Abstract
The Joint United Nations Programme on HIV/AIDS (UNAIDS) recently updated its global targets for antiretroviral therapy (ART) coverage for HIV-positive persons under which 90 % of HIV-positive people are tested, 90 % of those are on ART, and 90 % of those achieve viral suppression. Treatment policy is moving toward treating all HIV-infected persons regardless of CD4 cell count—otherwise known as treatment as prevention—in order to realize the full therapeutic and preventive benefits of ART. Mathematical models have played an important role in guiding the development of these policies by projecting long-term health impacts and cost-effectiveness. To guide future policy, new mathematical models must consider the barriers patients face in receiving and taking ART. Here, we describe the HIV care cascade and ART delivery supply chain to examine how mathematical modeling can provide insight into cost-effective strategies for scaling-up ART coverage in sub-Saharan Africa and help achieve universal ART coverage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antiretroviral therapy (ART), if taken consistently, reduces the viral load in people infected with HIV by 100 times within 1 month of starting treatment and by 10,000 times within 1 year of starting treatment [1] rendering HIV-infected persons uninfectious to others. The HIV Prevention Trial Network (HPTN) 052 study confirmed ART’s preventive benefits by demonstrating a 96 % reduction (95 % confidence interval [CI] 73–99 %) in HIV transmission with early provision of ART to HIV-positive persons (CD4 350–550 cells/μL) compared to late provision (CD4≤250 cells/μL) [2•]. To optimize the therapeutic and preventive benefits of ART, HIV prevention guidelines now focus on treatment as prevention strategies that increase ART coverage irrespective of CD4 cell count [3, 4]. Studies have found correlations between increasing community-level ART uptake and decreasing HIV incidence, with a 1.4 % (95 % CI 0.9–1.9 %) decline in the risk of HIV acquisition for every 1 % increase in ART coverage in South Africa [5] and similar results in Canada [6] and the USA [7]. Mathematical modeling studies have provided evidence in support of treatment as prevention by suggesting that high ART coverage can bring the HIV burden to low endemic levels or eventually eliminate HIV [8••, 9, 10]. Importantly, by providing projections and insights into the dynamics of ART scale-up and the economic costs and benefits, mathematical models have contributed to guiding clinical trial protocols and policies for HIV prevention strategies such as ART, circumcision, PrEP, and condom scale-up [11, 12].
Despite the potential impact of treatment as prevention, few studies have explored the implementation of providing universal access to ART for all HIV-infected persons [13]. In studies that have followed people who have tested positive for HIV, retention rates at several stages of the HIV care cascade have been low [14]. A systematic review of HIV care in sub-Saharan Africa estimated that only 18 % of HIV-positive patients were retained from diagnosis to ART eligibility [15]. Home-based HIV testing and counseling (HTC) [16, 17] and community campaign-based HTC [18, 19•] have increased rates of testing and linkage to care (83.3 % testing for home HTC, 95 % CI 80.4–86.1 %) relative to facility-based HTC, and present platforms for delivering an array of health services [20], but these strategies have yet to be implemented at the population level. With treatment as prevention becoming a broadly accepted framework for HIV prevention [4], models can simulate varying levels of program efficiency and coverage [21], thus providing insight into the impact of strategies that strengthen health programs on the HIV burden [22].
Mathematical models have played a large role in transforming HIV research into health policies, but given the clear benefits of early treatment for infected people and the impact on the epidemic, what is needed now is a shift toward implementation science [23, 24]. Whereas modeling has thus far been used to assess the health impact and cost-effectiveness of interventions, it also has the potential to evaluate system-level delivery strategies. From the clinical perspective, these strategies must increase the proportion of HIV patients who are identified, treated, and virally suppressed. From the programmatic perspective, these strategies must improve the efficiency of HIV supply chains in order to cost-effectively develop and deliver medication as well as initiate and retain patients in care [25–27]. Ultimately, the goals of the clinical and programmatic aspects are to save lives and improve health. Here, we present an overview of recent mathematical models for HIV prevention and describe how incorporating implementation science into mathematical modeling provides a more nuanced understanding of how to achieve universal access to ART and maximize the benefits of treatment as prevention.
