Abstract
Super-refractory status epilepticus (SRSE) is a devastating neurological condition with limited treatment options. We conducted an extensive literature search to identify and summarize the therapeutic options for SRSE. The search mainly resulted in case reports of various pharmacologic and non-pharmacologic treatments. The success rate of each of the following agents, ketamine, inhaled anesthetics, intravenous immunoglobulin G (IVIG), IV steroids, ketogenic diet, hypothermia, electroconvulsive therapy (ECT), transcranial magnetic stimulation (TMS), and vagal nerve stimulation (VNS), are discussed in greater detail. The choice of appropriate treatment options for a given patient is based on clinical presentation. This review focuses on evidence-based, pharmacotherapeutic strategies for patients in SRSE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Status epilepticus (SE) is a very devastating disease if not recognized and treated early. The hospitalization rate for patients with SE increased by more than 56 % between 1999 and 2010, with a high mortality rate of approximately two per 1,000,000 persons [2•]. As such, SE is newly defined as continuous clinical or electrographical seizures lasting 5 min or more and/or recurrent episodes without recovery in between episodes [1]. When seizures are not controlled despite the initial treatment, which include intravenous (IV) benzodiazepines (emergent therapy) plus an antiepileptic drug (AED) (urgent therapy), it is called refractory status epilepticus (RSE). Seizures continuing despite adding additional AEDs and/or after initiating continuous infusion anesthetic agents are considered super-refractory status epilepticus (SRSE).
Unfortunately, there are no strong data to help practitioners make therapeutic decisions beyond the initial treatments for SE. This concise review of the safety and efficacy of reported treatment strategies for SRSE will assist practitioners with critical decision-making in these patients.
Initial Status Epilepticus Management
Emergent treatment of SE should include a benzodiazepine, preferably lorazepam, in patients with IV access. For patients without IV access, midazolam can be used intramuscularly with similar effectiveness [3].
Urgent therapy should be started immediately after the benzodiazepine is administered for patients in whom the underlying cause is unable to be corrected or in patients with a history of epilepsy. Intravenous intermittent antiepileptic agents such as phenytoin, fosphenytoin, valproate sodium, phenobarbital, and levetiracetam are recommended as options for urgent therapy by recent guidelines [1]. Other AEDs, such as lacosamide and topiramate, have also been used for urgent control, but there is only low level of evidence to support their use. If these agents fail to halt SE, then IV continuous infusion anesthetic agents should be used to treat what is now considered RSE. The three most commonly recommended agents for RSE are propofol, midazolam, and pentobarbital [1].
At this stage, it is required to secure the airway and start mechanical ventilation if the patient is not already intubated for other reasons (e.g., hemodynamic instability, low Glasgow Coma Scale (GCS) score). The IV anesthetic dose should be increased to achieve seizure control or until a burst suppression pattern is achieved on electroencephalography (EEG) [1]. If intermittent AEDs are considered for RSE, topiramate, levetiracetam, and lacosamide (Vimpat®) use has been reported in the literature. Lacosamide is the newest IV AED and may be a promising option. Two recent small studies evaluating the use of IV lacosamide as an adjunctive therapy in treating RSE showed at least 50 % more seizure control compared to placebo [4, 5].
If seizures continue within 24 h despite the above treatment regimen, clinically and/or electrographically, or re-occur upon reduction or withdrawal of anesthetic agents, it is termed SRSE [6].
Super-Refractory Status Epilepticus Etiology
What causes status epilepticus to evolve into SRSE is not always clear. The neurophysiology at the cellular level can potentially be explained by the decrease in functional gamma-aminobutyric acid (GABA) receptors, which are the binding sites for GABA, the principle inhibitory neurotransmitter in the central nervous system (CNS) [7]. This can also explain the rationale for the decreased effectiveness of GABA agonists, such as benzodiazepines and barbiturates, in treating SE after prolonged seizure activity [8].
During SE, N-methyl-d-aspartic acid (NMDA) receptors are up-regulated. The activation of NMDA receptors by glutamate can result in calcium influx intracellularly, which not only makes seizure termination more difficult, but may also cause cellular damage and secondary brain injury [9, 10]. This can lead to vicious cycling in seizure activity.
Other etiologies to consider for SRSE are inflammatory processes, immunological processes, infectious processes, and intra-cellular apoptosis [9, 10].
