Abstract
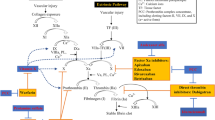
Anticoagulant therapies are increasingly being used for the treatment and prevention of thromboembolic diseases. A growing incidence of anticoagulant-associated intracranial hemorrhage (AICH) has accompanied the rise in their use. Although the rate of intracerebral hemorrhage (ICH) in patients receiving anticoagulation therapies such as heparin and target-specific oral anticoagulants (TSOAs) is significantly lower than that of vitamin K antagonists (VKAs), the mortality rate remains high. TSOAs have only recently become available for use in clinical practice, and presently, there is a paucity of both clinical data and evidence-based guidelines to assist in the management of TSOA-associated intracerebral hemorrhage. In this article, we review current literature and provide physicians with diagnostic and therapeutic considerations for the management of AICH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anticoagulants are increasingly being used for the prevention and treatment of thromboembolic disorders. At present, it is estimated that close to four million individuals within the USA receives oral anticoagulant therapy for thromboembolic indications with greater than 31 million prescriptions written annually for vitamin K antagonist (VKA) alone [1, 2]. The number of patients receiving anticoagulants is expected to more than double by the year 2050, corresponding with an aging population and increasing indications for their use [3]. A common and serious complication associated with anticoagulants is bleeding, with cases of anticoagulant-associated intracerebral hemorrhage (AICH) having the greatest degree of morbidity and mortality (up to 67 %) [4]. Patients receiving anticoagulants not only have an increased (seven- to tenfold) risk of intracerebral hemorrhage (ICH) but also greater ICH severity and mortality relative to aged-matched and non-anticoagulated cohorts [4, 5]. As the use of anticoagulants has risen over time so too has the incidence of AICH with one population-based study demonstrating a fivefold increase in ICH over a 10-year period, coinciding with a fourfold increase in VKA use [6].
VKAs have served as the mainstay of oral anticoagulant therapy in the treatment and prevention of thromboembolic disease for over six decades. Novel target-specific oral anticoagulants (TSOAs) have only recently become available for various indications. As a consequence, most studies evaluating the management and outcomes of patients presenting with AICH involve those treated with VKAs.
Hematoma expansion in patients with ICH correlates with both poor functional outcomes and increased mortality [7]. However, when compared to non-anticoagulated patients, those patients receiving VKAs are found to develop larger hematoma volumes and have a greater frequency of ventricular extension and an overall higher rate of mortality [8, 9]. The intensity of VKA anticoagulation is also of consequence, with larger baseline hematoma volumes seen with an international normalized ratio (INR) >3 [10]. Moreover, AICH patients demonstrate worse patient outcomes with a delayed correction in coagulopathy; therefore, timely (<2 h) and complete reversal of coagulopathy (INR ≤1.4) is often considered as the mainstay of managing AICH [11, 12].
Presently, there are no randomized controlled trials available to guide treatment of AICH. Therefore, the clinical management of AICH is largely founded on an expert opinion, an empiric evidence, animal studies, and a limited number of case reports often using surrogate markers of hemostasis such as INR [13]. The management of every patient presenting with AICH involves the discontinuation of the anticoagulant, administration of available hemostatic agents to counteract the anticoagulant effect, and the continuous clinical and laboratory monitoring of hemostasis.
Anticoagulant Pharmacology
The pharmacokinetic properties of anticoagulants are summarized in Table 1.
Vitamin K Antagonists
Although clinically very effective, VKAs exhibit considerable variability in their anticoagulant effects, influenced by a number of factors including diet, concomitant medications, and genetic composition. This variability can lead to difficulty in maintaining patients within a narrow therapeutic range (thromboembolic prophylaxis, INR 2.0–3.0; mechanical heart valve, INR 2.5–3.5) [14•]. The incidence of bleeding with supra-therapeutic INR (ST-INR) is high with a substantial risk of ICH with an INR of ≥4 [15]. Nonetheless, the majority of VKA-associated ICH occur with INR values within the therapeutic range with an overall ICH incidence of 0.03 to 3.7 % annually [16••, 17].
VKAs predominantly inhibit the C1 subunit of vitamin K epoxide reductase (VKORC1), a hepatic enzyme necessary for the generation of vitamin K hydroquinone, a cofactor required for the carboxylation of glutamate residues found on vitamin K-dependent clotting factors (VKDFs) II, VII, IX, and X and anticoagulant proteins C and S. Ultimately, this leads to under-carboxylation of factors secreted by the liver and reduction in their biologic activity (VKDF activity 30–15 % corresponding to INR 2–3, respectively) [18].
