and safety in moderate to severe RLS/WED, particularly patients with serum ferritin levels < 300 μg/L or transferrin saturation < 45% [106]. Anaphylaxis is a rare but life-threatening risk with intravenous iron dextran, particularly high-molecular weight dextran. Adverse events are more common with oral iron, consisting mainly of mild gastrointestinal events.
Others
Vitamins C and E are likely beneficial in uremic patients and do not require any special monitoring. The exact mechanism of action and long-term efficacy has not been established [107]. Magnesium is considered investigational in RLS/WED. One case series reports symptom improvement in 7 patients with RLS/WED and PLMS; no randomized-controlled trials have replicated this finding [108].
Non-pharmacologic Treatment
One randomized, sham-controlled trial has found pneumatic compression devices (PCDs) improve disease severity, quality life scores, and measures of fatigue; however, outcomes were based on patient self-report without any objective measurements. Additionally, PCDs require the patient to remain immobile for 1 h daily, which might be impractical for some patients [109].
There are two completed trials for devices that may be useful in RLS/WED, though the results have not yet been posted. The Scrambler device provides electrical stimulation to retrain peripheral sensation [110]. The RESTIFFIC™ device provides pressure on specific foot muscles and is being compared with ropinirole and placebo [111].
Single studies have reported symptom improvement with caffeine, alcohol, massage, hot baths, yoga, nerve stimulation or spinal current stimulation [51, 112,113,114].
Conclusion
Restless legs syndrome/Willis-Ekbom disease is a common neurological disorder with a specific set of clinical diagnostic criteria. We currently have a better understanding of the pathways involved in the pathophysiology of RLS/WED, but there are still several details that remain to be elucidated. Further description and validation of a regulated iron transport and storage model are necessary. An integral model involving iron, dopamine, glutamate, adenosine, and opiate pathways still needs to be fully understood. Genetic studies suggest that other monoamines, particularly serotonin, may be involved and warrant further analysis. Although many of the identified genes appear to be involved in neural development, their specific functions and their role in RLS/WED pathophysiology remain to be discovered. A better understanding of the pathophysiology will ultimately assist in the detection and development of new treatments that will specifically target the disease.
Several treatments are available for RLS/WED. Recently, α2δ ligands have been suggested as first-line treatment. Dopamine agonists are also effective but carry the risk of augmentation and compulsive behaviors. Although benzodiazepines and opioids are useful, more studies are needed to guide their utility. There is also a greater need to identify and develop effective non-pharmacologic strategies to treat RLS/WED.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Coccagna G, Vetrugno R, Lombardi C, Provini F. Restless legs syndrome: an historical note. Sleep Med. 2004;5:279–83.
Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria - history, rationale, description, and significance. Sleep Med. 2014;15:860–73. https://doi.org/10.1016/j.sleep.2014.03.025.
Walters AS. Toward a better definition of the restless legs syndrome. Mov Disord. 1995;10:634–42.
Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5.
Ferri R, Fulda S, Manconi M, Högl B, Ehrmann L, Ferini-Strambi L, et al. Night-to-night variability of periodic leg movements during sleep in restless legs syndrome and periodic limb movement disorder: comparison between the periodicity index and the PLMS index. Sleep Med. 2013;14:293–6.
Fantini ML, Michaud M, Gosselin N, Lavigne G, Montplaisir J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59:1889–94.
Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58:510–4.
Garcia-Borreguero D, Kohnen R, Boothby L, Tzonova D, Larrosa O, Dunkl E. Validation of the multiple suggested immobilization test: a test for the assessment of severity of restless legs syndrome. Sleep. 2013;36:1101–9.
Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32.
Fulda S, Wetter TC. Where dopamine meets opioids: a meta-analysis of the placebo effect in restless legs syndrome treatment studies. Brain. 2008;131:902–17.
Allen RP, Earley CJ. Validation of the Johns Hopkins restless legs severity scale. Sleep Med. 2001;2:239–42.
Kohnen R, Martinez-Martin P, Benes H, Trenkwalder C, Högl B, Dunkl E, et al. Rating of daytime and nighttime symptoms in RLS: validation of the RLS-6 scale of restless legs syndrome/Willis–Ekbom disease. Sleep Med. 2016;20:116–22.
Castillo PR, Kaplan J, Lin SC, Fredrickson PA, Mahowald MW. Prevalence of restless legs syndrome among native South Americans residing in coastal and mountainous areas. Mayo Clin Proc. 2006;81:1345–7.