If the etiology is known, it is appropriate to treat the underlying disease process while maintaining control over RSE and SRSE with the goal of seizure termination once the initial insult is treated. Classical examples here are treating underlying bacterial meningitis with IV antibiotics and treating the edema surrounding a brain tumor with IV steroids. Although antibiotics and steroids are not AEDs per se, they do help as adjunctive therapy in controlling SE.
In the setting of idiopathic SRSE, it is recommended to apply early and aggressive management strategies. This will mitigate the effects of neuronal damage caused by calcium influx into cells, which leads to apoptosis and necrosis of neuronal cells, in turn making the seizure control more challenging.
No definitive data exists regarding the duration of treatment after which SE or RSE treatment can be deemed futile. Prolonged therapy is suggested in young patients with no pre-morbid state and self-limiting disease processes [11, 12].
Treatment Strategies for SRSE
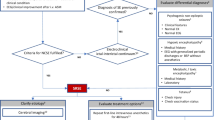
In an effort to provide optimal treatment strategies for SRSE, we divided the treatment into pharmacological options (Table 1) and non-pharmacological options (Table 2). An algorithmic approach to treatment for SRSE can be found in Fig. 1.
Ketamine
Most of the first-line AEDs and anesthetics for termination of seizures involve blocking GABAA receptors (e.g., benzodiazepines and propofol). It is suggested that with prolonged use of these agents, the receptors become less sensitive and hence the need for escalating doses or adding another agent to successfully halt seizure activity [8].
Ketamine is an NMDA receptor antagonist and has a potential therapeutic use in controlling SRSE. It also has hemodynamic advantage over the other anesthetics, as it tends not to decrease blood pressure. In fact, ketamine can eliminate the need for IV vasopressors when added to other agents [13].
The literature has limited data about ketamine use in SE. The available data is only small case series or retrospective reviews [14–16]. Ketamine is often used as a bolus followed by continuous infusion. The bolus dose is between 0.5 and 3 mg/kg. The infusion rate varies between 1 and 10 mg/kg/h, with no effect noted below 0.75 mg/kg/h [15, 17]. The duration for treatment varies between intermittent boluses and continuous infusions for up to 27 days [14, 18•].
Early use of ketamine in the treatment of SRSE may provide better and faster control of seizures. In a retrospective, multicenter study by Gaspard and colleagues, when ketamine was used as a third- or fourth-line agent, seizures were terminated in up to 60 % of patients as compared to 32 % if it was used later [18•].
Adverse Effects
Ketamine has been associated with tachycardia and acute elevation in blood pressure, all of which resolve after discontinuation of the drug [15, 17]. It may also increase intracranial pressure (ICP), especially in unstable patients that cannot autoregulate. Caution should be applied if the patient has an etiology that might increase ICP (e.g., severe brain edema from anoxic brain injury).
There are few reports on the theoretical risk of neurotoxic effects when ketamine is used for prolonged periods; however, this theory is not widely tested or proven [19].
Inhaled Anesthetics
In cases of SRSE, the use of inhaled anesthetics has been documented most notably with isoflurane and desflurane. These agents potentiate GABAA receptors and inhibit NMDA receptors. They are ideally administered in a closed environment to prevent intoxication of treating personnel [16]. Isoflurane and desflurane undergo significantly less metabolism than other volatile anesthetics and, thus, are preferred due to less organ toxicity.
One of the largest case series addressing the use of isoflurane was performed by Kofke and colleagues [20]. Nine patients with convulsive SE were administered isoflurane ranging from 1 to 5 % in concentration for 1–55 h and titrated to EEG burst suppression. Seizures ceased in all patients but returned with the discontinuation of the anesthetic agent in 8 of 11 occasions.
A retrospective review performed by Mirsattari and colleagues examined the use of inhaled isoflurane and desflurane in seven SRSE patients [21]. Burst suppression was achieved in all patients; however, seizures reoccurred once the inhaled anesthetics were stopped. End-tidal isoflurane concentrations ranged from 1.2 to 5 % and the mean duration was 11 days. Mortality was 43 % and all patients experienced hypotension and atelectasis.
Adverse Effects
The major concern with inhaled anesthetics is the induction of hypotension requiring IV vasopressor support. Other side effects include infection, paralytic ileus, deep vein thrombosis, and questionable cognitive dysfunction with prolonged use. A published, two-patient case report suggests neurotoxic side effects from prolonged isoflurane use [18•].