Target-Specific Oral Anticoagulants
TSOAs are being prescribed with increasing frequency because of their predictable pharmacokinetics and convenience, afforded by the elimination of routine monitoring. According to a recent meta-analysis involving major randomized trials, TSOAs demonstrate a greater than 50 % (OR 0.49; 95 % CI 0.36–0.65) reduction in the incidence of ICH relative to patients receiving VKAs for non-valvular atrial fibrillation [19•]. Although representing a significant decrease in the rate of ICH, the incidence in patients taking TSOAs for atrial fibrillation (AF) is still substantial with clinical trials demonstrating that annually, 0.2 to 0.5 % develop AICH [20].
Dabigatran
After oral administration, the prodrug dabigatran etexilate (Pradaxa; Boehringer Ingelheim, Ingelheim am Rhein, Germany) undergoes absorption and rapid transformation by esterase within the gastrointestinal mucosa, liver, and plasma to its active form, dabigatran (6–9 % of the original drug). Peak plasma concentrations occur within 1 to 3 h. Dabigatran exerts its anticoagulant effects through directly binding to active sites of both clot-bound and free thrombin, inhibiting the conversion of fibrinogen to fibrin and thrombin-mediated platelet activation. Approximately 85 % of dabigatran is eliminated by the kidneys with a half-life of 12 to 17 h in patients with normal renal function [21•].
Rivaroxaban
Following administration, rivaroxaban (Xarelto; Janssen Pharmaceuticals, Beerse, Belgium) undergoes rapid absorption with peak plasma concentrations occurring in 2.5 to 4 h. Anticoagulation with rivaroxaban occurs through the inhibition of activated factor X (FXa). The kidneys eliminate 66 % of rivaroxaban with the remaining 33 % undergoing hepatic metabolism and excretion in the feces with a half-life of 9 to 13 h in patients with normal renal function [21•].
Apixaban
Apixaban (Eliquis, Pfizer and Bristol-Meyers Squibb, New York, NY, USA) like rivaroxaban selectively inhibits FXa. It achieves peak plasma concentrations in 1 to 3 h following administration and demonstrates a half-life of 8 to 15 h. Of the three TSOAs, apixaban exhibits the smallest degree of renal excretion at 25 % with the remaining 75 % being excreted in the feces [21•].
Heparins
Heparins are the most commonly used parenteral anticoagulants within the hospital setting. The use of heparin may increase the risk of major bleeding up to 2 %, corresponding with the patient’s concurrent medications, underlying diseases (especially compromised renal function), and the intensity of anticoagulation [22].
Unfractionated Heparin
Unfractionated heparin (UFH) is a heterogeneous mixture of sulfated glycosaminoglycans. The lengths of UFH polymers vary, with molecular weights ranging from 5000 to 30,000 Da (mean 15,000 Da). UFH exerts its anticoagulant effects through a unique pentasaccharide chain that binds to antithrombin (AT), potentiating the inhibition of thrombin (FIIa) and, to a lesser extent, factor Xa [23]. UFH has an immediate onset of action following IV injection. At low doses (25 units/kg), UFH undergoes rapid, zero-order metabolism by the reticuloendothelial system, resulting in a half-life of 30 min. This zero-order mechanism is saturable, and at high doses (400 units/kg), UFH undergoes slower, first-order clearance by the kidneys, with a half-life of 150 min [24].
Low Molecular Weight Heparin
Low molecular weight heparin (LMWH) is derived from the chemical or enzymatic depolymerization of UFH, yielding polymers ranging from 2000 to 5000 Da that predominately inhibit factor Xa activity [23]. Following subcutaneous injection, peak plasma concentrations are seen at 3 to 5 h with a half-life of 4.5 to 7 h. LMWH is excreted entirely by the kidneys [24].
Effects of Anticoagulants on Laboratory Tests
Vitamin K Antagonists
The anticoagulant effects of VKAs are monitored by the INR. The INR value is derived from the patients’ measured prothrombin time (PT) (INR = (PTmeasured / PTmean)International sensitivity index). This calculated value corrects for variations in the sensitivities of thromboplastins toward VKDF levels in various PT reagents. However, the utility of this value is limited in patients with ST-INRs. A study correlating INR values with VKDF levels illustrated that INR values >5 demonstrate a poor linear relationship with concentrations of VKDF (II, VII, X) [25]. Thus, in accordance with the American College of Chest Physicians (AACP) guidelines, clinical considerations should ultimately dictate the management of patients with AICH as opposed to INR values [14•].
Target-Specific Oral Anticoagulants
Although TSOAs do not require monitoring in routine clinical practice, the laboratory measurements of their anticoagulant effects may prove useful in certain clinical situations including patients presenting with TAICH. The majority of data evaluating the effects of TSOAs on laboratory tests are from studies using plasma from either human volunteers or animals spiked with TSOAs or plasma from volunteers receiving TSOAs.
