Abstract
Purpose of Review
This review summarizes the latest science on hypertensive encephalopathy and posterior reversible encephalopathy syndrome (PRES). We review the epidemiology and pathophysiology of these overlapping syndromes and discuss best practices for diagnosis and management.
Recent Findings
Diagnosis of hypertensive encephalopathy largely relies on exclusion of other neurological emergencies. We review the extensive causes of PRES and its imaging characteristics. Management strategies have not changed substantially in the past decade, though newer calcium channel blockers simplify the approach to blood pressure reduction. While this alone may be sufficient for treatment of hypertensive encephalopathy in most cases, management of PRES also depends on modification of other precipitating factors.
Summary
Hypertensive encephalopathy and PRES are overlapping disorders for which intensive blood pressure lowering is critical. Further research is indicated to both in diagnosis and additional management strategies for these critical conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical Case Scenario
A 48-year-old male presented to the emergency department (ED) after being found confused at home by his family members. The family members were worried that he had a syncopal event earlier in the day and had been disoriented since. On presentation, the patient was awake but unable to answer any questions. Pupils were 2 mm bilaterally and reactive. He appeared to be in no distress and was moving all extremities well. His initial blood pressure was 280/111 mmHg. Labs were notable for troponin 0.56 ng/ml and creatinine 3.22 mg/dl. His electrocardiogram was normal. A chest radiograph showed mild pulmonary congestion, and a computed tomography (CT) scan of the head was unremarkable. The patient was started on nicardipine infusion for control of blood pressure and admitted to the intensive care unit (ICU) with a diagnosis of hypertensive encephalopathy.
Objectives
Uncontrolled hypertension remains a major health threat worldwide, and hypertensive encephalopathy reflects one of the most critical forms of uncontrolled hypertension. There exists a significant amount of uncertainty about its incidence, diagnosis, and relationship to posterior reversible encephalopathy syndrome (PRES). The primary aim of this review is to summarize the latest science and expert opinion on hypertensive encephalopathy and PRES. We review the pathophysiology, diagnosis, and management of these conditions in the ED and ICU settings and discuss considerations for further research.
Epidemiology
Hypertension, one of the most common disorders in the USA, is estimated to affect nearly 50% of the adult population under new guidelines [1••]. Although hypertension is prevalent in the American population, less than 2% of all hypertensive presentations can be classified as hypertensive emergencies [2, 3]. A recent study representing national data placed this estimate at only 2 per 1000 total visits and 6 per 1000 of hypertensive ED visits. Hypertensive encephalopathy itself accounts for approximately 15% of those presentations [4, 5]. Another recent study using administrative data sources indicated increasing diagnostic rates for hypertensive encephalopathy [6]. This rise, the authors argue, is likely due to changes in billing rather than a true change in epidemiology. There has been no rise in morbidity and mortality associated with hypertensive emergencies. While often due to hypertensive emergency, PRES is not always associated with severely elevated BP. This syndrome can also be the result of other disease processes and constitutes a small portion of hypertensive encephalopathy presentations [7•].
Hypertensive encephalopathy is a hypertensive emergency defined by acute neurological dysfunction associated with an acute and severe increase in blood pressure (typically > 220 mmHg systolic or > 120 mmHg diastolic blood pressure). Most patients have alterations in mental status. Some may present primarily with or have concomitant seizures, visual disturbance, or headache. Headache is the least specific symptom and alone cannot support the diagnosis of hypertensive encephalopathy. The presence of other significant life-threatening diagnoses that have overlapping symptoms creates a challenge in defining and diagnosing hypertensive encephalopathy in the ED. The subjective nature of mental status change or other neurological symptoms adds to this challenge. Furthermore, the specific diagnosis of PRES is challenging given its broad neurologic symptoms (discussed below), causes, and the lack of readily available confirmatory imaging in the ED.