Immunotherapy
Immunological therapies have been reported in several case studies for the treatment of SRSE and most often include steroids, immunoglobulins, and plasma exchange (PLEX).
Immunotherapy is mostly used in situations where paraneoplastic or autoimmune encephalitis is responsible for the development of seizures. Any process which causes inflammation may result in epilepsy. Similarly, inflammation caused by the seizures can further potentiate epileptogenesis. It is these scenarios which warrant the use of immunotherapy in addition to SE standards of care. Given the lower incidence of this type of cause of SRSE, available literature is mostly in the form of case series [22].
Two case series have been published reviewing adult patients receiving intravenous immunoglobulin G (IVIG) for new onset SRSE with no clear etiology [19, 20]. In Gall and colleagues case series, five patients with new onset SRSE were reviewed. Two patients received IVIG at 2 g/kg over 5 days, plus high dose steroids, and one patient received only high dose steroids. All three patients recovered without significant neurological deficits [23•].
Contrarily, three of the seven patients in Wilder Smith’s review received IVIG at 0.4 g/kg/day for 3 days. Specifics about the timing of IVIG and results on each patient are not clearly stated; however, five of the seven patients died. The two survivors remained in a vegetative state suffering severe encephalopathy and recurrent seizures [24].
While encephalitis was not mentioned in the two previously discussed case series, a review by Shorvon and Ferlisi suggest early immune therapy for patients with no clear, identifiable source for SRSE present [25]. Costello et al. described a 6-patient adult case series of SRSE in the setting of cryptogenic encephalitis for which no underlying cause was determined [26]. Four of the six patients had febrile illness in the previous 2 weeks. IV methylprednisolone at 1 g per day was used in two patients. One of these patients also received PLEX for 3 days plus IVIG 0.4 g/kg × 5 days without response. Of the five survivors, functionality at follow-up varied significantly.
PLEX is much less reported than steroids and immunoglobulins for the treatment of SRSE. PLEX non-selectively removes soluble factors and proteins which may contribute to SRSE.
One case series by Li and colleagues reports three patient cases for adult cryptogenic SE. Two patients had SRSE and presented with no past medical history in the setting of febrile illness in days preceding seizure activity. Both patients failed a course of high dose IV methylprednisone. PLEX was instituted on seizure days 18 and 29 for a 5-day course. One of the patient’s seizures stopped on PLEX day 4, and she was discharged to rehab 1 week later with mild impairments. The other patient was gradually weaned off of sedation after PLEX. She became more responsive but died due to ischemic bowel complications [27•].
In a review by LoPinto-Khoury summarizing SRSE and immunotherapy, the recommendation is made to consider these therapies for patients with a negative past medical history for seizures, presence of other acute psychiatric behavioral or dementia like changes, an underlying malignancy, or presence of other autonomic dysfunction. Management mainstay includes treating the underlying cause while treating seizure activity [28].
Adverse Effects
High-dose steroids can cause glucose intolerance, psychiatric disturbances, impaired immunological function, and adrenal suppression.
The most important concerns with the use of immunoglobulins are injection site and hypersensitivity reactions. Immunglobulin-induced renal dysfunction can occur with concentrated solutions and high infusion rates. Transfusion-related acute lung injury and thromboembolic events have also been associated with these infusions.
The use of PLEX requires insertion of large venous catheters which carries increased risk of infection and thrombosis. During the exchange, patients are at risk for hypotension and bleeding complications.
Ketogenic Diet
Ketogenic diet has been widely used especially among children for SRSE. The typical ketogenic diet of 4:1 (the ratio of fat to carbohydrate and protein) was implemented in multiple clinical studies with promising results. Nabbout et al. studied nine patients who all started on a 4:1 ketogenic diet after a 24-h fasting. All patients had FIRES (fever-induced refractory epileptic encephalopathy in school-age children) [29].
Glucose levels were monitored every 3 h for the first 3 days, then every 6 h thereafter. It is imperative to maintain a strict glucose level for treatment as well as to avoid adverse side effects secondary to severe hypoglycemia. This study found ketogenic diet to be efficacious in seven patients within 2–4 days following the onset of ketonuria and 4–6 days following the onset of the diet.