Qualitative Assays
Dabigatran demonstrates a curvilinear dose-response with activated partial thromboplastin time (APTT). As concentrations of dabigatran increase, APTT values plateau [21•]. This relationship makes APTT unsuitable for quantifying dabigatran concentrations. Therapeutic concentrations of dabigatran will typically prolong APTT. However, studies have shown that patients receiving dabigatran at doses of 150 mg twice daily can demonstrate APTT values within normal limits [26]. Therefore, each laboratory should evaluate the sensitivity of its own APTT reagent toward dabigatran. The thrombin time (TT) is exceedingly sensitive to dabigatran; therefore, a normal TT may be used to rule out the presence of therapeutic levels of dabigatran [27•, 28].
Therapeutic concentrations of rivaroxaban will typically prolong PT. However, PT clotting times are influenced by the sensitivity of reagents used with some reagents demonstrating a normal PT in the ex vivo samples from patients with therapeutic concentrations of rivaroxaban [29]. Therefore, each laboratory should evaluate the sensitivity of its PT reagent toward rivaroxaban. Alternatively, a recent study evaluating thrombelastography (TEG) in patients receiving rivaroxaban for ischemic stroke was predictive of its anticoagulant effects with variables drawn at 2, 4, and 6 h demonstrating slowed clot formation (prolonged R time and k time; reduced α angle) and lower clot strength (reduced G and maximum amplitude) [30•].
Both the PT and APTT have limited sensitivity to plasma concentrations of apixaban, and accordingly, they should not be used for the qualitative assessment of apixaban [27•]. However, a new rotational thromboelastometry (ROTEM) modification, low tissue factor ROTEM, has demonstrated a dose-dependent relationship with a wide range of both apixaban and rivaroxaban drug concentrations (50–400 ng/mL) and may prove useful in the qualitative testing of AICH [31•].
Quantitative Assays
According to published guidelines, the tests deemed appropriate for quantifying dabigatran plasma concentrations include the chromogenic anti-IIa, dilute thrombin time, and ecarin clotting time (ECT) [27•]. Depending on the methodology used, thrombin-based assays will be influenced by samples containing heparin. Likewise, ECT is affected by plasma concentrations of prothrombin.
Guidelines recommend the use of chromogenic anti-Xa assays for quantifying plasma concentrations of rivaroxaban and apixaban because they have demonstrated greater sensitivity and specificity relative to routine tests (i.e., PT, APTT) [27•].
Heparin
Stemming from the tendency of larger polymers of UFH to bind to plasma proteins (acute-phase reactants von Willebrand factor or fibrinogen) and activated cells (platelets and endothelium), a given patient’s anticoagulant response to UFH is exceedingly variable. Other contributing factors include the fact that UFH clearance and activity are dependent upon the size of heparin polymers. Larger polymers are cleared first leading to reduced inhibition of thrombin [23]. Consequently, patients receiving UFH therapy require frequent APTT measurements. The heparin sensitivity of APTT reagents demonstrates significant variability; therefore, each laboratory must establish its therapeutic range. The chromogenic anti-Xa activity is more reliable than APTT for assessing anticoagulant effects of UFH (therapeutic range 0.3–0.7 U/mL).
LMWH exhibits less binding to plasma proteins and cells relative to unfractionated heparin, leading to greater predictability in the anticoagulant response, and ultimately eliminates the need for routine monitoring. LMWH predominately inhibits the activity of FXa, and consequently, the PTT remains mostly unaffected by therapeutic doses. LMWH anticoagulant activity is measured by chromogenic anti-Xa assay (therapeutic range 0.5–1.0 U/mL).
Anticoagulant Reversal
Anticoagulant reversal strategies are summarized in Table 2.
Vitamin K Antagonists
VKA therapy produces a deficit in the concentrations of functioning VKDFs. Reversal strategies involve the replenishment of VKDFs to normalize hemostasis and avert hematoma expansion. Treatment options include vitamin K, plasma, and prothrombin complex concentrate (PCC).
Vitamin K
The immediate infusion of coagulation factor-containing products (PCC or plasma) leads to the rapid replacement of VKDFs. However, the correction in functional factors is only transient due to the short half-life of FVII (6 h) and other coagulation factors (36–48 h) relative to VKAs (20–60 h). Sustained reversal of VKAs requires the use of vitamin K1 (phytonadione). Vitamin K is available in subcutaneous (SC), oral, and intravenous (IV) formulations. The IV administration of vitamin K is more rapid and demonstrates a greater consistency in reversing anticoagulation (effect seen within 2–4 h) relative to both SC and oral forms (>12 h) [32, 33]. Accordingly, IV administration is the preferred route for reversing the coagulopathy of AICH.