Pathophysiology
The human brain receives 15% of the cardiac output and consumes 20% of the total body oxygen consumption. It does so while maintaining adequate cerebral perfusion pressure through an autoregulatory process that alters the pre-capillary arteriolar resistance in response to changing physiological conditions [8, 9]. Hypertensive encephalopathy, a term coined by Oppenheimer and Fishberg in 1928, occurs in the setting of acute rises in blood pressure that lead to arteriolar vasoconstriction in combination with a breakdown of the blood-brain barrier—resultant consequences include cerebral edema and petechial microhemorrhages [9, 10]. During hypertensive emergencies, the cerebral arteriolar endothelium responds initially with a release of NO and a forced hydrostatic dilation that in itself can lead to cerebral edema. However, when these mechanisms are saturated from persistent and sustained hypertension, the result is a state of increased resistance. This ongoing increased resistance contributes to endothelial damage and release of inflammatory cytokines that damage the blood-brain barrier, increasing its permeability, inhibiting fibrinolysis, and activating coagulation [9].
Posterior PRES, reversible posterior leukoencephalopathy syndrome (RPLS), and reversible posterior cerebral edema syndrome are synonyms for clinical and radiological spectrum of disease first described by Hinchey et al. as such in 1996 [11]. Although PRES was originally described in hypertensive patients, it has since been found in normotensive individuals and in association with numerous other clinical conditions such as renal disease, infections, immunosuppressive agents (cyclosporine, cytarabine), eclampsia, and rarely even anti-depressants such as venlafaxine (Table 1) [11, 12••, 13]. Changes identified on neuroimaging are consistent with a perivascular edema [12••]. This focal edema likely results from a breakdown of the blood-brain barrier and frequently has petechial hemorrhages. It predominates in the posterior portions of the cerebral hemisphere and in particular the parieto-occipital regions [12••].
The pathophysiology of PRES remains controversial, but the leading theory posits a relationship with cerebral autoregulatory dysfunction [11, 13]. This results in hyperperfusion that contributes to the development of perivascular edema, which subsequently compresses the surrounding microvessels and contributes to a profound endarteritis [11, 13, 14]. The posterior regions of the brain that have less sympathetic innervation for autoregulation are thus most susceptible to this type of injury [11]. Alternatively, direct endothelial damage from fluctuations in blood pressures, release of cytokines, or circulating inflammatory markers contributes to the breakdown of the blood-brain barrier and increases the risk of infarction and petechial hemorrhages, with or without hypertension [11, 14]. The typically reversible vascular changes of PRES include vasoconstriction, focal vasospasm, and string of bean signs of the cerebral vasculature [13].
Diagnosis
Hypertensive Encephalopathy
The identification of hypertensive encephalopathy in the ED usually relies on the exclusion of alternative causes of altered mentation. Patients without a history of hypertension may develop hypertensive encephalopathy with blood pressure > 180/110 mmHg, but most patients with or without a history of hypertension have a blood pressure > 220/120 mmHg. Symptoms usually include change in mental status, though this may be subtle, and fluctuate. Concomitant symptoms may include headache, blurred vision, nausea, and seizures. Focal motor or sensory deficits are uncommon and, when present, more likely indicate acute stroke. As in the clinical case we presented above, findings of acute kidney injury, fragmented red blood cells, and elevation in cardiac biomarkers can accompany hypertensive encephalopathy. How commonly these multi-organ system findings overlap is not well described in the literature. Computed tomography of the brain is critical to evaluate for other causes as a hypertensive response in the setting of alternative etiologies is far more common than encephalopathy due to markedly elevated blood pressure alone [15]. These include traumatic brain injury, subarachnoid hemorrhage, intracerebral hemorrhage, and ischemic stroke. Although petechial hemorrhage is possible in hypertensive encephalopathy and PRES, confluent intracerebral hemorrhage indicates an alternative diagnosis.
