Abstract
Purpose of Review
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system most often characterized by clinical relapses and periods of remission.
Recent Findings
The past decade has seen a dramatic increase in disease-modifying therapies for MS. Fourteen FDA-approved immunomodulatory drugs are currently available, and more medications are in development. A growing number of reported opportunistic infections, including progressive multifocal leukoencephalopathy (PML), highlight the serious complications of these new drugs and the need for specific screening guidelines.
Summary
Using data from Phase II and III randomized controlled trials, case reports, drug manufacturing data, and clinical experience, we outline the most common and serious infections associated with novel MS therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system (CNS) characterized by clinical relapses and periods of remission. The past decade has seen a dramatic increase in disease-modifying therapies for MS. Fourteen FDA-approved immunomodulatory drugs are currently available, and more medications are in development. The growing drug armamentarium allows providers to accommodate patient preference and to potentially achieve disease suppression. Nevertheless, infections represent serious complications of most intravenous and oral MS drugs. The data regarding infection risk derives from Phase II and III randomized controlled trials (RCT), post-marketing analyses, and case reports. The full spectrum of infectious complications may be unrecognized until months or years after drug approval. We highlight the known frequent and life-threatening infections associated with new MS therapies from the past decade. Where data is available, we discuss drug-specific monitoring and infection risk stratification for MS patients.
Intravenous Therapies
Natalizumab (Tysabri®)
Natalizumab is an intravenously administered, humanized monoclonal antibody approved for patients with relapsing remitting MS (RRMS) or Crohn’s disease. Natalizumab blocks the alpha-4 integrin on the surface of lymphocytes, preventing adherence to vascular cell adhesion molecule-1 (VAM-1) and inhibiting transmigration of activated T cells across the blood brain barrier [1]. By blocking transmural signaling, the drug also induces cellular apoptosis and reduction of peripheral T cell populations [2].
Natalizumab was the subject of two large randomized controlled trials that showed at least 60% reduction in annual MS relapse rates and over 80% decrease in MRI activity [3, 4]. Compared to placebo, systemic infections, such as urinary tract infections, lower respiratory tract infections, gastroenteritis, and tonsillitis were more common in the natalizumab arm, but did not reach statistical significance [3]. Subsequent research revealed that oral herpes outbreaks are three times more prevalent in MS patients treated with natalizumab compared to patients on other medications [5]. Case reports suggest a predisposition to CNS herpes simplex virus (HSV) [6,7,8] and varicella zoster virus (VZV) [8,9,10] infections.
Rarely, patients on natalizumab have acquired severe protozoan and fungal infections, requiring drug cessation [11,12,13]. A 27-year-old man who received natalizumab infusions over 10 months developed decreased visual acuity and floaters secondary to ocular toxoplasmosis [11]. His symptoms improved after initiation of appropriate antibiotic therapy. In a case report of cryptococcal meningitis, a 49-year-old man with RRMS on therapy for 2 years presented with headaches and vomiting [13]. Despite recovery with amphotericin and flucytosine, he acquired severe neurologic comorbidity from MS disease reactivation. Aside from natalizumab exposure, neither patient had obvious risk factors for opportunistic infections.
Natalizumab’s signature infectious complication is PML, in which reactivated JC polyomavirus destroys oligodendrocytes, causing CNS demyelination and progressive neurologic deterioration. Reports of natalizumab-associated PML first arose in 2005 [14,15,16]. These findings prompted the drug’s withdrawal from the market. The manufacturer later reintroduced it with more restrictive guidelines after investigators retrospectively evaluated thousands of patients enrolled in the original natalizumab trials and discovered no new cases of PML [17].
Natalizumab’s predisposition to PML is likely multifactorial. By disrupting T cell trafficking, natalizumab impairs immunosurveillance within the CNS and enables JCV proliferation [18]. Natalizumab also alters transcription of genes involved in B cell differentiation and maturation [19]. Since JCV lies dormant in B cell precursors, the drug may promote mobilization of infected cells from the bone marrow into the periphery [20, 21].