Current State of Mathematical Modeling for HIV Prevention
Modeling ART Scale-Up
Several mathematical models have been developed to explore the impact and cost-effectiveness of ART scale-up. Granich et al. [8••] used a deterministic, compartmental model to demonstrate that with annual HIV testing and immediate linkage to care, HIV incidence and prevalence could be reduced to less than 0.1 and 1 %, respectively, in 50 years. In a subsequent analysis [28], the authors assessed the cost-effectiveness of the same annual ART scale-up strategy but with varying ART-eligibility criteria. They found that under all scenarios, the intervention became cost-saving within 10 years. Walensky et al. [29] used a stochastic microsimulation model to evaluate ART scale-up as implemented in the HPTN 052 study, with similar ART initiation and dropout rates. They found that in South Africa and India, two countries with different HIV epidemics, early ART initiation as achieved in the HPTN 052 study would be very cost-effective at $590 and $530 per life-year saved, respectively. In South Africa, where the cost of medical care is higher than in India, the intervention was cost saving over 5 years due to the costs of medical care that are averted by early ART initiation. Finally, Cori et al. [30] used a deterministic, compartmental model to simulate the HPTN 071 trial (PopART), which is currently being implemented in Zambia and South Africa. The study assessed the feasibility of large-scale combination HIV prevention including home HTC, male circumcision, and ART provision regardless of CD4 cell count. The authors estimate that HIV incidence can be reduced by 60 % with universal ART regardless of CD4 cell count and by 20 % with ART coverage for persons with CD4≤350 cells/μL. The model does not include a cost-effectiveness analysis, but the PopART trial, as well as other trials of TasP [31], will collect data on the economic and financial costs for patients and healthcare programs, which will be incorporated into future modeling work.
Although the models discussed differed in their structures and assumptions, a comparison of 12 independently-developed mathematical models conducted by Eaton et al. [32] suggests that the effectiveness and cost-effectiveness of ART scale-up is robust across different models. Following the World Health Organization's (WHO) new recommendation of ART initiation at CD4≤500 cells/μL rather than CD4≤350 cells/μL, Eaton et al. [32] compared the estimated impact of the new guidelines among the different models and found that in all cases, scaling up ART according to the new WHO guidelines was expected to be cost-effective. Furthermore, providing ART to all those already known to be HIV-positive—thus, treatment as prevention—is very cost-effective over 20 years. The authors call for mathematical modeling to play a larger role in implementation science to provide insight into ART scale-up strategies and their costs.
Modeling Combination HIV Prevention
In addition to ART scale-up, other prevention interventions such as medical male circumcision [33] and pre-exposure prophylaxis (PrEP) [34, 35] may play important roles in reducing the HIV burden. Cremin et al. [9] evaluated the cost and effectiveness of varying levels of ART and PrEP coverage in KwaZulu-Natal, South Africa. If HIV-positive persons can be identified an average of 1 year following infection and ART coverage increases to 80 %, the model predicts that approximately 35 % of cumulative HIV infections will be averted over 10 years, with the impact being attenuated by the large proportion of infections coming from newly infected partners. In this scenario, PrEP provides an additional benefit by preventing infections from acutely-infected partners, though at an incremental cost-effectiveness ratio of $40,000 per infection averted, even with targeted PrEP delivery. Alsallaq et al. [36] modeled similar interventions in KwaZulu-Natal, South Africa, using four-yearly mass testing campaigns, to assess whether ART scale-up, medical male circumcision, and reduction in risky sexual behavior showed synergistic interactions. They estimated that synergy is at a maximum when interventions are sub-optimal due to the possibility of behavioral disinhibition, poor compliance, and dropout from ART care. Interventions that can further reduce transmission and are cost-effective, such as medical male circumcision and PrEP, will likely be incorporated in any HIV prevention program. Considering that HIV prevention received $19.1 billion USD of global funding in 2013 [37], even if all 34 million HIV-positive person received ART at an approximate cost of $500 per patient [38], there would remain $2 billion for these services.