Current AACP guidelines recommend rapid administration of PCC along with concomitant slow (over 30 min) IV infusion of 5–10 mg of IV vitamin K for AICH [14•]. A threefold reduction in the 7-day mortality of AICH patients has been demonstrated when both PCC and vitamin K are given within 8 h of presentation [34••].
Although correction typically occurs prior to 24 h, the INR should be reevaluated before this time with consideration of the second administration of IV vitamin K for consistently prolonged INR values.
Plasma
Plasma is derived from whole blood or plasma collected by apheresis techniques and frozen at −18 °C within 8 h to label as fresh-frozen plasma or within 24 h to label as frozen plasma 24. On average, 1 unit of plasma contains a little less than 1 IU/mL of each clotting factor. Thus, the transfusion of plasma can reverse anticoagulation through replenishment of all VKDFs.
Prior to transfusion, plasma must be matched for ABO blood type and frozen units must be thawed at 30–37 °C, a process that may take up to 30 min. These thawed units are then issued, transported, and transfused at varying rates. The necessary steps required to prepare and ultimately infuse plasma are time consuming and represent a considerable delay in treatment. Evidence suggests that an incomplete or delayed reversal in anticoagulation has significant impacts on the mortality and the long-term outcomes of patients [11]. One study evaluating treatment modalities in patients presenting with VKA-associated ICH demonstrated a 20 % decrease in the odds ratio of INR reversal within the first 24 h for each 30-min delay in the first dose of plasma [35].
Recommendations regarding plasma dosage vary. In a patient with ST-INR of >5 (FII and X <10 %), the rapid transfusion of 20–30 mL/kg of plasma (assuming factor concentrations 1 IU/mL) would be necessary to increase factors by 30–50 %. In an 80-kg-weight patient, this equates to an approximate plasma volume of 1600–2400 mL (5–8 units). These large volumes place patients at risk for transfusion-associated circulatory overload (TACO), posing problems in patients with volume limitations secondary to compromised cardiac or renal function [36]. Moreover, plasma has demonstrated an increased incidence of transfusion-related acute lung injury (TRALI) in critically ill patients [37]. Plasma also has the potential to transmit infectious diseases including hepatitis B virus, hepatitis C virus, and HIV.
Despite its shortcomings and the lack of data demonstrating efficacy of its use in hemorrhagic conditions, plasma is still widely used within the USA [2].
Prothrombin Complex Concentrate
PCCs are derived from the pooled human plasma in a manufacturing process involving both protein purification and viral inactivation via heat treatment, solvent detergent treatment, or nanofiltration. All manufactured formulations contain varying concentrations of factors II, VII, IX, and X. There are two types of PCCs, activated and nonactivated. Activated PCC (aPCC) contain activated factors VII and X and are used in the treatment of acute bleeding episodes in hemophilia A or B, who have developed antibodies against FVIII or IX. Non-activated three-factor PCC contains significant concentrations of factors II, IX, and X with only minimal concentrations of factor VII. Nonactivated four-factor PCC contains significant concentrations of all vitamin K-dependent factors (II, VII, IX, X, C, and S). A four-factor PCC (4F-PCC; Kcentra™, CSL Behring, King of Prussia, PA, USA) was recently licensed by the FDA for urgent VKA reversal in patients presenting with acute major bleeding or requiring urgent surgical intervention.
PCC comes as a lyophilized powder that can be reconstituted and available for infusion within minutes. Unlike plasma, PCC does not require ABO typing or thawing. Elimination of these steps represents a significant reduction in the time to initiation of treatment. In addition, the infusion volumes of PCC required for anticoagulant reversal are notably smaller than plasma (median, 99.4 mL 4F-PCC vs. 8135.5 mL plasma) and can be transfused over a shorter period (median, 17.0 min 4F-PCC vs. 148.0 min plasma) [16••]. Approximately 10 mL of PCC corresponds with 250 mL of plasma (≅1 unit). This small infusion volume mitigates concerns for volume overload, especially in patients with cardiac or renal compromise. Complete and rapid correction of the INR to <1.5 occurs within 10–130 min following administration of PCC [38]. The INR should be reevaluated 30 min following administration of PCC with consideration of an additional dose for consistently prolonged INR values (>1.5) after first excluding low levels of fibrinogen as the cause, which may indicate a rare instance of florid-disseminated intravascular coagulation.