Nomura T, Inoue Y, Kusumi M, Uemura Y, Nakashima K. Prevalence of restless legs syndrome in a rural community in Japan. Mov Disord. 2008;23:2363–9.
Cho SJ, Hong JP, Hahm BJ, Jeon HJ, Chang SM, Lee HB. Restless legs syndrome in a community sample of Korean adults: prevalence, impact on quality of life, and association with DSM-IV psychiatric disorders. Sleep. 2009;32:1069–76.
Chen NH, Chuang LP, Yang CT, Kushida CA, Hsu SC, Wang PC, et al. The prevalence of restless legs syndrome in Taiwanese adults. Psychiatry Clin Neurosci. 2010;64:170–8.
Manconi M, Ulfberg J, Berger K, Ghorayeb I, Wesström J, Fulda S, et al. When gender matters: restless legs syndrome. Report of the “RLS and woman” workshop endorsed by the European RLS Study Group. Sleep Med Rev. 2012;16:297–307.
Sander HH, Eckeli AL, Costa Passos AD, Azevedo LL, Fernandes do Prado LB, França Fernandes RM. Prevalence and quality of life and sleep in children and adolescents with restless legs syndrome / Willis-Ekbom disease. Sleep Med. 2017;30:204–9.
Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88:261–4.
Yang X, Liu B, Yang B, Li S, Wang F, Li K, et al. Prevalence of restless legs syndrome in individuals with migraine: a systematic review and meta-analysis of observational studies. Neurol Sci. 2018;39:1927–34.
Mucsi I, Molnar MZ, Rethelyi J, Vamos E, Csepanyi G, Tompa G, et al. Sleep disorders and illness intrusiveness in patients on chronic dialysis. Nephrol Dial Transpl. 2004;19:1815–22.
Trenkwalder C, Allen R, Högl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases a systematic review and new concept. Neurology. 2016;86:1336–43.
Szentkirályi A, Völzke H, Hoffmann W, Trenkwalder C, Berger K. Multimorbidity and the risk of restless legs syndrome in 2 prospective cohort studies. Neurology. 2014;82:2026–33.
• Chen SJ, Shi L, Bao YP, Sun YK, Lin X, Que JY, et al. Prevalence of restless legs syndrome during pregnancy: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:43–54. This review provides a detailed summary of RLS/WED in pregnancy.
Gupta R, Dhyani M, Kendzerska T, Pandi-Perumal S, BaHammam AS, Srivanitchapoom P, et al. Restless legs syndrome and pregnancy: prevalence, possible pathophysiological mechanisms and treatment, vol. 133; 2017. p. 320–9.
Wesström J, Skalkidou A, Manconi M, Fulda S, Sundström-Poromaa I. Pre-pregnancy restless legs syndrome (Willis-Ekbom disease) is associated with perinatal depression. J Clin Sleep Med. 2014;10:527–33.
Ramirez JO, Cabrera SA, Hidalgo H, Cabrera SG, Linnebank M, Bassetti CL, et al. Is preeclampsia associated with restless legs syndrome? Sleep Med. 2013;14:894–6.
Li Y, Li Y, Winkelman JW, Walters AS, Han J, Hu FB, et al. Prospective study of restless legs syndrome and total and cardiovascular mortality among women. Neurology. 2018;90:e135–41.
Ashraf A, Clark M, So PW. The aging of iron man. Front Aging Neurosci. 2018;10:65.
Berger K, von Eckardstein A, Trenkwalder C, Rothdach A, Junker R, Weiland SK. Iron metabolism and the risk of restless legs syndrome in an elderly general population - the MEMO-study. J Neurol. 2002;249:1195–9.
Kim KW, Yoon IY, Chung S, Shin YK, Lee SB, Choi EA, et al. Prevalence, comorbidities and risk factors of restless legs syndrome in the Korean elderly population - results from the Korean Longitudinal Study on Health and Aging. J Sleep Res. 2010;19:87–92.
Högl B, Stefani A. Restless legs syndrome and periodic leg movements in patients with movement disorders: specific considerations. Mov Disord. 2017;32:669–81.
Godau J, Klose U, Di Santo A, Schweitzer K, Berg D. Multiregional brain iron deficiency in restless legs syndrome. Mov Disord. 2008;23:1184–7.
Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698–700.