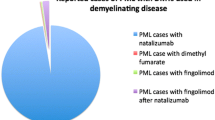
As of September 2016, the drug manufacturer has announced 685 confirmed PML cases, of which 23% were fatal [22]. The overall PML incidence is currently estimated at 4.2/1000 patients [22]. Recent attempts to classify PML risk rely on the presence or absence of JCV seropositivity, natalizumab duration ≥24 months, and prior immunosuppressant use [23]. Having all three risk factors confers an approximately 1/100 chance of developing PML [24]. Guidelines recommend further stratifying patients with no prior immunosuppressant use into low, intermediate, and high-risk groups based on an antibody index ≤0.9, 0.9–1.5, or >1.5 [25, 26••]. These values bestow a 0.1/1000, 0.1/1000, or 1/1000 risk within the first 24 months and a 0.3/1000, 1.2/1000, or 8.1/1000 risk between 25 and 48 months, respectively [25]. Regardless of the exact JCV titer, patients in the highest risk category should have more frequent MRI surveillance for PML and consider alternative therapies after 2 years [26••].
PML treatment requires rapid drug removal and immune reconstitution with plasmapheresis (PLEX) or immunoabsorption [27]. Almost all patients develop an immune reconstitution inflammatory syndrome (IRIS), characterized by clinical exacerbations or enlarging MRI lesions that are difficult to distinguish from PML progression. JCV may be detectable in the CSF for months to years after IRIS, mandating continued vigilance for new symptoms [28]. As our detection and treatment of natalizumab-associated PML improves, we expect the rates of fatal JCV infection to decline.
Rituximab (Rituxan®)
Rituximab is a chimeric anti-CD20 monoclonal antibody that targets and depletes B cell lymphocytes, thereby indirectly inhibiting T cell activation and pro-inflammatory cytokine expression [29]. Rituximab is approved for the treatment of B cell lymphomas and rheumatoid arthritis (RA) and has off-label use in MS. The risk of systemic infections is low. In a randomized controlled Phase II trial for RRMS, investigators found no difference in the incidence of infections among the rituximab and placebo cohorts [30]. A Phase II/III study of patients with primary progressive MS (PPMS) revealed slightly higher rates of pneumonias, urinary tract infections, and cellulitis, in those receiving rituximab (4.5%) vs. placebo (1.0%) [31]. Since rituximab can reactivate chronic hepatitis B virus (HBV), guidelines recommend checking HBV serologies prior to treatment [32].
To date, there have been no reported cases of PML in MS patients on rituximab. However, the overall incidence of PML is approximately 1/4000 and 1/25,000, among those treated for systemic lupus erythematosis (SLE) and RA, respectively [33, 34]. Among 57 non-HIV patients who developed PML after exposure to rituximab, 90% had lymphoproliferative disorders [35]. Notably, those affected had chronic lymphopenia and prior immunosuppressant exposure, risk factors that are generally absent in MS patients [36]. Compared to other diseases, our experience with rituximab in the MS population is limited, so we may not know the full extent of PML until years after treatment onset.
PML’s pathogenesis with rituximab administration is unclear. Depletion of B lymphocytes may trigger peripheral mobilization of premature lymphocytes infected with latent neurotropic JCV [33]. Indirect impairment of the T cell immune response may also facilitate viral replication in the CNS [36]. In general, PLEX has limited efficacy with rituximab since CD20 counts remain suppressed up to 6–9 months after treatment [33]. However, since rituximab is detectable in the serum for up to 2 months after administration, PLEX may be useful in patients who develop PML early into therapy [34].