Ketogenic diet has been used in treating SRSE in adults as well. In a meta-analysis of 12 studies, 270 adult SRSE patients receiving a ketogenic diet had a combined efficacy rate of 42 %. The efficacy rate of all types of ketogenic diet, a classic ketogenic diet, and a modified Atkins diet were 42, 52, and 34 %, respectively. However, the overall compliance rate was low at 45 % [30].
In a case series of 10 adult patients treated with ketogenic diet for SRSE, nine demonstrated seizure resolution within a median of 3 days. Patients continued to receive adjunctive treatment with multiple AEDs as well as treatment of the etiology for the seizure if known [31].
Therapeutic success rates were reported to be up to 70 % at the 3rd International Symposium on Dietary Therapies by several groups using a ketogenic diet [32].
Adverse Effects
Major contraindications to ketogenic diet include possible pyruvate carboxylase and beta-oxidation deficiency. Other side effects include hyperlipidemia and weight loss [33, 34]. Practical difficulties in achieving and maintaining ketogenic diet might limit its efficacy.
Hypothermia
Hypothermia protocol is typically used after cardiac/respiratory arrest as an effort to minimize anoxic/hypoxic brain injury and improve outcomes, and it has been studied in patients with intractable intracranial hypertension [35, 36].
The effect of hypothermia in slowing down metabolism to allow for efficient recovery is a widely noted concept. It also prevents neuronal loss during SE while suppressing the duration and frequency of ictal discharges. It has a neuroprotective mechanism by conserving energy and reducing nitric oxide production [37].
The temperature at which successful seizure cessation can be obtained is equivocal. Hypothermia can be broken down into three levels: mild (34–35.9 °Celsius (C)), moderate (32–33.9 °C), and moderate to deep (30–31.9 °C). A temperature less than 32 °C should be avoided due to serious side effects [38].
However, the notion of inducing hypothermia in the setting of SRSE is not a prevalent practice. In one case series, four SRSE patients who had failed benzodiazepine and/or barbiturate infusions were treated with hypothermia (target temperature 31–35 °C). Seizure control was achieved in all four patients with minimal morbidity [37].
Isoflurane and hypothermia have been used in combination, resulting in mild synergism for lowering body temperature and therapeutic efficacy in seizure management [39].
Hypothermia has successfully been used even in the setting of two failed burst suppression attempts using either pentobarbital, or propofol and midazolam. Target temperature of 33 °C was maintained for 24 h with controlled rewarming over 8 h. No rebound seizures were noted upon achieving normothermia [40].
Adverse Effects
Adverse effects of hypothermia include coagulation disorders, venous thrombosis, cardiac arrhythmia, electrolyte abnormalities, infections, pharmacokinetic and pharmacodynamics changes, and acute intestinal ischemia/necrosis. These adverse effects are more noticeable at temperatures lower than 30 °C, but begin as temperatures begin to fall below 37 °C [41].
Electroconvulsive Therapy
Electroconvulsive therapy (ECT) is not considered a first- or even a second-line treatment in SRSE. Its use for this indication is still premature and controversial. ECT is more commonly used in advanced psychiatric illnesses such as severe depression and mood disorder [42]. Even for these indications, ECT remains a second-line therapy after other treatment modalities have failed or have caused unacceptable side effects.
ECT is used by applying two electrodes in the head under anesthesia and inducing a convulsive seizure. The exact mechanism of how ECT can help in stopping seizures is not completely clear. It is suggested that ECT enhances GABAA receptor transmission prolonging the refractory threshold after inducing seizures [43].
Few case reports are available regarding the acute use of ECT in SRSE. No clinical trial is available [44–47]. In a recent systematic review for the use of ECT in SRSE by Lambrecq et al., the authors found eight case reports with 11 patients treated (seven adults and four children) [48]. ECT was used significantly later in the treatment protocol, 30–40 days after SRSE onset. Also, most protocols continued to use other intermittent AEDs while minimizing or eliminating one or more continuous AEDs (e.g., midazolam or pentobarbital) to achieve an actual seizure by applying ECT. The success rate of controlling SRSE from combining all the cases together was about 80 %. However, the ECT protocols and the AED choices varied significantly.
Adverse Effects
ECT works by inducing seizures, so convulsive and non-convulsive status epilepticus following the treatment is a concern. Close monitoring with continuous EEG is required. Other side effects include cognitive impairment, amnesia, and headache [49, 50].
Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is a relatively newer technique in the field of neuroscience and psychiatry. Its original use was as a non-invasive way of recording cortical brain activity, localizing seizure origin, and detecting the effect of AEDs on seizure control.
When the magnetic stimulation is repeated at regular intervals, this is called repetitive TMS or rTMS. Repetitive TMS is a non-invasive technique where pulsed intracranial electrical current is induced by electromagnetic induction. When rTMS is used with lower frequency, it can suppress the cortex. However, with higher frequency (>5 Hz), it can excite the cortex [51].
This unique property of rTMS found its way as a therapeutic tool in treating some psychiatric diseases such as depression and mood disorder [52]. More recently, it was applied as a therapeutic tool in treating intractable focal seizures [53–55]. Repetitive TMS is able to stimulate the brain in a way similar to ECT; however, it is more focal and localized compared to ECT. Although most of rTMS use is in the inter-ictal state (i.e., not in actively seizing patients), there are few reports about the use of rTMS in acute focal SE treatment [56]. Liu et al. has reported the use of low frequency rTMS in two ICU patients with refractory focal SE. Both patients showed at least 50 % reduction in seizures frequency after rTMS application [56]. Although no therapeutic conclusion can be obtained from such a small sample, there is an important point here, which is rTMS does not interfere with the ICU equipment and can be safely used in the ICU setting.
Adverse Effects
TMS is considered very safe. In contrast to vagal nerve stimulation or deep brain stimulation, TMS does not require surgery or device implantation. Additionally, TMS does not require the cessation of all or some AEDs (in contrast to ECT). TMS can rarely induce seizures and can sometimes provoke headache, dizziness, and other neurological side effects [57].
Vagal Nerve Stimulation
Vagal nerve stimulation (VNS) is a relatively newer therapy for controlling seizures (FDA approval in 1997 as adjunctive therapy for patients >12 years old) [58]. Despite its wide usage in epilepsy, there is not much data to support its efficacy. The American Association of Neurology (AAN) 2013 guidelines stated that VNS may be considered for seizures in children, for Lennox-Gastaut Syndrome (LGS)-associated seizures, and for improving mood in adults with epilepsy (level C) [59]. Furthermore, urgent VNS insertion and utilization in acute SE treatment is not widely studied.
VNS mechanism in seizure control is not completely understood. There is little evidence in humans that VNS increase GABA levels in the CSF and upregulate GABAA receptors which may help with controlling the seizures [60]. Other hypothesized mechanisms include locus ceruleus stimulation with increase in noradrenergic secretion and raphe magnus enhancement of serotonin transmission that may lead to seizure inhibition [61, 62].
Recent systemic review for acute VNS use in SE by Zeiler et al. found only 17 eligible articles with only 28 subjects [63]. The overall success rate of controlling generalized seizures was more than 75 %, and more than 25 % in focal seizures. The duration needed to achieve seizure control after inserting urgent VNS ranged between 3 and 16 days. All other AEDs were continued in addition to VNS. No correlation was noted with any specific stimulation parameters (current strength, frequency, or pulse width) [64].
Adverse Effects
Side effects were generally low. The most common side effects are voice hoarseness, infection risk at the VNS implantation site, and rare bradycardia.
Urgent Neurosurgical Intervention
Surgical intervention for the management of SRSE has been done in the past for carefully selective cases. Some of the surgical interventions included focal cortical resection, lobar and multi-lobar resection, anatomic and functional hemispherectomy, corpus callosotomy, and multiple subpial transection [65–67].
In a small case series using surgical intervention in acute SRSE in three patients, SRSE was successfully terminated in two patients. All three patients were able to leave the ICU eventually. Interventions in this series included temporoparietal lobectomy, multiple subpial transections in motor cortex, and occipital resection. [65].
Experimental Treatment for SE
Newer therapies are being tested every day to help control SE. Although this is beyond the scope of this review, some therapies worth mentioning are neuroactive steroids (allopregnanolone) [68], pharmaceutical-grade cannabidiol [69], and some other compounds such as brivaracetam, bumetanide, everolimus, ganaxolone, huperzine A, imepitoin, minocycline, and valnoctamide [70].