Earley CJ, Connor J, Garcia-Borreguero D, Jenner P, Winkelman J, Zee PC, et al. Altered brain iron homeostasis and dopaminergic function in restless legs syndrome. Sleep Med. 2014;15:1288–301.
Wilkinson N, Pantopoulos K. The IRP/IRE system in vivo: insights from mouse models. Front Pharmacol. 2014;5:176.
Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106:1559S–66S.
•• Connor JR, Patton SM, Oexle K, Allen RP. Iron and restless legs syndrome: treatment, genetics and pathophysiology. Sleep Med. 2017;31:61–70. https://doi.org/10.1016/j.sleep.2016.07.028 This reference provides a concise and complete review on the current knowledge on iron pathway involvement in RLS/WED, including the genetic variants, and provides iron supplementation recommendations.
Connor JR, Ponnuru P, Wang XS, Patton SM, Allen RP, Earley CJ. Profile of altered brain iron acquisition in restless legs syndrome. Brain. 2011;134:959–68.
Simpson IA, Ponnuru P, Klinger ME, Myers RL, Devraj K, Coe CL, et al. A novel model for brain iron uptake: introducing the concept of regulation. J Cereb Blood Flow Metab. 2015;35:48–57.
McCarthy RC, Kosman DJ. Iron transport across the blood-brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol Life Sci. 2015;72:709–27.
Nnah IC, Wessling-Resnick M. Brain iron homeostasis: a focus on microglial iron. Pharmaceuticals. 2018;11:E129.
Gutsaeva D, Carraway M, Suliman H, Demchenko I, Shitara H, Yonekawa H, et al. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015–24.
Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–12.
Unger EL, Wiesinger JA, Hao L, Beard JL. Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J Nutr. 2008;138:2487–94.
Allen RP, Connor JR, Hyland K, Earley CJ. Abnormally increased CSF 3-ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009;10:123–8.
Earley C, Hyland K, Allen R. Circadian changes in CSF dopaminergic measures in restless legs syndrome. Sleep Med. 2006;7:263–8.
Allen RP. Restless leg syndrome/Willis-Ekbom disease pathophysiology. Sleep Med Clin. 2015;10:207–14. https://doi.org/10.1016/j.jsmc.2015.05.022.
Magalhães SC, Kaelin-Lang A, Sterr A, do Prado GF, Eckeli AL, Conforto AB. Transcranial magnetic stimulation for evaluation of motor cortical excitability in restless legs syndrome/Willis-Ekbom disease. Sleep Med. 2015;16:1265–73.
Lanza G, Cantone M, Lanuzza B, Pennisi M, Bella R, Pennisi G, et al. Distinctive patterns of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome, restless legs syndrome, insomnia, and sleep deprivation. Sleep Med Rev. 2015;19:39–50.
Heide AC, Winkler T, Helms HJ, Nitsche MA, Trenkwalder C, Paulus W, et al. Effects of transcutaneous spinal direct current stimulation in idiopathic restless legs patients. Brain Stimul. 2014;7:636–42.
Jung KY, Koo YS, Kim BJ, Ko D, Lee GT, Kim KH, et al. Electrophysiologic disturbances during daytime in patients with restless legs syndrome: further evidence of cognitive dysfunction? Sleep Med. 2011;12:416–21.
•• Ferré S, García-Borreguero D, Allen RP, Earley CJ. New insights into the neurobiology of restless legs syndrome. Neuroscientist. 2019;25:113–25. This review details the current understanding of pathophysiology of RLS/WED, including involvement of adenosinergic and glutamatergic pathways, and provides a model linking brain iron deficiency to these neurotransmitters and symptoms of RLS/WED.
Yepes G, Guitart X, Rea W, Newman A, Allen R, Earley C, et al. Targeting hypersensitive corticostriatal terminals in restless legs syndrome. 2017;82:951–960.
Ferré S, Bonaventura J, Zhu W, Hatcher-Solis C, Taura J, Quiroz C, et al. Essential control of the function of the striatopallidal neuron by pre-coupled complexes of adenosine A2A-dopamine D2 receptor heterotetramers and adenylyl cyclase. Front Pharmacol. 2018;9:243.
Ferré S, Quiroz C, Guitart X, Rea W, Seyedian A, Moreno E, et al. Pivotal role of adenosine neurotransmission in restless legs syndrome. Front Neurosci. 2018;11:722. https://doi.org/10.3389/fnins.2017.00722.