Ocrelizumab (Ocrevus®)
Recently, the FDA granted ocrelizumab, a humanized anti-CD20 antibody, “breakthrough therapy designation,” to expedite drug review and approval by the end of 2016 [37]. With its humanized backbone, ocrelizumab is potentially less immunogenic and more effective than rituximab [38]. Ocrelizumab is currently under investigation for treatment of RRMS and PPMS. In a Phase II, randomized, placebo-controlled multicentre trial, the rates of serious infections were comparable among the ocrelizumab and placebo groups [39]. Unpublished Phase III trials have yielded similar safety analyses without reports of PML or other opportunistic infections [40, 41].
Ocrelizumab has a different safety profile in studies for systemic rheumatological conditions. Investigators terminated Phase III trials for RA and SLE early due to a higher incidence of opportunistic infections, such as Pneumocystis jiroveci pneumonia, cytomegalovirus pneumonia, pulmonary tuberculosis, atypical mycobacterial infections, and systemic candidiasis in the treatment arms [42, 43]. The disparity between the data for MS and systemic rheumatological conditions may derive from a lower ocrelizumab dose in the former trials and adjuvant immunosuppressants in the latter studies [29]. If and when ocrelizumab receives FDA approval, we expect to learn more about the specific infectious complications in MS.
Alemtuzumab (Lemtrada®)
Alemtuzumab, a monoclonal antibody approved for RRMS, lymphoproliferative disorders, and graft versus host disease, binds to CD52 on T cells, B cells, thymocytes, and natural killer cells, resulting in cell lysis and lymphocyte depletion for up to 16 months post infusion [44]. Repopulation of regulatory T lymphocyte subsets may cause long-standing inflammatory suppression [45]. In the Phase III trials for RRMS, CARE-MS I, and CARE-MS II, higher infection rates occurred in the alemtuzumab group compared to the interferon beta-1a cohort [46, 47]. Infections were predominantly mild or moderate in severity and involved the upper respiratory and urinary tracts. Mucocutaneous Candida infections were significantly more common in the population receiving the study drug [47, 48].
Herpesvirus infections, most often cutaneous, occurred in 16 and 2% of patients treated with alemtuzumab and interferon beta 1a, respectively [46]. Serious VZV infections led to hospitalization in two patients [47]. Consequently, the investigators amended the Phase III trial protocol to include oral acyclovir prophylaxis from drug infusion to day 28. The drug package insert now recommends antiviral prophylaxis for a minimum of 2 months following treatment or until CD4+ lymphocyte count is ≥200 cells/μL [48]. Providers should also test patients for VZV antibodies and consider VZV vaccination for non-immune individuals 6 weeks prior to therapy.
Among the non-MS patient population, alemtuzumab is associated with an increased incidence of HHV-6 encephalitis, systemic cytomegalovirus (CMV) infection, toxoplasmosis, Pneumocystis jirovcei pneumonia, and PML [49,50,51]. Lower doses of alemtuzumab and lack of adjuvant immunosuppressant exposure may account for the relative absence of PML cases and other opportunistic infections in the MS patient cohort. Nevertheless, emerging cases of Listeria meningitis, active tuberculosis, and nocardiosis have raised safety concerns [47, 52, 53]. The manufacturer now advises patients to avoid dietary sources of Listeria monocytogenes, such as deli meat and unpasteurized dairy products [48]. Physicians should also screen patients at risk for tuberculosis before treatment.
Oral Therapies
Fingolimod (Gilenya®)
Fingolimod, a sphingosine 1-receptor modulator, was the first oral agent approved for RRMS. The drug prevents lymphocytes from exiting lymph nodes and from recirculating within the blood and CNS [54]. Redistribution of lymphocytes within lymphatic tissue causes a significant, albeit reversible lymphopenia. Two large randomized controlled trials, FREEDOMS and TRANSFORMS, demonstrated a reduction in relapse rates and MRI disease activity with fingolimod compared to placebo or interferon-beta [55, 56]. The overall incidence of infections was similar among treatment groups, although lower respiratory tract infections were more common. A retrospective analysis showed no definite association between absolute lymphocyte count and incidence of infection [57]. After 2 years of therapy, the rate of serious infections in patients with chronic lymphopenia (defined as ALC <400 cells/mm3 for ≥60% of lab values over time) was not significantly increased compared to short-term drug therapy [58].