Summary
Limited data exist on the treatment of SRSE as the armamentarium of AEDs has grown and the treatment is highly variable due to the various etiologies of SRSE. Currently, IV AEDs and anesthetics are being used as adjunctive therapy and have shown some promise, but the most appropriate sequence of SRSE therapy is yet to be defined. Identifying the etiology of SRSE and understanding the pharmacologic properties of each AED is key to identifying the best individualized treatment strategy for patients in SRSE and preventing unwanted adverse effects in this patient population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Trends in status epilepticus-related hospitalizations and mortality: redefined in US practice over time. - PubMed - NCBI [Internet]. [cited 6/9/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25915004.
Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. This reference is important as it is the most recent guidelines from the neurocritical care society that describe status epilepticus definitions and classification, etiology, diagnostic evaluation, prognosis, monitoring, and management in critically ill patients.
Kossoff EH. A shot in the arm for prehospital status epilepticus: the RAMPART study. Epilepsy Curr. 2012;12(3):103–4.
Mnatsakanyan L, Chung JM, Tsimerinov EI, Eliashiv DS. Intravenous lacosamide in refractory nonconvulsive status epilepticus. Seizure. 2012;21(3):198–201.
Miro J, Toledo M, Santamarina E, Ricciardi AC, Villanueva V, Pato A, et al. Efficacy of intravenous lacosamide as an add-on treatment in refractory status epilepticus: a multicentric prospective study. Seizure. 2013;22(1):77–9.
Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134(Pt 10):2802–18.
Smith KR, Kittler JT. The cell biology of synaptic inhibition in health and disease. Curr Opin Neurobiol. 2010;20(5):550–6.
Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25(23):5511–20.
Kapur J, Lothman EW. NMDA receptor activation mediates the loss of GABAergic inhibition induced by recurrent seizures. Epilepsy Res. 1990;5(2):103–11.
Mazarati AM, Wasterlain CG. N-methyl-D-asparate receptor antagonists abolish the maintenance phase of self-sustaining status epilepticus in rat. Neurosci Lett. 1999;265(3):187–90.
Bramstedt KA, Morris HH, Tanner A. Now we lay them down to sleep: ethical issues with the use of pharmacologic coma for adult status epilepticus. Epilepsy Behav. 2004;5(5):752–5.
Dara SI, Tungpalan LA, Manno EM, Lee VH, Moder KG, Keegan MT, et al. Prolonged coma from refractory status epilepticus. Neurocrit Care. 2006;4(2):140–2.
The effects of ketamine on cardiovascular dynamics during ha… : Anesthesia & Analgesia [Internet]. [cited 4/28/2015]. Available from: http://journals.lww.com/anesthesia-analgesia/Abstract/1975/09000/The_Effects_of_Ketamine_on_Cardiovascular_Dynamics.5.aspx.
Kofke WA, Bloom MJ, Van Cott A, Brenner RP. Electrographic tachyphylaxis to etomidate and ketamine used for refractory status epilepticus controlled with isoflurane. J Neurosurg Anesthesiol. 1997;9(3):269–72.
Kramer AH. Early ketamine to treat refractory status epilepticus. Neurocrit Care. 2012;16(2):299–305.
Rossetti AO. Novel anesthetics and other treatment strategies for refractory status epilepticus. Epilepsia. 2009;50 Suppl 12:51–3.
Rosati A, L’Erario M, Ilvento L, Cecchi C, Pisano T, Mirabile L, et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology. 2012;79(24):2355–8.
Gaspard N, Foreman B, Judd LM, Brenton JN, Nathan BR, McCoy BM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia. 2013;54(8):1498–503. This reference is important as it is one of the largest studies for ketamine use in SE. It is a retrospective multi-center study and included 58 subjects. Ketamine was felt to have contributed to permanent control (“possible” or “likely” responses) in about one third of the cases.
Yan J, Jiang H. Dual effects of ketamine: neurotoxicity versus neuroprotection in anesthesia for the developing brain. J Neurosurg Anesthesiol. 2014;26(2):155–60.
Kofke WA, Young RS, Davis P, Woelfel SK, Gray L, Johnson D, et al. Isoflurane for refractory status epilepticus: a clinical series. Anesthesiology. 1989;71(5):653–9.
Mirsattari SM, Sharpe MD, Young GB. Treatment of refractory status epilepticus with inhalational anesthetic agents isoflurane and desflurane. Arch Neurol. 2004;61(8):1254–9.
Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40.