In a recent randomized clinical trial, the hemostatic efficacy of PCC was demonstrated to be as good as plasma when utilized for urgent VKA reversal in patients presenting with major bleeding. Patients receiving PCC also showed rapid correction of INR and reconstitution of all VKDFs. Moreover, similar rates of thromboembolic events were observed when comparing four-factor PCC to plasma. However, patients who received plasma exhibited a higher incidence of volume overload [16••].
The growing body of evidence evaluating the safety and efficacy of PCC has led to widespread support for its use as the standard of care. Underscoring this support are the various professional organizations that endorse PCC use for patients requiring urgent VKA reversal [14•, 39–41].
Target-Specific Oral Anticoagulants
The current principles underlying appropriate management of TAICH are based upon experiences treating patients with VKA-AICH. At present, it is unknown if hematoma expansion occurs in patients receiving TSOAs who presents with AICH, although studies evaluating anticoagulation with dabigatran or rivaroxaban in murine models have shown greater hematoma expansion relative to non-anticoagulated mice [42, 43••].
Similar to the management of VKA-AICH, the TSOA in question should be discontinued immediately. TSOAs have a much shorter half-lives compared to VKAs, and thus, the normalization of hemostasis occurs rapidly following their discontinuation. If possible, appropriate laboratory studies should be conducted to assess the extent of anticoagulation.
In patients presenting within 2 to 3 h of dabigatran ingestion administration of orally activated charcoal may limit gastrointestinal absorption [44]. In addition, dabigatran is mostly free in the plasma (65 %), thus making it a suitable candidate for dialysis especially in patients with significant renal dysfunction. In patients with end-stage renal disease, treated with 50 mg of dabigatran, up to 68 % of dabigatran was removed following 4 h of dialysis [45]. Because rivaroxaban and apixaban are highly protein bound (65 %), plasma exchange would be a more appropriate consideration. The benefits of these extracorporeal therapies are limited by the constraints of central venous access placement in the setting of a fully anticoagulated patient.
Hemostatic Agents
Presently, no specific antidotes are available to reverse the anticoagulant effects of TSOAs. Numerous studies evaluating the efficacy of nonspecific hemostatic agents have been performed using animal models or non-bleeding, healthy volunteers. However, the design and outcomes of these studies are heterogeneous and difficult to interpret. Currently, there are no well-designed, high-quality studies to confirm the safety and efficacy of nonspecific hemostatic agents for reversing the anticoagulant effects of TSOAs in bleeding patients [46•].
Prothrombin Complex Concentrate
Nonactivated PCC could serve as a possible treatment modality used to reverse the anticoagulant effects of TSOAs. Infusion of PCC provides an excess of factors II and X that can potentially enhance the generation of thrombin and FXa and overcome the anticoagulant effects of dabigatran and FXa inhibitors, respectively [47].
In one in vivo study, mice were given dabigatran (4.5 or 9.0 mg/kg), and ICH was induced by striatal collagenase injection. Mice that were administered a 4F-PCC (100 U/kg) demonstrated a reduction in hematoma expansion and mortality, while recombinant factor VIIa (8.0 mg/kg) failed to reduce either [42]. In a similar murine model, ICH hematoma expansion was observed following the administration of rivaroxaban (30 mg/kg). The two highest doses of PCC (50 and 100 U/kg), rFVIIa (1 mg/kg), and plasma were found to be equal in reducing the extent of hematoma expansion and the degree of neurologic deficit. Although the lowest dose of PCC (25 U/kg) did not demonstrate a significant reduction in hematoma volumes, it was still found to improve neurologic outcomes [43••].
In patients receiving TSOAs, coagulation tests have shown a limited value in predicting the incidence of bleeding following the use of hemostatic agents [48]. However, human studies evaluating the effects of PCC on TSOA-induced anticoagulation are primarily based on these surrogate markers of hemostasis. In a randomized, double-blinded, placebo-controlled, phase I study, healthy male volunteers received rivaroxaban (20 mg once or twice daily) or dabigatran (150 mg twice daily) followed by treatment with PCC (Cofact, 50 U/kg) or normal saline. In volunteers receiving rivaroxaban, PCC corrected both the endogenous thrombin potential (ETP) and the PT. In volunteers receiving dabigatran, PCC exhibited no correction in the ECT, APTT, ETP, or TT [49]. In a cohort study, patients receiving dabigatran (110 mg) complicated by intestinal bleeding were administered 4F-PCC (15–25 IU/kg) with demonstration of adequate clinical control in four out of the five patients without significantly affecting INR values (1.8 ± 1.2 to 1.2 ± 0.2) or the APTT ratio (2.4 ± 1.5 to 1.5 ± 0.6) [50].