Two patients who received fingolimod in the TRANSFORMS trial developed fatal viral infections, including disseminated varicella zoster virus (VZV) and herpes simplex virus (HSV) encephalitis [55]. Pooled data from all trials revealed that VZV infections occurred almost twice as frequently in the fingolimod cohorts compared to placebo [59•]. Fingolimod diminishes the VZV-specific T cell response in vitro and correlates with a subclinical VZV reactivation in the saliva [60]. Current guidelines recommend ascertaining a patient’s VZV immune status and vaccinating non-immune individuals at least 1 month before treatment [59•].
Other opportunistic infections, although uncommon, have raised additional safety concerns. Reports of systemic and cutaneous cryptococcal infections prompted an updated warning to the drug’s prescribing information [61,62,63]. PML risk remains uncertain, since antecedent natalizumab therapy can confound interpretation of new cases. Investigators reported 11 patients diagnosed with PML after switching from natalizumab to fingolimod, although five patients may have actually acquired the infection prior to transition. The appropriate natalizumab washout period is debatable. Some investigators have proposed limiting the washout to 8–12 weeks, given an increase in MS disease reactivation after this time interval [64]. Nevertheless, as of August 2016, six cases of PML developed in MS patients without previous natalizumab treatment and two patients (one definite PML and one probable PML) had no prior immunosuppressant exposure [65, 66]. The aforementioned individuals had taken fingolimod for over 2 years and did not have a history of grade 4 lymphopenia (ALC <200 cells/mm3) [66]. No fatalities occurred after stopping fingolimod. Checking JCV antibody status is not currently recommended. Providers should factor immunosuppression history and antecedent natalizumab therapy, in particular, in their decision-making.
Teriflunomide (Aubagio®)
Teriflunomide is a dihydroorotate dehydrogenase inhibitor that blocks pyrimidine synthesis in activated, proliferating T lymphocytes [67]. Teriflunomide is the active metabolite of leflunomide, an agent employed for many years in rheumatoid arthritis. In Phase III trials for RRMS, the incidence of severe infections was similar among treatment and placebo groups, although investigators reported three cases of serious pyelonephritis in the high-dose terifluonimde cohort [68, 69]. Based on over 8 years of follow-up data, neither PML nor other opportunistic infections have emerged [70]. A case of PML in a 55-year-old man on leflunomide for SLE has been described. This patient had previously received several other immunosuppressants and had switched from methotrexate 5 months before the onset of PML symptoms [71].
Dimethyl Fumarate (Tecfidera®)
Dimethyl Fumarate (DMF) is a fumaric acid ester that appears to shift CD4+ T cell populations from pro-inflammatory, pathogenic Th1 subsets to immunomodulatory Th2 subsets [72]. The drug may also act on the NrF2 biochemical pathway and upregulate antioxidant molecules responsible for neuroprotection. Prior to the FDA approval of DMF in 2013, fumaric acid esters treated psoriasis and other immune-mediated diseases. In the Phase III RCTs for RRMS, DEFINE, and CONFIRM, the most common adverse events included nasopharyngitis, urinary tract infections, and upper respiratory tract infections [73, 74]. The incidence of serious infections was overall low and similar between placebo and DMF groups [75].