Confirmatory diagnostic testing is challenging in the ED. Head CT imaging that shows cerebral edema supports the diagnosis; however, CT is not sufficiently sensitive to rule out edema on its own. While magnetic resonance imaging (MRI) has greater sensitivity, its availability is limited in many EDs and treatment will usually precede such imaging. Retinal findings can support the diagnosis of hypertensive encephalopathy. However, clinicians should be cautious to use findings of hypertensive retinopathy alone to support the diagnosis of hypertensive encephalopathy as the chronicity of retinal findings without papilledema can be difficult to determine. When present, papilledema does strongly support the diagnosis of hypertensive encephalopathy, provided pressure is adequately high and altered mentation is present. Advancements in retinal imaging in the ED setting may improve detection of papilledema [16]. Despite such considerations, a diagnosis of hypertensive encephalopathy is frequently established only retrospectively, once a patient’s mental status improves with reduction of blood pressure. While this improvement can occur rapidly in the ED setting, it is often not established until later in a patient’s hospitalization.
Posterior Reversible Encephalopathy Syndrome
There are currently no set guidelines or specific diagnostic criteria for PRES. Common symptoms include non-localized headaches, visual disturbances, and altered consciousness ranging from somnolence to agitation, confusion, and behavioral changes [12••, 17]. Seizures are a common presenting symptom, including status epilepticus [15, 18].
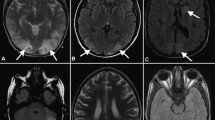
Neuroimaging with MRI is the modality of choice for diagnosis of PRES. Symmetrical white matter edema in the posterior cerebral hemispheres (usually posterior and occipital lobes) with eventual resolution of the findings is typically observed [17, 19]. The lesions can also frequently involve the cerebellar and brain stem and rarely may involve the spinal cord [20,21,22]. Typical MRI features of lesions display increased signal on T2 and fluid-attenuated inversion recovery (FLAIR) [15, 23]. Distinguishing PRES from an acute stroke can be challenging. Distribution of abnormalities beyond a single vascular territory, sparing of calcarine and paramedian occipital lobes, usually helps distinguish PRES from cerebrovascular infarction [12••, 17]. Moreover, diffusion weighted imaging (DWI) can help distinguish between PRES and bilateral posterior cerebral artery infarctions. In PRES, the edema is vasogenic and is represented by isointense or slightly hyperintense signals on DWI, whereas in an acute infarction, the edema is cytotoxic which manifests as marked hyperintensity on DWI [23, 24].
Although PRES affects the posterior hemispheres and is reversible in the overwhelming majority of patients, recent cases have shown more widespread findings than previously considered. Lesions involving the frontal lobes have been described and also isolated cases of irreversible PRES, sometimes progressing to a fulminant variant causing death [25, 26••, 27]. Despite the widespread involvement of lesions, currently, there is limited prognostic data to suggest specific anatomic distribution would affect outcomes [24].
Management
Blood Pressure Management
For patients with suspected hypertensive encephalopathy, the primary treatment aim is acute blood pressure reduction without over correction. No randomized clinical trials have been conducted to define end points of treatment or the efficacy of one anti-hypertensive related to another. Consensus opinion among experts and extrapolation from large animal studies has led to guidelines that recommend blood pressure reduction of approximately 20–25% as soon as possible once the diagnosis is suspected [3, 28]. Rapid correction beyond this threshold potentially exposes a patient to cerebral hypoperfusion [29]. The most commonly recommended medications include intravenous labetalol, nicardipine, and nitroprusside infusions for immediate and titratable control (Table 2). Although nitroprusside was a mainstay of treatment for hypertensive emergencies, newer agents such as nicardipine and clevidipine have supplanted its use in many centers. Nicardipine, for instance, is inexpensive, easier to titrate, and does not carry the concern for possible cyanide toxicity [4, 30, 31•, 32].