Activated Prothrombin Complex Concentrate
aPCC is used to treat bleeding in hemophiliac patients who have developed inhibitors against FVIII and FIX. Similarly, direct thrombin (dabigatran) or factor Xa inhibitors (rivaroxaban or apixaban) can be likened to inhibitors found in patients with acquired hemophilia. Thus, principles underlying the use of aPCC in acquired hemophilia can be applied to its use in the treatment of TAICH.
In one in vivo study, rats were given high-dose rivaroxaban (2 mg/kg; 30 times greater than standard human concentration) followed by incision of mesenteric vessels. Rats receiving the highest doses of aPCC (100 U/kg), PCC (50 U/kg), or rFVIIa (400 μg/kg) demonstrated significantly reduced mesenteric bleeding times. Partial normalization of PT occurred with aPCC (50 and 100 U/kg), PCC (50 U/kg), or rFVIIa (400 μg/kg). Complete correction of thrombin-antithrombin complex (TAT) was seen with the highest dose of PCC (50 U/kg) with only partial correction using aPCC (50 and 100 U/kg), and no correction demonstrated rFVIIa (100 and 400 μg/kg) [51].
A recent case report describes the use of aPCC (27.5 U/kg) in a patient presenting with dabigatran-associated intracerebral hemorrhage. Following aPCC administration, the patient exhibited improvement on neurological examination but demonstrated intraventricular hematoma expansions and the APTT failed to normalize [52].
Recombinant Factor VIIa
Recombinant factor VIIa initiates the formation of thrombin through the activation of factor X. Much like aPCC, it is commonly used in hemophiliacs following the development of inhibitors to ameliorate bleeding episodes. However, based upon current data, the therapeutic benefit of rFVIIa in reversing TSOA-related anticoagulation is unclear [49].
Selective Reversal Agents
Efforts to develop selective reversal agents are ongoing with early data from phase 2 clinical trials having generated promising results. An antibody fragment (Fab) designed to target and neutralize dabigatran is under development by Boehringer Ingelheim [53••]. Another candidate reversal agent, andexanet alfa, is a catalytically inactive analog of FXa, designed to bind small-molecule FXa inhibitors (rivaroxaban and apixaban) and reduce their capacity to inactivate endogenous factor Xa [54••].
Published data have demonstrated the efficacy of these specific antidotes within animal models and in vitro settings. Unfortunately, their safety and efficacy has not yet been evaluated in bleeding patients.
Heparin
Protamine has been widely used to neutralize the anticoagulant effects of UFH for over 30 years. Protamine sulfate is a positively charged polypeptide that avidly binds the acidic polymer of heparin forming an inactive salt that undergoes rapid clearance by the reticuloendothelial system [55]. Care must be taken when calculating the dose of protamine sulfate, due to its inherent anticoagulant properties when given in excess. Therefore, it is recommended that a dose needed to neutralize 80 % of the anticipated units of UFH should be administered within the previous 2 h (maximum protamine dose of 50 mg). The neutralization of UFH should be followed by APTT.
Protamine only partially neutralizes the anticoagulant effects of LMWH with reports in the return of only 60 % of FXa activity following treatment [56]. If the last dose of enoxaparin was administered <8 h, we would recommend 1 mg protamine for every 1 mg LMWH (maximum protamine dose of 50 mg). If the last enoxaparin dose occurred between 8 and 24 h ago, we recommend 0.5 mg protamine for every 1 mg LMWH (maximum protamine dose of 50 mg). The neutralization of LMWH should be followed by an anti-Xa assay.
Conclusion
The clinical use of anticoagulants in the management of thromboembolic disorders is increasing. Intracerebral hemorrhage remains to be the most feared complication of oral anticoagulant therapy. Until specific antidotes become available for TSOAs, we recommend the use of 4F-PCC in all cases of AICH. This allows for the correction of VKDFs in VKA-associated ICH and also provides a rapid increase of FII and X for possible generation of excess IIa and Xa, binding to direct IIa and Xa inhibitors, respectively.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414–9.
Magee G, Peters C, Zbrozek A. Analysis of inpatient use of fresh frozen plasma and other therapies and associated outcomes in patients with major bleeds from vitamin K antagonism. Clin Ther. 2013;35:1432–43. Elsevier.
Freedman JE, Gersh BJ. Atrial fibrillation and stroke prevention in aging patients: what’s good can be even better. Circulation. 2014;130:129–31.
Veltkamp R, Rizos T, Horstmann S. Intracerebral bleeding in patients on antithrombotic agents. Semin Thromb Hemost. 2013;39:963–71.
Virjo I, Mäkelä K, Aho J, Kalliola P, Kurunmäki H, Uusitalo L, et al. Who receives anticoagulant treatment with warfarin and why? A population-based study in Finland. Scand J Prim Health Care. 2010;28:237–41.