Mizoguchi H, Takagi H, Watanabe C, Yonezawa A, Sato T, Sakurada T, et al. Involvement of multiple μ-opioid receptor subtypes on the presynaptic or postsynaptic inhibition of spinal pain transmission. Peptides. 2014;51:15–25. https://doi.org/10.1016/j.peptides.2013.10.012.
Gupta R, Ali R, Ray R. Willis-Ekbom disease/restless legs syndrome in patients with opioid withdrawal. Sleep Med. 2018;45:39–43.
Walters AS, Ondo WG, Zhu W, Le W. Does the endogenous opiate system play a role in the restless legs syndrome? A pilot post-mortem study. J Neurol Sci. 2009;279:62–5. https://doi.org/10.1016/j.jns.2008.12.022.
Youdim MBH. Brain iron deficiency and excess; cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotox Res. 2008;14:45–56.
Hawes SL, Salinas AG, Lovinger DM, Blackwell KT. Long-term plasticity of corticostriatal synapses is modulated by pathway-specific co-release of opioids through κ-opioid receptors. J Physiol. 2017;595:5637–52.
Campos-Jurado Y, Martí-Prats L, Zornoza T, Polache A, Granero L, Cano-Cebrián MJ. Regional differences in mu-opioid receptor-dependent modulation of basal dopamine transmission in rat striatum. Neurosci Lett. 2017;638:102–8. https://doi.org/10.1016/j.neulet.2016.12.024.
Winkelmann J, Wetter TC, Collado-Seidel V, Gasser T, Dichgans M, Yassouridis A, et al. Clinical characteristics and frequency of the hereditary restless legs syndrome in a population of 300 patients. Sleep. 2000;23:597–602.
Xiong L, Jang K, Montplaisir J, Levchenko A, Thibodeau P, Gaspar C, et al. Canadian restless legs syndrome twin study. Neurology. 2007;68:1631–3.
Desai AV, Cherkas LF, Spector TD, Williams AJ. Genetic influences in self-reported symptoms of obstructive sleep apnoea and restless legs: a twin study. Twin Res. 2004;7:589–95.
Mathias RA, Hening W, Washburn M, Allen RP, Lesage S, Wilson AF, et al. Segregation analysis of restless legs syndrome: possible evidence for a major gene in a family study using blinded diagnoses. Hum Hered. 2006;62:157–64.
Zimprich A. Phenocopies in families with essential tremor and restless legs syndrome challenge Mendelian laws. Epigenetics might provide answers. Park Relat Disord. 2012;18:711–6.
• Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG. Genetics of restless legs syndrome: an update. Sleep Med Rev. 2018;39:108–21. This reference provides an up-to-date summary of genetics of RLS/WED.
• Schormair B, Zhao C, Bell S, Tilch E, Salminen AV, Pütz B, et al. Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Lancet Neurol. 2017;16:898–907. This meta-analysis provides an updated GWAS confirms three previously known variants (MEIS1, PTPRD, and TOX3) and provides evidence for involvement of three new variants (SEMA6D, SETBP1, and MYT1).
Yang Q, Li L, Chen Q, Foldvary-Schaefer N, Ondo WG, Wang QK. Association studies of variants in MEIS1, BTBD9, and MAP2K5/SKOR1 with restless legs syndrome in a US population. Sleep Med. 2011;12:800–4.
Catoire H, Dion PA, Xiong L, Amari M, Gaudet R, Girard SL, et al. Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann Neurol. 2011;70:170–5.
Silver N, Allen RP, Earley CJ. MEIS1 as a potential mediator of the RLS-iron pathology. Mov Disord. 2010;25:S513–4.
Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–47.
DeAndrade MP, Johnson RL, Unger EL, Zhang L, van Groen T, Gamble KL, et al. Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in BTBD9 mutant mice. Hum Mol Genet. 2012;21:3984–92.
Freeman A, Pranski E, Miller RD, Radmard S, Bernhard D, Jinnah HA, et al. Sleep fragmentation and motor restlessness in a drosophila model of restless legs syndrome. Curr Biol. 2012;22:1142–8.
•• Winkelmann J, Allen RP, Högl B, Inoue Y, Oertel W, Salminen AV, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017)§. Mov Disord. 2018;33:1077–91. This reference provides updated treatment recommendations for RLS/WED from the Movement Disorders Society Task Force.
Garcia-Borreguero D, Larrosa O, De La Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59:1573–9.