A 2008 Cochrane review of 15 randomized clinical trials concluded that there was insufficient evidence to determine which drug is most effective in the setting of hypertensive emergencies [33]. More recent studies still have not clearly defined a single agent of choice for hypertensive encephalopathy, although most support acute reduction of baseline blood pressure up to 25% using intravenous and titratable anti-hypertensive medications. The seventh report of the Joint National Committee (JNC) agreed with such treatment goals and did not recommend one medication over others [34]. Neither the eighth report from the JNC members nor the latest American College of Cardiology and American Heart Association guidelines did not tackle management strategies in hypertensive encephalopathy [1••, 35].
For hypertensive encephalopathies, nicardipine has become a common initial choice given its rapid onset of action and ability to titrate without invasive monitoring. It is also dose-independent to body weight with relatively few major side effects or contraindications. Labetalol would also be an acceptable choice; it can be administered as an intravenous infusion or by repeated bolus and can be titrated to effect without invasive monitoring. Moreover, it is generally well tolerated. Bronchospasm and heart block at higher doses are possible but rarely reported. Nitroprusside has very rapid onset but typically requires invasive and constant blood pressure monitoring. This, in conjunction with its potential for cyanide toxicity, makes it less desirable and renders it second-line therapy after other available anti-hypertensives. Esmolol is a rapid onset beta adrenergic blockade but has a higher fluid burden with administration and is considered primarily an adjunct (rather than primary) therapy for blood pressure control with use generally restricted to perioperative patients or aortic dissection. Nitroglycerin is readily available but typically requires higher doses for blood pressure control and patients can develop tolerance with prolonged use. Finally, clevidipine is a rapid onset calcium channel blocker similar to nicardipine. Its potential pharmacological advantage is its shorter half-life compared to nicardipine [36].
Following the initial 25% reduction in blood pressure, patients require further gradual reduction over the next 24 h. There is no literature to support the exact timing of this blood pressure reduction and when it is safe to intensify treatment to reach normal blood pressure. While on a continuous anti-hypertensive infusion, a patient can initiate oral anti-hypertensive medications as soon as the individual clinical condition allows. Clinicians may then wean the intravenous infusion to produce a gradual reduction in blood pressure.
Posterior Reversible Encephalopathy Syndrome
Treatment of PRES also involves early blood pressure reduction with concurrent intervention for contributing etiologies. There are currently no specific treatment regimens or societal guidelines advocated for PRES. When severely elevated blood pressure is causative of PRES, blood pressure treatment goals are identical to hypertensive encephalopathy. When patients have elevated blood pressure and a different cause for PRES, such as medication-induced, there is no specific literature suggesting a targeted lowering of blood pressure. Normalization of blood pressure to pre-morbid levels is a reasonable approach for these patients. Drugs causing PRES such as cytotoxic agents require reduction in dosages or complete discontinuation [37,36,39]. Complete discontinuation in the case of immunosuppressant medications may not be feasible.
Further management of PRES depends on associated conditions contributing to this syndrome as described in Table 1. Treatment of seizures which commonly accompany PRES includes benzodiazepines followed by phenytoin or leviteracetam. In cases of PRES leading to status epilepticus, anesthetic agents like propofol or midazolam infusion should be used along with anti-epileptic drugs. However, in cases of PRES associated with eclampsia, magnesium is considered the drug of choice. Eclampsia causing PRES may also necessitate emergent cesarean section delivery.
Given the pathophysiology of PRES and the associated vasogenic edema, the role of corticosteroids has been widely debated. Corticosteroids have been both implicated as causative of PRES and used for treatment in a number of case reports [40, 41]. There exist conflicting schools of thought pertaining to steroid induced hypertension as an etiological factor versus anti-inflammatory effects of corticosteroids which may contribute to reduction of vasogenic edema and resolution of PRES. A recent retrospective analysis by Parikh et al. showed a possible association of corticosteroid therapy and the development of PRES [40]. The mean duration corticosteroid use was 6 days before the onset of PRES. The study showed no benefit with continued corticosteroid therapy and no association of corticosteroid dose with the extent of edema. At this time, current literature does not support the use of corticosteroids in the treatment of PRES.