Gall CR, Jumma O, Mohanraj R. Five cases of new onset refractory status epilepticus (NORSE) syndrome: outcomes with early immunotherapy. Seizure. 2013;22(3):217–20. This paper is one of the larger, more recent case series of the potential efficacy immunotherapy for the treatment of new onset refractory status epilepticus without known cause. This data adds guidance to the support of using steroids in combination with IVIG for SRSE in adult patients.
Wilder-Smith EP, Lim EC, Teoh HL, Sharma VK, Tan JJ, Chan BP, et al. The NORSE (new-onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singap. 2005;34(7):417–20.
Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135(Pt 8):2314–28.
Costello DJ, Kilbride RD, Cole AJ. Cryptogenic new onset refractory status epilepticus (NORSE) in adults—infectious or not? J Neurol Sci. 2009;277(1–2):26–31.
Plasma exchange in cryptogenic new onset refractory status epilepticus. - PubMed - NCBI [Internet]. [cited 5/14/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23068971. This article is of importance because the use of plasma exchange for SRSE is much less reported when compared to other treatment modalities. These authors provide data on 3 adult patients who responded well to plasma exchange after failing several other standard therapies.
Autoimmune status epilepticus. - PubMed - NCBI [Internet]. [cited 5/14/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23852708.
Nabbout R, Mazzuca M, Hubert P, Peudennier S, Allaire C, Flurin V, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES). Epilepsia. 2010;51(10):2033–7.
Ye F, Li XJ, Jiang WL, Sun HB, Liu J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis. J Clin Neurol. 2015;11(1):26–31.
Thakur KT, Probasco JC, Hocker SE, Roehl K, Henry B, Kossoff EH, et al. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. 2014;82(8):665–70.
Cervenka MC, Henry B, Nathan J, Wood S, Volek JS. Worldwide dietary therapies for adults with epilepsy and other disorders. J Child Neurol. 2013;28(8):1034–40.
Wusthoff CJ, Kranick SM, Morley JF, Christina Bergqvist AG. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. 2010;51(6):1083–5.
Kumada T, Miyajima T, Kimura N, Saito K, Shimomura H, Oda N, et al. Modified Atkins diet for the treatment of nonconvulsive status epilepticus in children. J Child Neurol. 2010;25(4):485–9.
Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002 02/21; 2015/04;346(8):549–56.
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24 Suppl 1:S1–106.
Corry JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care. 2008;9(2):189–97.
Motamedi GK, Lesser RP, Vicini S. Therapeutic brain hypothermia, its mechanisms of action, and its prospects as a treatment for epilepsy. Epilepsia. 2013;54(6):959–70.
Zhumadilov A, Gilman CP, Viderman D. Management of super-refractory status epilepticus with isoflurane and hypothermia. Front Neurol. 2015;5:286.
Therapeutic hypothermia for status epilepticus: a report, historical perspective, and review. - PubMed - NCBI [Internet]. [cited 5/16/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=Therapeutic+hypothermia+for+status+epilepticus:+A+report,+historical++perspective,+and+review.
Potential mechanisms and clinical applications of mild hypothermia and electroconvulsive therapy on refractory status epilepticus. - PubMed - NCBI [Internet]. [cited 5/16/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25495421.
Versiani M, Cheniaux E, Landeira-Fernandez J. Efficacy and safety of electroconvulsive therapy in the treatment of bipolar disorder: a systematic review. J ECT. 2011;27(2):153–64.
Sackeim HA. Convulsant and anticonvulsant properties of electroconvulsive therapy: towards a focal form of brain stimulation. Clin Neurosci Res. 2004;4(1–2):39–57.
Shin HW, O’Donovan CA, Boggs JG, Grefe A, Harper A, Bell WL, et al. Successful ECT treatment for medically refractory nonconvulsive status epilepticus in pediatric patient. Seizure. 2011;20(5):433–6.
Kamel H, Cornes SB, Hegde M, Hall SE, Josephson SA. Electroconvulsive therapy for refractory status epilepticus: a case series. Neurocrit Care. 2010;12(2):204–10.
Morales OG, Henry ME, Nobler MS, Wassermann EM, Lisanby SH. Electroconvulsive therapy and repetitive transcranial magnetic stimulation in children and adolescents: a review and report of two cases of epilepsia partialis continua. Child Adolesc Psychiatr Clin N Am. 2005;14(1):193–210.