Micozkadioglu H, Ozdemir FN, Kut A, Sezer S, Saatci U, Haberal M. Gabapentin versus levodopa for the treatment of restless legs syndrome in hemodialysis patients: an open-label study. Ren Fail. 2004;26:393–7.
Kim ES, Deeks ED. Gabapentin enacarbil: a review in restless legs syndrome. Drugs. 2016;76:879–87.
Kushida CA, Becker PM, Ellenbogen AL, Canafax DM, Barrett RW, XP052 Study Group. Randomized, double-blind, placebo-controlled study of XP13512 / GSK1838262 in patients with RLS. Neurology. 2009;72:439–46.
Lee DO, Ziman RB, Perkins AT, Poceta JS, Walters AS, Barrett RW, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011;7:282–92.
Lal R, Ellenbogen A, Chen D, Zomorodi K, Atluri H, Luo W, et al. A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin Neuropharmacol. 2012;35:165–73.
VanMeter SA, Kavanagh ST, Warren S, Barrett RW. Dose response of gabapentin enacarbil versus placebo in subjects with moderate-to-severe primary restless legs syndrome. CNS Drugs. 2012;26:773–80.
• Garcia-Borreguero D, Cano-Pumarega I, Garcia Malo C, Cruz Velarde JA, Granizo JJ, Wanner V. Reduced response to gabapentin enacarbil in restless legs syndrome following long-term dopaminergic treatment. Sleep Med. 2019;55:74–80. This new study showed that prior exposure to dopaminergic agonists can decrease response to gabapentin enacarbil.
Ahmed M, Hays R, Steven Poceta J, Jaros MJ, Kim R, Shang G. Effect of gabapentin enacarbil on individual items of the international restless legs study group rating scale and post-sleep questionnaire in adults with moderate-to-severe primary restless legs syndrome: pooled analysis of 3 randomized trials. Clin Ther. 2016;38:1726–1737.e1.
Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49:661–9.
Griffin E, Brown JN. Pregabalin for the treatment of restless legs syndrome. Ann Pharmacother. 2016;50:586–91. https://doi.org/10.1177/1060028016643097.
Garcia-Borreguero D, Patrick J, DuBrava S, Becker PM, Lankford A, Chen C, et al. Pregabalin versus pramipexole: effects on sleep disturbance in restless legs syndrome. Sleep. 2014;37:635–43.
Allen RP, Chen C, Garcia-Borreguero D, Polo O, DuBrava S, Miceli J, et al. Comparison of pregabalin with pramipexole for restless legs syndrome. N Engl J Med. 2014;370:621–31.
Högl B, Garcia-Borreguero D, Trenkwalder C, Ferini-Strambi L, Hening W, Poewe W, et al. Efficacy and augmentation during 6 months of double-blind pramipexole for restless legs syndrome. Sleep Med. 2011;12:351–60.
Inoue Y, Shimizu T, Hirata K, Uchimura N, Ishigooka J, Oka Y, et al. Efficacy and safety of rotigotine in Japanese patients with restless legs syndrome: a phase 3, multicenter, randomized, placebo-controlled, double-blind, parallel-group study. Sleep Med. 2013;14:1085–91.
Garcia-Borreguero D, Allen R, Hudson J, Dohin E, Grieger F, Moran K, et al. Effects of rotigotine on daytime symptoms in patients with primary restless legs syndrome: a randomized, placebo-controlled study. Curr Med Res Opin. 2016;32:77–85.
Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case-control study. Sleep. 2010;33:81–7.
García-Borreguero D, Allen RP, Kohnen R, Högl B, Trenkwalder C, Oertel W, et al. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a World Association of Sleep Medicine - International Restless Legs Syndrome Study group consensus conference at the Max Planck Institute. Sleep Med. 2007;8:520–30.
Giorgi L, Asgharian A, Hunter B. Ropinirole in patients with restless legs syndrome and baseline IRLS total scores ≥24: efficacy and tolerability in a 26-week, double-blind, parallel-group, placebo-controlled study followed by a 40-week open-label extension. Clin Ther. 2013;35:1321–36.
Trenkwalder C, Beneš H, Poewe W, Oertel WH, Garcia-Borreguero D, de Weerd AW, et al. Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2008;7:595–604.
Dinkins M, Lallemand P, Clemens S. Long-term treatment with dopamine D3 receptor agonists induces a behavioral switch that can be rescued by blocking the dopamine D1 receptor. Sleep Med. 2017;40:47–52.
Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults—an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of sleep medicine clinical practice guideline. Sleep. 2012;35:1039–62.
Peled R, Lavie P. Double-blind evaluation of clonazepam on periodic leg movements in sleep [Internet]. J Neurol Neurosurg Psychiatry. 1987;50:1679–81.
Carlos K, Prado GF, Teixeira CD, Conti C, de Oliveira MM, Prado LB, et al. Benzodiazepines for restless legs syndrome. Cochrane Database Syst Rev. 2017;(3):CD006939.
Roshi TVR, Mahajan A, Sharma S, Khajuria V. A prospective, randomized, open-label study comparing the efficacy and safety of clonazepam versus nortriptyline on quality of life in 40+ years old women presenting with restless leg syndrome. J Mid-life Heal. 2018;9:135–9. https://doi.org/10.4103/jmh.JMH_25_18.
Walters AS, Wagner ML, Hening WA, Grasing K, Mills R, Chokroverty S, et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16:327–32.
Trenkwalder C, Zieglgänsberger W, Ahmedzai SH, Högl B. Pain, opioids, and sleep: implications for restless legs syndrome treatment. Sleep Med. 2017;31:78–85.
Silber MH, Becker PM, Buchfuhrer MJ, Earley CJ, Ondo WG, Walters AS, et al. The appropriate use of opioids in the treatment of refractory restless legs syndrome. Mayo Clin Proc. 2018;93:59–67.
Avni T, Reich S, Lev N, Gafter-Gvili A. Iron supplementation for restless legs syndrome – a systematic review and meta-analysis. Eur J Intern Med. 2019;Epub ahead;63:34–41.
Allen RP, Picchietti DL, Auerbach M, Cho YW, Connor JR, Earley CJ, et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: an IRLSSG task force report. Sleep Med. 2018;41:27–44. https://doi.org/10.1016/j.sleep.2017.11.1126.
Sagheb MM, Dormanesh B, Fallahzadeh MK, Akbari H, Sohrabi Nazari S, Heydari ST, et al. Efficacy of vitamins C, E, and their combination for treatment of restless legs syndrome in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Sleep Med. 2012;13:542–5.
Hornyak M, Voderholzer U, Hohagen F, Berger M, Riemann D. Magnesium therapy for periodic leg movements-related insomnia and restless legs syndrome: an open pilot study. Sleep. 1998;21:501–5.
Lettieri CJ, Eliasson AH. Pneumatic compression devices are an effective therapy for restless legs syndrome: a prospective, randomized, double-blinded, sham-controlled trial. Chest. 2009;135:74–80.
ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US). 2000 Feb 29- . Identifier NCT03249779, Treatment of RLS/WED symptoms through sensory counter-stimulation (RLS/WED); 2017 August 15 [cited 2019 Feb 22]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03249779.
ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US). 2000 Feb 29- . Identifier NCT02386423 RESTIFFIC™ foot wrap reduces moderate to severe restless leg syndrome; 2015 March 11 [cited 2019 Feb 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT02386423.
Innes KE, Selfe TK, Agarwal P, Williams K, Flack KL. Efficacy of an eight-week yoga intervention on symptoms of restless legs syndrome (RLS): a pilot study. J Altern Complement Med. 2013;19:527–35.
Rozeman AD, Ottolini T, Grootendorst DC, Vogels OJ, Rijsman RM. Effect of sensory stimuli on restless legs syndrome: a randomized crossover study. J Clin Sleep Med. 2014;10:893–6.
Aukerman MM, Aukerman D, Bayard M, Tudiver F, Thorp L, Bailey B. Exercise and restless legs syndrome: a randomized controlled trial. J Am Board Fam Med. 2006;19:487–93.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
Roneil Malkani reports grant support from the Alzheimer’s Association, Illinois Department of Public Health, National Institutes of Health, and Northwestern University Parkinson’s Disease Advisory Council, and speaking honoraria for Advocate Healthcare and for American Academy of Neurology. Paulina Gonzalez-Latapi declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Movement Disorders
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Gonzalez-Latapi, P., Malkani, R. Update on Restless Legs Syndrome: from Mechanisms to Treatment. Curr Neurol Neurosci Rep 19, 54 (2019). https://doi.org/10.1007/s11910-019-0965-4
Published:
DOI: https://doi.org/10.1007/s11910-019-0965-4