Abstract
Purpose of Review
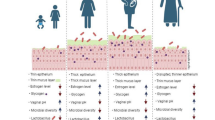
The vaginal milieu in women differs from that of other mammals, including non-human primates, in composition of secretions, the endogenous microbiota, and level of acidity. These changes apparently reflect evolutionary variations that maximized productive responses to a uniquely human vaginal environment. This review will highlight recent findings on properties of human vaginal epithelial cells that contribute to maintenance of a healthy vaginal environment.
Recent Findings
Vaginal epithelial cells are responsive to the composition of the vaginal microbiome even in women who are in apparently good health and do not exhibit any adverse physical symptoms. This is especially important during pregnancy when immune defenses are modified and an effective epithelial cell-derived anti-microbial activity is essential to prevent the migration to the uterus of bacteria potentially harmful to pregnancy progression. When Lactobacillus crispatus numerically predominates in the vagina, epithelial cell activity is low. Conversely, predominance of Lactobacillus iners, Gardnerella vaginalis, or other non-Lactobacilli evokes production and release of a large variety of compounds to minimize the potentially negative consequences of an altered microbiome. The extent of autophagy in vaginal epithelial cells, a basic process that functions to maintain intracellular homeostasis and engulf microbial invaders, is also sensitive to the external microbial environment Vaginal epithelial cells bind and release norepinephrine and upregulate their anti-microbial activity in response to external stress.
Summary
Vaginal epithelial cells in women are responsive to local conditions that are unique to humans and, thereby, contribute to maintenance of a healthy milieu.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vaginal microbiome in most reproductive age women is dominated by species of Lactobacilli and vaginal fluid is acidic due to production of lactic acid by these bacteria [1••]. This differs from what is present in all other mammals, including non-human primates, where Lactobacilli are scarce and vaginal pH approaches neutrality. These changes are likely a consequence of the unique behavioral, dietary, and environmental exposures of humans [2, 3]. Vaginal epithelial cells in women have had to adapt to these species differences to optimize a lower genital tract environment most compatible with health and reproductive efficacy. This communication will review properties of human vaginal epithelial cells that monitor and respond to external variations in the local environment.
Epithelial Cell Turnover and Survival in an Acidic Environment
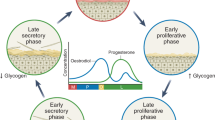
Several layers of rapidly regenerating stratified squamous epithelial cells line the human vagina. The layer closest to the vaginal lumen exfoliates approximately every 4 h [4]. This serves a protective function as any potential microbial pathogen that have gained entry and adhered to the vaginal epithelium will be rapidly disengaged from the vagina and eliminated. In addition, the degradation of sloughed epithelial cells results in the release of glycogen into the vaginal lumen. The breakdown of glycogen by alpha amylase, an enzyme present in human vaginal secretions [5], results in production of small carbohydrates that are utilized by Lactobacilli to preferentially facilitate their proliferation in the vagina [6].
Resident Lactobacilli are the principal source of lactic acid, the major acid in the vagina [7, 8], responsible for maintaining an acidified vaginal lumen. The acidic pH lyses many non-endogenous bacterial species and prevents their maintenance at this site [9, 10]. To remain viable in this acidic environment, the vaginal epithelial cells must be able to regulate the influx and egress of hydrogen ions. This is accomplished by the activity of a protein, monocarboxylate transporter 1 (MCT-1), on the epithelial cell surface. MCT-1 activity requires the presence of an essential co-factor, extracellular matrix metalloproteinase inducer (EMMPRIN) [11]. EMMPRIN is also produced by vaginal epithelial cells and its concentration in vaginal fluid is directly proportional to the lactic acid level [8]. This suggests that the vaginal epithelial cells are sensitive to the level of acidity and respond by adjusting their production of EMMPRIN.