DMF can cause sustained lymphopenia. In the RCTs, mean lymphocyte counts decreased by approximately 30% during the first year of treatment, but remained overall stable thereafter [73, 74]. A retrospective study of 221 patients on DMF revealed that grade 2 (ALC 500–799 cells/mm3) or grade 3 lymphopenia (ALC <500 cells/mm3) developed in 17% of the total cohort and did not resolve during DMF treatment [76]. Older age (>55), lower baseline ALC, and previous natalizumab exposure increased the risk of developing moderate to severe lymphopenia. DMF decreases both CD4+ and CD8+ memory T cells, but preferentially impacts CD8+ lymphocytes and Th1 subsets, which are essential for cell-mediated antiviral immunity [77, 78]. Although monitoring specific lymphocyte subsets may prevent certain viral complications, guidelines do not currently advise checking CD4+ or CD8+ cell levels.
Cases of PML were first identified in association with fumaric acid esters for psoriasis, and most of the affected patients had prior immunosuppressant exposure [79,80,81]. Four MS patients on DMF have since acquired PML, and three out of the four had sustained lymphopenia during treatment [82•, 83]. The first patient had received the drug for 4 years prior to the diagnosis of PML and ultimately died from the infection [82•]. She, like two others, had sustained grade 3 lymphopenia [82•, 83]. The fourth case involved a patient whose ALC dropped quickly to 600 cells/mm3 within the first 13 months, but never declined further [83]. While the role of lymphopenia in these cases is uncertain, the drug manufacturer now recommends obtaining a cell blood count every 6 months and either interrupting or stopping DMF therapy if ALC falls below 500 cells/mm3 for more than 6 months [84]. ALC recovery is often prolonged. Among nine patients with grade 3 lymphopenia, their ALCs did not reach normal values until at least 5 months after drug cessation [76].
Subcutaneous Therapy
Daclizumab (ZinbrytaTM)
Daclizumab is the newest FDA-approved medication for the treatment of relapsing remitting MS. Originally developed as a prophylactic agent against solid organ allograft rejection, this humanized monoclonal antibody selectively targets the IL-2 receptor and inhibits activated T cell signaling [85]. Subcutaneous daclizumab therapy led to a 50% or more reduction in annual MS disease relapse and MRI activity compared to placebo or interferon beta in clinical trials [86, 87].
In a Phase II RCT, the rate of serious infections was 2% in the daclizumab cohort and 0% in the placebo cohort [86]. A subsequent Phase III RCT similarly demonstrated that infections were overall more common with daclizumab than with interferon beta (65 vs. 57% overall and 4 vs. 2% serious infections) [87]. Infections included at least one case of urinary tract infection, pneumonia, appendicitis, and cellulitis. At 3-year follow-up, the incidence of infections among patients taking daclizumab remained stable [88]. Less than 1% of individuals acquired opportunistic complications, such as CMV infection, pulmonary tuberculosis, and oral/vulvovaginal candidiasis. No reports of PML or systemic fungal infections have surfaced to date.
Conclusions
The novel immunomodulatory therapies provide increasing opportunities to suppress MS inflammation and improve patients’ qualities of life. Nevertheless, since most MS drugs received FDA approval within the last decade, their long-term safety is unknown. The rising cases of PML, in particular, illustrate a rare, but devastating, consequence of these drugs. JCV antibody titers provide an innovative opportunity to risk stratify patients on natalizumab, but their application to other medications remains unstudied. While sustained lymphopenia correlates with PML development in most cases of patients on dimethyl fumarate, the relationship is less straightforward in other therapies. The absence of consistent guidelines provides a predicament for MS providers, who must counsel patients daily about their drug options and risks.
Fortunately, more recommendations regarding appropriate screening and infection prophylaxis are available. Many of the drugs, especially fingolimod and alemtuzumab, predispose patients to herpesviridae infections. Obtaining VZV immune status and vaccinating non-immune patients early may prevent severe cutaneous and systemic zoster infections on these drugs. Screening for latent TB and chronic HBV infection, and treating when present, are also essential tasks. Providers have a responsibility to ensure that basic immunizations are up to date before initiating any disease-modifying therapy (Table 1).