Critical questions remain regarding how best to increase ART coverage. The models discussed above have made different assumptions about the frequency of testing, the proportion of people that are linked to care, and compliance and dropout rates, and each stage requires careful consideration. One aspect to consider is the cascade of care, which describes how patients link to care, start ART, receive support to increase compliance and reduce loss-to-follow-up, and finally, achieve sustainable viral suppression. Another aspect is operational research using dynamical models, which helps to determine the optimal allocation of resources given an appropriate objective function and to identify the logistical barriers and bottlenecks that are most important in relation to health outcomes. Current models do not adequately address these important issues (Table 1), but by clearly analyzing these stages, mathematical modeling can provide insight into the implementation science aspect of HIV prevention.
HIV Care Cascade
Pre-ART Care Cascade
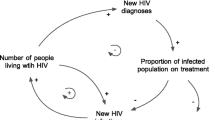
The steps for achieving viral suppression in an HIV-positive person, known as the care cascade (Fig. 1), can broadly be divided into pre-ART—HIV testing and linkage to and retention in pre-ART care—and post-ART—ART initiation, retention in care, and viral suppression.
Pre-ART stages of the cascade have historically been inefficient, but testing rates have increased in recent years. In Kenya, the proportion of adults ever tested for HIV increased from 34 % in 2007 to 71 % in 2012 [39] while in South Africa, it increased from 51 % in 2008 to 66 % in 2012 [40, 41]. New strategies for further increasing testing are also being studied. Chamie et al. [18] describe a community-wide health campaign in rural Uganda that provided HIV testing in addition to screening for malaria, TB, hypertension, and diabetes. Of the 63 % of residents who attended the campaign, 98 % received an HIV test and 38 % of them had never been previously tested. In Western Kenya, Lugada et al. [42] also used a week-long multi-disease prevention campaign to promote HIV testing and achieved 99.7 % testing uptake among the 96 % of participants who received the counseling package. Implementing these programs may help countries increase HIV testing as well as improve overall health outcomes.
Under past and current guidelines for ART initiation using a criterion based on CD4 cell count, not all infected people start ART when they test positive for HIV, and as a result, retention in pre-ART care has been low. A review of 28 studies suggested that only 18 % of HIV-positive persons across sub-Saharan Africa are in continuous care until linked to ART [15]. However, several studies have developed and implemented methods for improving HIV testing and linkage to care [43]. Van Rooyen et al. [16] used community-wide home HTC in Uganda and South Africa to achieve high levels of HIV testing (91 % tested) and linkage to ART (80 % among ART-eligible persons by month three). Involving sexual partners during HTC has also resulted in increased condom use in HIV-discordant couples in South Africa [44] and in higher uptake of HIV prevention services in Zimbabwe [45]. These studies suggest that substantial engagement with people as soon as they are found to be infected with HIV, such as ART initiation and involving sexual partners, would greatly reduce the initial loss of patients.
Few mathematical models have incorporated these testing strategies into their model scenarios, but three modeling studies that included periodic testing campaigns found that most of the benefits are realized before even the second testing campaign. Alsallaq et al. [36] used HIV testing campaigns every 4 years to link HIV-positive persons to ART and found that with 100 % ART uptake for persons with CD4≤350 cells/μL, as well as increases in circumcision and reductions in risky sexual behavior, HIV incidence could be reduced by 50 % within 4 years. Similarly, Barnabas et al. [46] suggest that five-yearly campaigns of home HTC in which 48 % of HIV-positive persons are virally suppressed can reduce HIV incidence by 33 % in 10 years. Dodd et al. [47] modeled HIV testing in different epidemiological contexts and found that testing and treating 80 % of the population every 2 years reduces HIV incidence by 95 % in regions with generalized HIV epidemics, but only by 60 % in concentrated HIV epidemics. Although these models estimate the impact of periodic testing campaigns, they do not include key steps in the pre-ART cascade. To model the cascade, Birger et al. [48•] developed a model for Newark, New Jersey, and estimated that if 68 % of HIV-positive people were virally suppressed, HIV incidence would decrease by only 16 % in 10 years, which they attribute to HIV testing not occurring randomly among all HIV-positive people, and instead, consistently missing certain individuals who do not test or seek HIV care. This study suggests that without a focus on all steps of the care cascade, even a high rate of testing may have a relatively modest impact.