Epithelial Cell Recognition of Bacteria
The dominant Lactobacilli species in most reproductive age women are Lactobacillus crispatus and L. iners. The dominant non-Lactobacillus species is usually Gardnerella vaginalis [12]. Recent studies have demonstrated that the expression of genes in vaginal epithelial cells differs according to the dominant bacterium that is present. One mechanism regulating gene transcription is the epigenetic alteration of the acetylation state of histones that are associated with a particular gene. Acetylated histones do not bind as tightly to chromosomes as do histones whose acetyl groups have been removed. A specific enzyme, histone deacetylase (HDAC), removes acetyl groups from histones and, thereby, inhibits gene activation [13]. In a study of 150 pregnant women, the HDAC concentration in vaginal epithelial cells was lowest when L. crispatus was the dominant vaginal bacterium and significantly elevated when non-Lactobacilli dominated the microbiome [14]. The HDAC level was positively associated with the concentration of matrix metalloproteinase-8 (MMP-8) and the stress-inducible 70 kDa heat shock protein (hsp70) in vaginal secretions. Many studies have concluded that L. crispatus numerical dominance is associated with vaginal health and maintenance of conditions conducive to successful pregnancy progression and outcome [15, 16]. Thus, it appears that the differential regulation of HDAC production by vaginal epithelial cells in response to the bacterium that is dominant in the vagina influences the production and release of specific bioactive compounds from these cells. Extracellular hsp70 is a potent inducer of pro-inflammatory immunity [17], while MMP-8 releases hyaluronan from the extracellular matrix leading to activation of anti-microbial immunity [18]. A second study has provided additional evidence of epigenetic gene regulation in vaginal epithelial cells by Lactobacilli. Expression of the gene coding for human beta defensin-1, DEFB1, in an immortalized vaginal epithelial cells line was shown to vary between different strains of Lactobacilli through species-specific alterations in DNA methylation and histone modification [19].
Production of other compounds by vaginal epithelial cells has also been shown to be sensitive to the bacterial milieu. Neutrophil gelatinase-associated lipocalin (NGAL) production is induced in these cells when Lactobacilli predominate [20]. This protein inhibits bacterial uptake of iron and, therefore, inhibits proliferation of those microorganisms that require iron for growth [21]. G. vaginalis is an iron-dependent bacterium [22], while Lactobacilli can proliferate in the absence of this element [23]. Other iron-binding compounds released from vaginal epithelial cells include lactoferrin [24] and calprotectin [25]. Thus, the capability of vaginal epithelial cells to influence the vaginal level of iron, and possibly other elements, will impact the likelihood of specific bacterial dominance at this site. A direct comparison of levels of compounds in vaginal fluid from pregnant women when either L. crispatus or L. iners was numerically dominant revealed that levels of NGAL and calprotectin, as well as other compounds involved in anti-microbial defense—stress-induced hsp70, MMP-8, and hyaluronan—were preferentially induced by L. iners [26]. This reinforces studies demonstrating that L. iners dominance in the vaginal microbiota is associated with induction of a stress response in the vaginal epithelium [27].
Other anti-microbial compounds differentially released by vaginal epithelial cells in response to the presence of specific microorganisms include secretory leukocyte protease inhibitor [28], mannose-binding lectin [29], beta defensins [30], and cathelicidins [31]. The surface of vagina epithelial cells contains a number of Toll-like receptors (TLR) that recognize invariant molecular patterns on microorganisms [32]. TLR-agonist binding results in the production of a large number of pro-inflammatory cytokines—interleukin (IL)-1, IL-6, IL-8, IL-12, and tumor necrosis factor-alpha [33, 34]. The release of these cytokines from in vitro-cultivated vaginal epithelial cells could not be demonstrated when either L. crispatus or L. jensenii was present. Furthermore, these two Lactobacillus strains inhibited epithelial cell cytokine expression when TLR agonists were added to the cultures [35]. This further highlights the sensitivity of vaginal epithelial cells to the local external environment and its influence on composition of the vaginal milieu.
Epithelial Cell Responses to Stress
Studies in a mouse model have demonstrated that maternal stress changes the composition of the vaginal microbiota, resulting in a decreased level of Lactobacilli [36]. Evidence for a possible involvement of vaginal epithelial cells in the stress-related alteration of vaginal microbial abundance comes from a recent study demonstrating that two human vaginal epithelial cell lines secreted both norepinephrine and dopamine. In addition, these cells recognized and bound exogenous norepinephrine [37]. Interestingly, norepinephrine by itself did not induce production of pro-inflammatory compounds from vaginal epithelial cells, but its presence resulted in a significant upregulation of the release of these mediators in the simultaneous presence of immune system activators. These observations led us to postulate a stress-vaginal dysbiosis relationship based on vaginal epithelial cell responses [1••]. Production of norepinephrine in woman experiencing prolonged stress results in its appearance in the vagina due to transduction from the circulation. Norepinephrine binding to vaginal epithelial cells coupled with its local production by these cells leads to the decrease or loss of Lactobacilli dominance in the vagina. The subsequent increased production of non-Lactobacilli and their induction of inflammation are enhanced by the presence of norepinephrine. Thus, disparities between individual women in the magnitude of their response to various stressors may determine their propensity to develop a symptomatic vaginal disorder. A strain of Lactobacillus, L. salivarius, has very recently been shown to possess receptors for the uptake of neuroactive biogenic amines [38••]. If Lactobacilli in the vagina possess a similar mechanism, still to be determined, then vaginal epithelial cell-Lactobacilli cross-communication may have even a more enhanced influence on the stress-associated vaginal environment.