In summary, the infectious complications of the novel MS therapies can be serious and life-threatening, requiring special vigilance and recognition on the part of providers. Opportunistic infections, although infrequent, pose significant obstacles to patients, who face the dilemma of switching from an effective medication to a safer, albeit less potent alternative. The distinct drug mechanisms and pharmacokinetic profiles highlight the need for specific disease-monitoring criteria. Until then, physicians should account for a patient’s age, prior immunosuppressant exposure, current immune status, and duration of therapy in their decision-making and screening.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–42.
Leussink VI, Zettl UK, Jander S, et al. Blockade of signaling via the very late antigen (VLA-4) and its counterligand vascular cell adhesion molecule-1 (VCAM-1) causes increased T cell apoptosis in experimental autoimmune neuritis. Acta Neuropathol. 2002;103:131–6.
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–23.
Schiess N, Zong J, Hayward G, et al. Reactivation of herpes virus in multiple sclerosis patients on natalizumab therapy [P03.163]. Seattle: American Academy of Neurology; 2009.
Shenoy ES, Mylonakis E, Hurtado RM, et al. Natalizumab and HSV meningitis. J Neurovirol. 2011;17:288–90.
Kwiatkowski A, Gallois J, Bilbault N, et al. Herpes encephalitis during natalizumab treatment in multiple sclerosis. Mult Scler. 2012;18:909–11.
Fine AJ, Sorbello A, Kortepeter C, et al. Central nervous system herpes simplex and varicella zoster virus infections in natalizumab-treated patients. Clin Infect Dis. 2013;57:849–52.
Bourre B, Lefaucheur R, Ahtoy P, et al. Varicella-zoster virus acute myelitis in a patient with MS treated with natalizumab. Neurology. 2013;81:1966–7.
Yeung J, Cauquil C, Saliou G, et al. Varicella-zoster virus acute myelitis in a patient with MS treated with natalizumab. Neurology. 2013;80:1812–3.
Zecca C, Nessi F, Bernasconi E, et al. Ocular toxoplasmosis during natalizumab treatment. Neurology. 2009;73:1418–9.
Gutwinski S, Erbe S, Munch C, et al. Severe cutaneous Candida infection during natalizumab therapy in multiple sclerosis. Neurology. 2010;74:521–3.
Valenzuela RM, Pula JH, Garwacki D, et al. Cryptococcal meningitis in a multiple sclerosis patient taking natalizumab. J Neurol Sci. 2014;340:109–11.
Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74.
Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81.
Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–8.
Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–33.
Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab—unforeseen consequences. N Engl J Med. 2005;353:414–6.
Lindberg RL, Achtnichts L, Hoffmann F, et al. Natalizumab alters transcriptional expression profiles of blood cell subpopulations of multiple sclerosis patients. J Neuroimmunol. 2008;194:153–64.
Houff SA, Berger J, Major EO. Response to Linberg et al. natalizumab alters transcriptional expression profiles of blood cell subpopulations of multiple sclerosis patients. J Neuroimmunol 2008;204:155–6; author reply 157
Major EO. Reemergence of PML in natalizumab-treated patients—new cases, same concerns. N Engl J Med. 2009;361:1041–3.
Biogen. TYSABRI® (natalizumab): PML in patients receiving TYSABRI. http://medinfo.biogen.com: Biogen; 2016.
Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–80.
Koendgen H, Chang I, Sperling B, et al. New algorithm to estimate risk of natalizumab-associated Progressive Multifocal Leukoencephalopathy (PML) in anti-JCV antibody positive patients: analyses of clinical trial data to provide further temporal precision and inform clinical practice [P1249]. 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis. London, UK; 2016.
Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802–12.
•• McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. 2016;87:117–25. McGuigan and colleagues outline practical consensus guidelines for monitoring patients on natalizumab based on current evidence. The authors break down the risk of PML using JCV antibody titers, previous immunosuppressant exposure, and duration of natalizumab therapy.
Clifford DB, De Luca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–46.