Cline JS, Roos K. Treatment of status epilepticus with electroconvulsive therapy. J ECT. 2007;23(1):30–2.
Lambrecq V, Villega F, Marchal C, Michel V, Guehl D, Rotge JY, et al. Refractory status epilepticus: electroconvulsive therapy as a possible therapeutic strategy. Seizure. 2012;21(9):661–4.
UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799–808.
Gangadhar BN, Kapur RL, Kalyanasundaram S. Comparison of electroconvulsive therapy with imipramine in endogenous depression: a double blind study. Br J Psychiatry. 1982;141:367–71.
Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(4):847.
Aleman A. Use of repetitive transcranial magnetic stimulation for treatment in psychiatry. Clin Psychopharmacol Neurosci. 2013;11(2):53–9.
Misawa S, Kuwabara S, Shibuya K, Mamada K, Hattori T. Low-frequency transcranial magnetic stimulation for epilepsia partialis continua due to cortical dysplasia. J Neurol Sci. 2005 7/15;234(1–2):37–9.
Fregni F, Otachi PTM, Do Valle A, Boggio PS, Thut G, Rigonatti SP, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60(4):447–55.
Sun W, Mao W, Meng X, Wang D, Qiao L, Tao W, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study. Epilepsia. 2012;53(10):1782–9.
Liu A, Pang T, Herman S, Pascual-Leone A, Rotenberg A. Transcranial magnetic stimulation for refractory focal status epilepticus in the intensive care unit. Seizure. 2013;22(10):893–6.
Gilbert DL, Garvey MA, Bansal AS, Lipps T, Zhang J, Wassermann EM. Should transcranial magnetic stimulation research in children be considered minimal risk? Clin Neurophysiol. 2004;115(8):1730–9.
Recently-approved devices > VNS therapy system - P970003s050 [Internet]. [cited 6/9/2015]. Available from: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm078532.htm.
Morris 3rd GL, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the guideline development subcommittee of the American Academy of Neurology. Epilepsy Curr. 2013;13(6):297–303.
Vonck K, Van Laere K, Dedeurwaerdere S, Caemaert J, De Reuck J, Boon P. The mechanism of action of vagus nerve stimulation for refractory epilepsy: the current status. J Clin Neurophysiol. 2001;18(5):394–401.
Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318(2):890–8.
Raedt R, Clinckers R, Mollet L, Vonck K, El Tahry R, Wyckhuys T, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 2011;117(3):461–9.
Zeiler FA, Zeiler KJ, Teitelbaum J, Gillman LM, West M. VNS for refractory status epilepticus. Epilepsy Res. 2015;112(0):100–13.
VNS for refractory status epilepticus. - PubMed - NCBI [Internet]. [cited 6/9/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25847345.
Urgent, resective surgery for medically refractory, convulsive status epilepticus. - PubMed - NCBI [Internet]. [cited 4/28/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18460420.
Ma X, Liporace J, O’Connor MJ, Sperling MR. Neurosurgical treatment of medically intractable status epilepticus. Epilepsy Res. 2001;46(1):33–8.
Life-saving epilepsy surgery for status epilepticus caused by cortical dysplasia. - PubMed - NCBI [Internet]. [cited 4/28/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12446223.
Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54 Suppl 6:93–8.
Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. - PubMed - NCBI [Internet]. [cited 6/2/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25845492.
Progress report on new antiepileptic drugs: a summary of the twelfth Eilat Conference (EILAT XII). - PubMed - NCBI [Internet]. [cited 6/2/2015]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=SAGE-547.
Compliance with Ethics Guidelines
Conflict of Interest
Ahmad Bayrlee, Nimalya Ganeshalingam, and Lisa Kurczewski declare that they have no conflict of interest.
Gretchen M. Brophy has received consultancy fees from Edge Therapeutics, honoraria payments, and paid travel accommodations from UCB Pharma and payment for development of educational presentations from the American College of Clinical Pharmacy.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Epilepsy
Rights and permissions
About this article
Cite this article
Bayrlee, A., Ganeshalingam, N., Kurczewski, L. et al. Treatment of Super-Refractory Status Epilepticus. Curr Neurol Neurosci Rep 15, 66 (2015). https://doi.org/10.1007/s11910-015-0589-2
Published:
DOI: https://doi.org/10.1007/s11910-015-0589-2