Future Directions
There remain many unanswered questions regarding the evaluation and management of patients with suspected hypertensive encephalopathy. In the absence of an accessible and simple gold standard for diagnosis, diagnostic uncertainty persists. It is probable that higher reimbursement tied to diagnostic codes for hypertensive encephalopathy leads to overdiagnosis of the disease in administrative data sources [42]. Future clarification of diagnostic criteria that include structured assessment of mental status change, imaging for PRES, digital retinal artery scans, and consideration of systemic vasculopathy could add substantially to our understanding of hypertensive encephalopathy. There may additionally be a role for non-invasive cerebral blood flow monitoring with either transcranial Doppler monitoring or evolving perfusion devices as both a diagnostic tool and measure to guide therapy [43]. In the management of PRES, there remains uncertainty as to whether corticosteroids and routine anti-epileptics may play a role in management. Research in these areas remains challenging due to the low incidence of true hypertensive encephalopathy and the critical nature of the diagnosis.
Conclusions
The patient in the case presentation had rapid improvement in encephalopathy with early blood pressure reduction. He manifested concomitant vascular pathology resultant of a hypertensive emergency. His nicardipine infusion was weaned as oral agents were added, and he required a short stay in the ICU without further complication.
Hypertensive encephalopathy and PRES are uncommon but critical diagnoses. Hypertensive encephalopathy is often considered in patients with markedly elevated blood pressure, but it is typically a diagnosis of exclusion that becomes fully recognized when symptoms improve with anti-hypertensive therapy. Imaging with MRI can confirm the presence of cerebral edema, particularly in patients with PRES, whether due to severely elevated blood pressure or other albeit less common etiologies such as those shown in Table 1. Blood pressure management targets up to a 25% reduction as soon as possible with subsequent more gradual reduction. The preferred agent in our experience is nicardipine. Further research is indicated both in improving the diagnosis of these critical diagnoses and defining treatment goals.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance.
•• Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. doi:https://doi.org/10.1161/HYP.0000000000000065. Newest guidelines from multiple specialties that redifines hypertension and provides extensive recommendations for evaluation and management.
Johnson W, Nguyen ML, Patel R. Hypertension crisis in the emergency department. Cardiol Clin. 2012;30(4):533–43. https://doi.org/10.1016/j.ccl.2012.07.011.
Adebayo O, Rogers RL. Hypertensive emergencies in the emergency department. Emerg Med Clin North Am. 2015;33(3):539–51. https://doi.org/10.1016/j.emc.2015.04.005.
Manning L, Robinson TG, Anderson CS. Control of blood pressure in hypertensive neurological emergencies. Curr Hypertens Rep. 2014;16(6):436. https://doi.org/10.1007/s11906-014-0436-x.
Suneja M, Sanders ML. Hypertensive emergency. Med Clin North Am. 2017;101(3):465–78. https://doi.org/10.1016/j.mcna.2016.12.007.
Polgreen LA, Suneja M, Tang F, Carter BL, Polgreen PM. Increasing trend in admissions for malignant hypertension and hypertensive encephalopathy in the United States. Hypertension. 2015;65(5):1002–7. https://doi.org/10.1161/HYPERTENSIONAHA.115.05241.
• Fischer M, Schmutzhard E. Posterior Reversible encephalopathy syndrome. J Neurol. 2017;264(8):1608–16. https://doi.org/10.1007/s00415-016-8377-8. Excellent review focusing on posterior reversible encephalopathy.
Brust JCM. Cerebral circulation: stroke. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. Nowalk, Connecticut: Appleton & Lange; 1991. p. 1041–9.