Ryschkewitsch CF, Jensen PN, Monaco MC, et al. JC virus persistence following progressive multifocal leukoencephalopathy in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2010;68:384–91.
Milo R. Therapeutic strategies targeting B-cells in multiple sclerosis. Autoimmun Rev. 2016;15:714–8.
Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88.
Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–71.
Buch MH, Smolen JS, Betteridge N, et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:909–20.
Carson KR, Focosi D, Major EO, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10:816–24.
Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156–64.
Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–40.
Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–37.
Ocrelizumab granted “Breakthrough Therapy Designation” for primary-progressive MS by FDA. UPDATE: priority review granted by Dec. 28, 2016. http://www.nationalmssociety.org/About-the-Society/News/Ocrelizumab-Granted-Breakthrough-Therapy-Designat: National Multiple Sclerosis Society; 2016.
Sorensen PS, Blinkenberg M. The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Ther Adv Neurol Disord. 2016;9:44–52.
Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–87.
Hauser S, Comi G, Hartung HP, et al. Efficacy and safety of ocrelizumab in relapsing multiple sclerosis – results of the phase III double-blind, interferon beta-1a-controlled OPERA I and II Studies. 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Barcelona, Spain; 2015.
Montalban X, Hemmer B, Rammohan K, et al. Efficacy and safety of ocrelizumab in primary progressive multiple sclerosis – results of the phase III, double-blind, placebo-controlled ORATORIO Study. 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Barcelona, Spain; 2015.
Mysler EF, Spindler AJ, Guzman R, et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. 2013;65:2368–79.
Emery P, Rigby W, Tak PP, et al. Safety with ocrelizumab in rheumatoid arthritis: results from the ocrelizumab phase III program. PLoS One. 2014;9, e87379.
Hartung HP, Aktas O, Boyko AN. Alemtuzumab: a new therapy for active relapsing-remitting multiple sclerosis. Mult Scler. 2015;21:22–34.
De Mercanti S, Rolla S, Cucci A, et al. Alemtuzumab long-term immunologic effect: Treg suppressor function increases up to 24 months. Neurol Neuroimmunol Neuroinflamm. 2016;3, e194.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–28.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–39.
Corporation G. Lemtrada Prescribing Information. https://www.lemtradahcp.com/: Genzyme Corporation; 2016.
Martin SI, Marty FM, Fiumara K, et al. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin Infect Dis. 2006;43:16–24.
Peleg AY, Husain S, Kwak EJ, et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis. 2007;44:204–12.
Vu T, Carrum G, Hutton G, et al. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:705–9.
Rau D, Lang M, Harth A, et al. Listeria meningitis complicating alemtuzumab treatment in multiple sclerosis—report of two cases. Int J Mol Sci. 2015;16:14669–76.
Penkert H, Delbridge C, Wantia N, et al. Fulminant central nervous system nocardiosis in a patient treated with alemtuzumab for relapsing-remitting multiple sclerosis. JAMA Neurol. 2016;73:757–9.
Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–77.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15.
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401.
Francis G, Kappos L, O’Connor P, et al. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult Scler. 2014;20:471–80.
Cohen JA, Kappos L, Selmaj K, et al. Long-term safety and effectiveness of fingolimod: 7 year data from the LONGTERMS study [P591]. 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Barcelona, Spain; 2015.
• Arvin AM, Wolinsky JS, Kappos L, et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol. 2015;72:31–9. By retrospectively analyzing Phase 2 and 3 studies, Arvin and colleagues report an almost two fold increased rate of VZV infections in patients on fingolimod compared to those patients on placebo. Consequently, the authors recommend ascertaining a patient’s VZV immune status and vaccinating non-immune patients prior to treatment.
Ricklin ME, Lorscheider J, Waschbisch A, et al. T-cell response against varicella-zoster virus in fingolimod-treated MS patients. Neurology. 2013;81:174–81.
Achtnichts L, Obreja O, Conen A, et al. Cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol. 2015;72:1203–5.