Flaherty ML, Kissela B, Woo D, Kleindorfer D, Alwell K, Sekar P, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–21.
Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–44.
Towfighi A, Greenberg SM, Rosand J. Treatment and prevention of primary intracerebral hemorrhage. Semin Neurol. 2005;25:445–52.
Bechtel BF, Nunez TC, Lyon JA, Cotton BA, Barrett TW. Treatments for reversing warfarin anticoagulation in patients with acute intracranial hemorrhage: a structured literature review. Int J Emerg Med. 2011;4:40. Springer Open Ltd.
Flaherty M, Tao H, Haverbusch M. Warfarin use leads to larger intracerebral hematomas. Neurology. 2008;71:1084–9.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44. Elsevier Ltd.
Huttner HB, Schellinger PD, Hartmann M, Köhrmann M, Juettler E, Wikner J, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–70.
Schaefer JH, Leung W, Wu L, Van Cott EM, Lok J, Whalen M, et al. Translational insights into traumatic brain injury occurring during dabigatran or warfarin anticoagulation. J Cereb Blood Flow Metab. 2014;34:870–5. Nature Publishing Group.
Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e152S–84. The most recent guidelines from the American College of Chest Physicians summarizing recommendations for the management of antithrombotic therapy.
Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141:745–52.
Sarode R, Milling TJ, Refaai MA, Mangione A, Schneider A, Durn BL, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–43. A randomized multicenter trial investigating the safety and efficacy of 4F-PCC for patients with warfarin-associated coagulopathy. Trial results led to the approval of 4F-PCC in the United States for urgent warfarin reversal.
Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke. 2006;37:256–62.
Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, Ansell J, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest J. 2001;119:8S–21. American College of Chest Physicians.
Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol. 2013;70:1486–90. A meta-analysis of randomized clinical trials evaluating the incidence of intracranial hemorrhage in patients receiving warfarin and TSOAs.
Hankey GJ. Intracranial hemorrhage and novel anticoagulants for atrial fibrillation: what have we learned? Curr Cardiol Rep. 2014;16:480.
Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29:S24–33. This article provides an overview of TSOAs and their pharmacologic characteristics.
Suryanarayan D, Schulman S. Potential antidotes for reversal of old and new oral anticoagulants. Thromb Res. 2014;133(Suppl):S158–66.
Weitz DS, Weitz JI. Update on heparin: what do we need to know? J Thromb Thrombolysis. 2010;29:199–207.
Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the seventh ACCP conference on antithrombotic and thrombolytic therapy heparin and low-molecular-weight heparin. Chest. 2004;126:188S–203.
Sarode R, Rawal A, Lee R, Shen Y-M, Frenkel EP. Poor correlation of supratherapeutic international normalised ratio and vitamin K-dependent procoagulant factor levels during warfarin therapy. Br J Haematol. 2006;132:604–7.
Hawes EM, Deal AM, Funk-Adcock D, Gosselin R, Jeanneret C, Cook AM, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost. 2013;11:1493–502.
Kitchen S, Gray E, Mackie I, Baglin T, Makris M, committee the B. Measurement of non-Coumarin anticoagulants and their effects on tests of haemostasis: guidance from the British Committee for Standards in Haematology. Br J Haematol. 2014;166:830–41. The most recent guidelines from the British Committee for Standards in Haematology characterizing appropriate tests for evaluating the effects of non-Coumarin anticoagulants.
Baglin T. The role of the laboratory in treatment with new oral anticoagulants. J Thromb Haemost. 2013;11:122–8.
Mueck W, Lensing AWA, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675–86.
Bowry R, Fraser S, Archeval-Lao JM, Parker SA, Cai C, Rahbar MH, et al. Thrombelastography detects the anticoagulant effect of rivaroxaban in patients with stroke. Stroke. 2014;45:880–3. This article characterizes the use of thrombelastography to detect the anticoagulant effects of rivaroxaban in patients presenting with ischemic stroke.
Adelmann D, Marion W, Wohlgemuth RK, Koch S, Frantal S, Quehenberger P, et al. Measuring the activity of apixaban and rivaroxaban with rotational thrombelastometry. Thromb Res. 2014;134(4):918–23. This article characterizes the use of rotational thrombelastography for detecting anticoagulant effects in patients receiving varying doses of either rivaroxaban or apixaban.
Dezee KJ, Shimeall WT, Douglas KM, Shumway NM, O’malley PG. Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis. Arch Intern Med. 2006;166:391–7.
Watson HG, Baglin T, Laidlaw SL, Makris M, Preston FE. A comparison of the efficacy and rate of response to oral and intravenous vitamin K in reversal of over-anticoagulation with warfarin. Br J Haematol. 2001;115:145–9.