Orjuela K, Ruland SD. Hypertensive encephalopathy, posterior reversible encephalopathy syndrome, and eclampsia. In: Aiyagari V, Gorelick P, editors Hypertension and stroke clinical hypertension and vascular diseases Humana Press, Cham; 2016.
Oppenheimer BS, Fishbery AM. Hypertensive encephalopathy. Arch Intern Med. 1928;41(2):264–78. https://doi.org/10.1001/archinte.1928.00130140126010.
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. https://doi.org/10.1056/NEJM199602223340803.
•• Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–25. Overview of radiological findings in PRES.
Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry. 2017; https://doi.org/10.1136/jnnp-2017-316225.
Hamati AI. Neurological complications of systemic disease: children. In: Daroff RB, Jankovic J, Mazziotta JC, Pomeroy S, editors. Bradley’s neurology in clinical practice. China: Elsevier; 2016. p. 835–849.
Lee SY, Dinesh SK, Thomas J. Hypertension-induced reversible posterior leukoencephalopathy syndrome causing obstructive hydrocephalus. J Clin Neurosci. 2008;15(4):457–9. https://doi.org/10.1016/j.jocn.2006.12.019.
Bruce BB, Thulasi P, Fraser CL, Keadey MT, Ward A, Heilpern KL, et al. Diagnostic accuracy and use of nonmydriatic ocular fundus photography by emergency physicians: phase II of the FOTO-ED study. Ann Emerg Med. 2013;62(1):28–33 e1. https://doi.org/10.1016/j.annemergmed.2013.01.010.
Lamy C, Oppenheim C, Meder JF, Mas JL. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging. 2004;14(2):89–96. https://doi.org/10.1111/j.1552-6569.2004.tb00223.x.
Kozak OS, Wijdicks EF, Manno EM, Miley JT, Rabinstein AA. Status epilepticus as initial manifestation of posterior reversible encephalopathy syndrome. Neurology. 2007;69(9):894–7. https://doi.org/10.1212/01.wnl.0000269780.45472.16.
Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036–42. https://doi.org/10.3174/ajnr.A0928.
McKinney AM, Jagadeesan BD, Truwit CL. Central-variant posterior reversible encephalopathy syndrome: brainstem or basal ganglia involvement lacking cortical or subcortical cerebral edema. AJR Am J Roentgenol. 2013;201(3):631–8. https://doi.org/10.2214/AJR.12.9677.
McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189(4):904–12. https://doi.org/10.2214/AJR.07.2024.
de Havenon A, Joos Z, Longenecker L, Shah L, Ansari S, Digre K. Posterior reversible encephalopathy syndrome with spinal cord involvement. Neurology. 2014;83(22):2002–6. https://doi.org/10.1212/WNL.0000000000001026.
Ahn KJ, You WJ, Jeong SL, Lee JW, Kim BS, Lee JH, et al. Atypical manifestations of reversible posterior leukoencephalopathy syndrome: findings on diffusion imaging and ADC mapping. Neuroradiology. 2004;46(12):978–83. https://doi.org/10.1007/s00234-004-1276-1.
Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23(6):1038–48.
Toledano M, Fugate JE. Posterior reversible encephalopathy in the intensive care unit. Handb Clin Neurol. 2017;141:467–83. https://doi.org/10.1016/B978-0-444-63599-0.00026-0.
•• Jacquot C, Glastonbury CM, Tihan T. Is posterior reversible encephalopathy syndrome really reversible? Autopsy findings 4.5 years after radiographic resolution. Clin Neuropathol 2015;34(1):26–33. doi:https://doi.org/10.5414/NP300771. Interesting evaluation of potential non-reversible damage from PRES.
Zhang R, Jin L, Cheng H, Yang J, Duan YL, Huang S, et al. Reversible posterior leukoencephalopathy syndrome sometimes could be irreversible: a case following tumor lysis syndrome in childhood Burkitt's lymphoma. Chin Med J. 2016;129(4):480–3. https://doi.org/10.4103/0366-6999.176075.