Huang D. Disseminated cryptococcosis in a patient with multiple sclerosis treated with fingolimod. Neurology. 2015;85:1001–3.
Forrestel AK, Modi BG, Longworth S, et al. Primary cutaneous cryptococcus in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol. 2016;73:355–6.
Kappos L, Radue EW, Comi G, et al. Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology. 2015;85:29–39.
FDA. FDA warns about cases of rare brain infection with MS drug Gilenya (fingolimod) in two patients with no prior exposure to immunosuppressant drugs. http://www.fda.gov/Drugs/DrugSafety/ucm456919.htm: FDA Drug Safety Communications; 2015.
Novartis. Gilenya – Progressive Multifocal Leukoencephalopathy (PML). https://www.novartis.it. Novartis; 2016.
Fragoso YD, Brooks JB. Leflunomide and teriflunomide: altering the metabolism of pyrimidines for the treatment of autoimmune diseases. Expert Rev Clin Pharmacol. 2015;8:315–20.
O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–303.
Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247–56.
Confavreux C, Li DK, Freedman MS, et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult Scler. 2012;18:1278–89.
Warnatz K, Peter HH, Schumacher M, et al. Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Ann Rheum Dis. 2003;62:50–7.
Fox RJ, Kita M, Cohan SL, et al. BG-12 (dimethyl fumarate): a review of mechanism of action, efficacy, and safety. Curr Med Res Opin. 2014;30:251–62.
Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–97.
Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107.
Gold R, Giovannoni G, Phillips JT, et al. Efficacy and safety of delayed-release dimethyl fumarate in patients newly diagnosed with relapsing-remitting multiple sclerosis (RRMS). Mult Scler. 2015;21:57–66.
Longbrake EE, Naismith RT, Parks BJ, et al. Dimethyl fumarate-associated lymphopenia: risk factors and clinical significance. Mult Scler J Exp Transl Clin. 2015;1:2055217315596994.
Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, et al. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2015;2, e76.
Gross CC, Schulte-Mecklenbeck A, Klinsing S, et al. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3, e183.
Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med. 2013;368:1657–8.
van Oosten BW, Killestein J, Barkhof F, et al. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368:1658–9.
Dammeier N, Schubert V, Hauser TK, et al. Case report of a patient with progressive multifocal leukoencephalopathy under treatment with dimethyl fumarate. BMC Neurol. 2015;15:108.
• Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372:1476–8. Rosenkranz and colleagues report the first patient with RRMS who developed PML on dimethyl fumarate. The patient’s ALL was consistently < 600 cells/mm3. The case highlights the potential relationship between lymphopenia and PML risk with dimethyl fumarate.
Biogen. TECFIDERA® (dimethyl fumarate): PML Case Reports. https://medinfo.biogen.com: Biogen; 2016.
Biogen. TECFIDERA® (dimethyl fumarate): Treatment Interruption or Discontinuation Due to Low Lymphocyte Levels. https://medinfo.biogen.com: Biogen; 2016.
Pfender N, Martin R. Daclizumab (anti-CD25) in multiple sclerosis. Exp Neurol. 2014;262 Pt A:44–51.
Gold R, Giovannoni G, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:2167–75.
Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2015;373:1418–28.
Giovannoni G, Kappos L, Gold R, et al. Safety and tolerability profile of daclizumab in patients with relapsing-remitting multiple sclerosis: an integrated analysis of clinical studies. Mult Scler Relat Disord. 2016;9:36–46.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Levin and Dr. Kaplan declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neurological Infections
Rights and permissions
About this article
Cite this article
Levin, S.N., Kaplan, T.B. Infectious Complications of Novel Multiple Sclerosis Therapies. Curr Infect Dis Rep 19, 7 (2017). https://doi.org/10.1007/s11908-017-0562-0
Published:
DOI: https://doi.org/10.1007/s11908-017-0562-0