The stress-induced reduction in the vaginal Lactobacillus level is also a consequence of the effect of cortisol on vaginal epithelial cells [39]. Elevations in cortisol as a result of prolonged stress inhibit glycogen deposition in the epithelial cells. This reduces the glycogen concentration in the vaginal lumen resulting in local conditions that are less than ideal for the preferential proliferation of Lactobacilli.
Vaginal Epithelial Cell Autophagy
Autophagy is a mechanism present in all multicellular organisms to maintain intracellular homeostasis [40]. Misfolded or aggregated proteins, aged mitochondria as well as fungi, bacteria and viruses that have entered the cytoplasm are engulfed in autophagosomes. The subsequent fusion with a lysosome results in degradation of the engulfed macromolecules or microorganisms by lysosomal enzymes and the resulting amino acids, nucleotides, carbohydrates, and lipids are returned to the cytoplasm for reutilization. The induction of a stress response results in upregulation of hsp70, an inhibitor of autophagy [41]. Unlike autophagy, which removes non-functional proteins, hsp70 binds to intracellular proteins to maintain their functional activity and prevent their misfolding [42]. This latter process appears to take precedence over autophagy under non-physiological conditions [43, 44]. A recent study evaluated the level of autophagy in vaginal epithelial cells from pregnant women in relation to bacterial dominance in the vaginal microbiome [45•]. It was determined that L. crispatus dominance was associated with the highest level of epithelial cell autophagy and the lowest intracellular hsp70 concentration. These levels were significantly different from what was observed when L. iners was dominant. The highest hsp70 level and lowest autophagy corresponded to Streptococcus and Bifidobacterium dominance. It thus appears that vaginal epithelial cells modulate their level of autophagy in response to the vaginal bacterial composition and the predominance of L. crispatus at this site results in the maintenance of an optimal intracellular environment. This may account, at least in part, for the association between L. crispatus vaginal dominance and normal pregnancy progression [15, 16]. A low level of autophagy has been associated with elevations in reactive oxygen species, possibly from defective or aged mitochondria, and induction of preterm birth [46]. In addition, vaginal epithelial cell homeostasis would maximize the ability of these cells to recognize and respond to potential pathogens by the induction of innate immunity [47].
Candida albicans that has penetrated into vaginal epithelial cells is sequestered into autophagosomes and destroyed by autophagy [48••]. Inhibition of autophagy in these cells permitted C. albicans to proliferate and kill the infected cell [49]. Conversely, the detection of Streptococci in vaginal secretions from pregnant women is followed by the inhibition of autophagy in vaginal epithelial cells and the upregulation of the autophagy inhibitor, hsp70 [45•, 50]. Thus, the differential capacity of microorganisms that are present in the vagina to either promote or inhibit autophagy modulates this anti-microbial defense mechanism in vaginal epithelial cells.
Conclusion
There is a symbiotic relationship between human vaginal epithelial cells and the resident vaginal microbiota in the exchange of macromolecules and creation of an environment that protects against the invasion and establishment of potential pathogens. Changes in the bacterial environment and the presence of factors that alter the vaginal microbiome induce differential gene activity and the upregulation by vaginal epithelial cells of compounds with anti-microbial and immune-inducing activity. Properties of vaginal epithelial cells that are responsive to variations in the local environment are summarized in Table 1. This adaptation to a milieu—namely vaginal acidity and Lactobacilli dominance—specific to humans maximizes conditions conducive to pregnancy progression.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Witkin SS, Linhares IM. Why do lactobacilli dominate the human vaginal microbiota? BJOG. 2017;124:606–11. This article highlights and summarizes why Lactobacilli dominance in the vagina is unique to humans.
Witkin SS, Ledger WJ. Complexities of the uniquely human vagina. Sci Transl Med. 2012;4:132fs11.
Stumpf RM, Wilson BA, Rivera A, Yidirim S, Yeoman CJ, Polk JD, et al. The primate vaginal microbiome: comparative context and implications for human health and disease. Am J Phys Anthropol. 2013;152:119–34.
Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AR, Eschenbach DA. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol. 2000;183:967–73.
Nasioudis D, Beghini J, Bongiovanni AM, Giraldo PC, Linhares IM. Witkin SS. α-amylase in vaginal fluid: association with conditions favorable to dominance of Lactobacillus. Reprod Sci. 2015;22(1):1393–8. https://doi.org/10.1177/1933719115581000rs.sagepub.com.
Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis. 2014;210(7):1019–28. https://doi.org/10.1093/infdis/jiu231.
O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One. 2013;8:e80074.
Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. Influence of vaginal bacteria and D- and L- lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. mBio. 2013;4(4):e00460–13.
O’Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200.
Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permealizes Gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000;66:2001–5.
Wilson MC, Meredith D, Fox JE, Manoharan C, Davies AJ, Halestrap AP. Besigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4; the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J Biol Chem. 2005;280:27213–21.
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–7.
Kelly RD, Cowley SM. The physiological roles of histone deacetylase (HDAC) 1 and 2: complex co-stars with multiple leading parts. Biochem Soc Trans. 2013;41:741–9.
• Witkin SS, Nasioudis D, Leizer J, Minis E, Boester A, Forney LJ. Epigenetics and the vaginal microbiome: influence of the microbiota on the histone deacetylase level in vaginal epithelial cells from pregnant women. Minerva Ginecol. 2019;71:171–5. https://doi.org/10.23736/S0026-4784.18.04322-8. This provides evidence that the vaginal microbiota exerts influence on vaginal epithelial cells by an epigenetic mechanism.
Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5(1):6. https://doi.org/10.1186/s40168-016-0223-9.
Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci U S A. 2017;114(37):9966–71. https://doi.org/10.1073/pnas.1705899114.
Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45.
Powell JD, Horton MR. Threat matrix: low-molecular weight hyaluronan (HA) as a danger signal. Immunol Res. 2005;31:207–18.
Lee J, Jang A, Kim JW, Han JH, Chun BH, Jung HS, et al. Distinct histone modifications modulate DEFB1 expression in human vaginal keratinocytes in response to Lactobacillus spp. Probiotics & Antimicro Prot. 2017;9:406–14. https://doi.org/10.1007/s12602-017-9286-6.
Beghini J, Giraldo PC, Linhares IM, Ledger WJ, Witkin SS. Neutrophil gelatinase- associated lipocalin concentration in vaginal fluid: relation to bacterial vaginosis and vulvovaginal candidiasis. Reprod Sci. 2015;22:964–8.
Nasioudis D, Witkin SS. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med Microbiol Immunol. 2015;204:471–9.
Jarosik GP, Land CB, Duhon P, Chandler R Jr, Mercer T. Acquisition of iron by Gardnerella vaginalis. Infect Immun. 1998;66:5041–7.
Inbert M, Blondeau R. On the iron requirement of lactobacilli grown in chemically defined medium. Curr Microbiol. 1998;37:64–6.
Rein MF, Shih LM, Miller JR, Guerrant RL. Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis. 1996;23:517–21.
Kostakis ID, Cholidou KG, Kallianidis K, Perrea D, Antsaklis A. The role of calprotectin in obstetrics and gynecology. Eur J Obstet Gynecol Reprod Biol. 2010;151:3–9.
Leizer J, Nasioudis D, Forney LJ, Schneider GM, Gliniewicz K, Boester A, et al. Properties of epithelial cells and vaginal secretions in pregnant women when. Lactobacillus crispatus or Lactobacillus iners dominate the vaginal microbiome. Reprod Sci. 2018;25(6):854–60. https://doi.org/10.1177/1933719117698583.
Vaneechoutte M. Lactobacillus iners, the unusual suspect. Res Microbiol. 2017;168(9–10):826–36. https://doi.org/10.1016/j.resmic.2017.09.003.
Draper DL, Landers DV, Krohn MA, Sl H, Wiesenfeld HC, Heine RP. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am J Obstet Gynecol. 2000;183:1243–8.
Bulla R, De Seta F, Radillo O, Agostinis C, Durigutto P, Pellis V, et al. Mannose- binding lectin is produced by vaginal epithelial cells and its level in the vaginal fluid is influenced by progesterone. Mol Immunol. 2010;48(1–3):281–6. https://doi.org/10.1016/j.molimm.2010.07.016.
Pivarcsi A, Nagy I, Koreck A, Kenderessy-Szabo A, Szell M, Dobozy A, et al. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005;7(9–10):1117–27.
Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbial Immunol. 2006;306:199–230.
Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20:1372–8.
Anderson DJ, Marathe J, Putney J. The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol. 2014;71:618–23.
Witkin SS. The vaginal microbiome, vaginal anti-microbial defence mechanisms and the clinical challenge of reducing infection-related preterm birth. BJOG. 2014;122:213–8. https://doi.org/10.1111/1471-0528.13115.
Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol. 2015;6:164.
Jasarevic E, Howerton CL, Howrd CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinol. 2015;156(9):3265–76. https://doi.org/10.1210/en.2015-1177.
Brosnahan AJ, Vulchanova L, Witta SR, Dai Y, Jones BJ, Brown DR. Norepinephrine potentiates proinflammatory responses of human vaginal epithelial cells. J Neuroimmunol. 2013;259:8–16. https://doi.org/10.1016/j.jneuroim.2013.03.005.
•• Lyte M, Brown DR. Evidence for PMAT- and OCT-like biogenic amine transporters in a probiotic strain of Lactobacillus: implications for interkingdom communication within the microbiota-gut-brain axis. PLoS One. 2018;13(1):e0191037. https://doi.org/10.1371/journal.pone.0191037. Recent evidence that Lactobacilli are responsive to stress hormones.
Amabebe E, Anumba DOC. Psychosocial stress, cortisol levels, and maintenance of vaginal health. Front Endocrinol. 2018;9:568. https://doi.org/10.3389/fendo.2018.00568.
Wang C-W, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9:65–76.
Dokladny K, Myers OB, Mosley PL. Heat shock response and autophagy-cooperation and control. Autophagy. 2015;11:200–13. https://doi.org/10.1080/15548627.2015.1009776.
Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77.
Dokladny K, Zuhl MN, Mandell M, Bhattacharya D, Schneider S, Deretic V, et al. Regulatory coordination between two major intracellular homeostatic systems. Heat shock response and autophagy. J Biol Chem. 2013;288:14959–72. https://doi.org/10.1074/jbc.M113.462408.
Kanninen TT, Sisti G, Witkin SS. Induction of the 70kDa heat shock protein stress response inhibits autophagy: possible consequences for pregnancy outcome. J Matern Fetal Neonatal Med. 2016;29:159–62.
• Nasioudis D, Forney LJ, Schneider GM, Gliniewicz K, France MT, Boester A, et al. The composition of the vaginal microbiome in first trimester pregnant women influences the level of autophagy and stress in vaginal epithelial cells. J Reprod Immunol. 2017;123:35–9. https://doi.org/10.1016/j.jri.2017.08.009. The relationship between vaginal microbiome composition, stress and autophagy is highlighted.
Ramos BR, Witkin SS. The influence of oxidative stress and autophagy cross regulation on pregnancy outcome. Cell Stress Chaperones. 2016;21:755–62.
Fichorova R, Anderson DJ. Differential expression of immunobiological mediators by immortalize human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–14.
•• Shroff A, Sequeira R, Reddy KVR. Human vaginal epithelial cells augment autophagy marker genes in response to Candida albicans infection. Am J Reprod Immunol 2017;77(4): https://doi.org/10.1111/aji.12639. Evidence that human vaginal epithelial cells defend against Candida albicans infection by induction of autophagy.
Shroff A, Reddy KVR. Autophagy gene ATG5 knockdown upregulates apoptotic cell death during Candida albicans infection in human vaginal epithelial cells. Am J Reprod Immunol. 2018;80(6):e13056. https://doi.org/10.1111/aji.13056.
Scholl J, Nasioudis D, Boester SM, Grunebaum A, Witkin SS. Group B streptococcus alters properties of vaginal epithelial cells in pregnant women. Am J Obstet Gynecol. 2016;214(3):383.e1–5. https://doi.org/10.1016/j.ajog.2015.12.053.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Iara M. Linhares, Giovanni Sisti, Evelyn Minis, Gabriela B. de Freitas, Antonio F. Moron, and Steven S. Witkin declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Female Genital Tract Infections
Rights and permissions
About this article
Cite this article
Linhares, I.M., Sisti, G., Minis, E. et al. Contribution of Epithelial Cells to Defense Mechanisms in the Human Vagina. Curr Infect Dis Rep 21, 30 (2019). https://doi.org/10.1007/s11908-019-0686-5
Published:
DOI: https://doi.org/10.1007/s11908-019-0686-5