Muiesan ML, Salvetti M, Amadoro V, di Somma S, Perlini S, Semplicini A, et al. An update on hypertensive emergencies and urgencies. J Cardiovasc Med (Hagerstown). 2015;16(5):372–82. https://doi.org/10.2459/JCM.0000000000000223.
Strandgaard S. Autoregulation of cerebral circulation in hypertension. Acta Neurol Scand Suppl. 1978;66:1–82.
Udeh CI, Ting M, Arango M, Mick S. Delayed presentation of nitroprusside-induced cyanide toxicity. Ann Thorac Surg. 2015;99(4):1432–4. https://doi.org/10.1016/j.athoracsur.2014.05.097.
• Miller JB, Kinni H, Amer A, Levy PD. Therapies to reduce blood pressure acutely. Curr Hypertens Rep. 2016;18(6):43. https://doi.org/10.1007/s11906-016-0651-8. Updated literature on medications for rapid blood pressure reduction.
Ipek E, Oktay AA, Krim SR. Hypertensive crisis: an update on clinical approach and management. Curr Opin Cardiol. 2017;32(4):397–406. https://doi.org/10.1097/HCO.0000000000000398.
Perez MI, Musini VM. Pharmacological interventions for hypertensive emergencies. Cochrane Database Syst Rev. 2008;1:CD003653. https://doi.org/10.1002/14651858.CD003653.pub3.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. https://doi.org/10.1001/jama.289.19.2560.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20. https://doi.org/10.1001/jama.2013.284427.
Deeks ED, Keating GM, Keam SJ. Clevidipine: a review of its use in the management of acute hypertension. Am J Cardiovasc Drugs. 2009;9(2):117–34. https://doi.org/10.2165/00129784-200909020-00006.
Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: a review. J Neurol. 1999;246(5):339–46. https://doi.org/10.1007/s004150050360.
Hayes D Jr, Adler B, Turner TL, Mansour HM. Alternative tacrolimus and sirolimus regimen associated with rapid resolution of posterior reversible encephalopathy syndrome after lung transplantation. Pediatr Neurol. 2014;50(3):272–5. https://doi.org/10.1016/j.pediatrneurol.2013.11.006.
Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–25. https://doi.org/10.1016/S1474-4422(15)00111-8.
Parikh NS, Schweitzer AD, Young RJ, Giambrone AE, Lyo J, Karimi S, et al. Corticosteroid therapy and severity of vasogenic edema in posterior reversible encephalopathy syndrome. J Neurol Sci. 2017;380:11–5. https://doi.org/10.1016/j.jns.2017.06.044.
Eaton JM. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(26):1744–5. author reply 6
• Janke AT, McNaughton CD, Brody AM, Welch RD, Levy PD. Trends in the incidence of hypertensive emergencies in US emergency departments from 2006 to 2013. J Am Heart Assoc. 2016;5(12) https://doi.org/10.1161/JAHA.116.004511. Key epidemiological data on hypertensive emergencies.
Tsalach A, Ratner E, Lokshin S, Silman Z, Breskin I, Budin N, et al. Cerebral autoregulation real-time monitoring. PLoS One. 2016;11(8):e0161907. https://doi.org/10.1371/journal.pone.0161907.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Levy reports grants and funds in part by the National Heart, Lung and Blood Institute, grants from Novartis, Trevena, Cardiorentis, and BMS, and personal fees from Novartis, Trevena, and Cardiorentis, outside the submitted work. The other authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and Emergency Medicine
Rights and permissions
About this article
Cite this article
Miller, J.B., Suchdev, K., Jayaprakash, N. et al. New Developments in Hypertensive Encephalopathy. Curr Hypertens Rep 20, 13 (2018). https://doi.org/10.1007/s11906-018-0813-y
Published:
DOI: https://doi.org/10.1007/s11906-018-0813-y