Tazarourte K, Riou B, Tremey B, Samama C-M, Vicaut E, Vigué B. Guideline-concordant administration of prothrombin complex concentrate and vitamin K is associated with decreased mortality in patients with severe bleeding under vitamin K antagonist treatment (EPAHK study). Crit Care. 2014;18:R81. A prospective observational study of patients presenting with vitamin K-antagonist-associated severe bleeding, demonstrating that administration of vitamin K and PCC within 8 h of presentation resulted in a threefold decrease in the 7-day mortality of ICH.
Goldstein JN, Thomas SH, Frontiero V, Joseph A, Engel C, Snider R, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151–5.
Li G, Rachmale S, Kojicic M, Shahjehan K, Malinchoc M, Kor DJ, et al. Incidence and transfusion risk factors for transfusion-associated circulatory overload among medical intensive care unit patients. Transfusion. 2011;51:338–43.
Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52 Suppl 1:65S–79.
Bershad EM, Suarez JI. Prothrombin complex concentrates for oral anticoagulant therapy-related intracranial hemorrhage: a review of the literature. Neurocrit Care. 2010;12:403–13.
Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. Guidelines on oral anticoagulation with warfarin—fourth edition. Br J Haematol. 2011;154:311–24.
Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS. An update of consensus guidelines for warfarin reversal. Med J Aust. 2013;198:1–7.
Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17:R76. BioMed Central Ltd.
Zhou W, Schwarting S, Illanes S, Liesz A, Middelhoff M, Zorn M, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–9.
Zhou W, Zorn M, Nawroth P, Bütehorn U, Perzborn E, Heitmeier S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke. 2013;44:771–8. This article characterizes an experimental mouse model of rivaroxaban-associated cerebral hemorrhage and the effects of various hemostatic agents on intracerebral hematoma expansion.
Van Ryn J, Stangier J, Haertter S, Liesenfeld K-H, Wienen W, Feuring M, et al. Dabigatran etexilate-a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116.
Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate. Clin Pharmacokinet. 2010;49:259–68.
Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost. 2014;111:189–98. This article provides a comprehensive review of data regarding the use of PCC for reversing the anticoagulant effects of TSOAs.
Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of Beriplex P/N prothrombin complex concentrate in healthy volunteers. Thromb Haemost. 2007;98:790–7.
Kaatz S, Kouides PA, Garcia DA, Spyropolous AC, Crowther M, Douketis JD, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87:S141–5. Wiley Subscription Services, Inc., A Wiley Company.
Lazo-Langner A, Lang E, Douketis J. Clinical review: clinical management of new oral anticoagulants: a structured review with emphasis on the reversal of bleeding complications. Crit Care. 2013;17:230.
Díaz MQ, Borobia AM, Núñez MAR, Virto AMM, Fabra S, Casado MS, et al. Use of prothrombin complex concentrates for urgent reversal of dabigatran in the emergency department. Haematologica. 2013;98:e143–4.
Perzborn E, Gruber A, Tinel H, Marzec UM, Buetehorn U, Buchmueller A, et al. Reversal of rivaroxaban anticoagulation by haemostatic agents in rats and primates. Thromb Haemost. 2013;110:162–72.
Faust AC, Peterson EJ. Management of dabigatran-associated intracerebral and intraventricular hemorrhage: a case report. J Emerg Med. 2014. doi:10.1016/j.jemermed.2013.11.097.
Schiele F, Van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–62. This article characterizes the first specific antidote for dabigatran, aDabi-Fab, demonstrating reversal of the anticoagulant effects in human plasma in vitro and in rats in vivo.
Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446–51. This article characterizes the study of a specific antidote for factor Xa inhibitors, andexanet alfa, demonstrating its ability to reverse the anticoagulant effects of both fondaparinux and enoxaparin.
Carr JA, Silverman N. The heparin-protamine interaction. A review. J Cardiovasc Surg (Torino). 1999;40:659–66.
Makris M, Van Veen JJ, Tait CR, Mumford AD, Laffan M. Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol. 2013;160:35–46.
Compliance with Ethics Guidelines
Conflict of Interest
Sean Yates declares no conflict of interest.
Ravi Sarode is a consultant for CSL Behring and Octapharma.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Stroke
Rights and permissions
About this article
Cite this article
Yates, S., Sarode, R. Reversal of Anticoagulant Effects in Patients with Intracerebral Hemorrhage. Curr Neurol Neurosci Rep 15, 504 (2015). https://doi.org/10.1007/s11910-014-0504-2
Published:
DOI: https://doi.org/10.1007/s11910-014-0504-2