Post-ART Care Cascade
Once people have been tested, found positive, and linked to pre-ART care, they must then initiate ART, be retained in care, and achieve viral suppression. In a study in South Africa, Katz et al. [49] found that 20 % of ART-eligible persons refused to initiate ART despite multiple counseling session and free medication, suggesting the need for improved counseling messages about the benefits of ART. Other studies indicate consistent late presentation to HIV care with the average CD4 count at ART initiation being 159 cells/μL (interquartile range [IQR] 65–299) in a South African cohort [50]. After initiating ART, many patients are lost to follow-up with a recent estimate of only 64.6 % (range 57.5–72.1 %) of patients remaining in care after 3 years across sub-Saharan Africa [51]. To study the outcomes of patients lost to follow-up in Mbarara, Uganda, Geng et al. [52] followed a cohort in which 39 % of ART patients were lost to follow-up in 3 years. They found that among the patients whose outcomes were collected, 36 % had died within 1 year and 83 % of the remaining patients had visited a different health facility within the previous 3 months. In Malawi, Yu et al. [53] found that 69 % of traced patients had died and 36 % of the remaining patients were still engaged in care. Despite the heterogeneity in outcomes, for patients truly lost from care, reengagement is critical.
To re-engage patients lost to follow-up, Rosen et al. [54] developed a strategy in which a social worker followed the 27 % of patients lost to follow-up who had discontinued ART and successfully re-engaged 30 % of them at a cost of $432 per patient returned. Rich et al. [55] describe a Rwandan ART program in which 92 % of patients were retained in care after 2 years and only 3 % were lost to follow-up. Their program addressed specific steps of the care cascade, including transportation allowance and community health worker (CHW) visits to increase retention, and directly observed ART delivery by CHWs, suggesting that by increasing engagement in care, the costs of patient tracing may be avoided. The importance of high retention in the care cascade also extends beyond ART initiation. In an analysis of data from Vancouver, Canada, increasing ART coverage was associated with a reduction in ART-resistant HIV [56]. Although it has been suggested that ART resistance may increase if treatment is more widely provided, Montaner et al. [57] show that with high levels of compliance and triple-drug therapy, early treatment can reduce levels of resistance. Although increasing retention in care may require up-front costs, it will be a critical aspect of a treatment as prevention program in order to reduce morbidity, mortality, and ART resistance.
Several models have been developed to analyze the issues of the post-ART care cascade. Based on the data from the “Back 2 Care program” in Malawi, Estill et al. [58] developed a mathematical model to estimate the impact of tracing persons who are lost to follow-up. They estimate that these patients contribute 2.1 HIV infections (95 % CI 1.8–2.2) per 100 patients over 5 years due to a doubling of cohort viral load. The authors emphasize that ensuring viral suppression by virological monitoring prevents more infections than tracing because of the immediate feedback that virological monitoring provides. In another modeling study of the care cascade, Klein et al. [59] found that increasing ART re-initiation and retention in care are the most cost-effective approaches for reducing DALYs and, as was also found by Eaton et al. [32], that starting all patients currently in care on ART is a cost-effective strategy for ART scale-up. To assess the consequences of “leakage’ in the cascade, Hoffman et al. [60] used a mathematical model to evaluate the excess mortality due to delays between ART eligibility and ART initiation and estimated that for patients with CD4≤150 cells/μL, a 6-month delay in ART initiation resulted in a 12 % increased risk of death.
These models analyze specific steps of the care cascade, but to estimate health system-level impacts, all steps must be included. Furthermore, detailed clinic-level data on the care cascade are necessary in order to understand leakages at specific steps and what strategies work best to improve the cascade. Hallett et al. [61] have suggested that the typical depiction of the care cascade is supplemented by a “side door” into ART programs, in which a patient initiates ART without engaging in pre-ART care, implying a nonlinear process that researchers must consider when assessing clinic data and evaluating interventions. Further exploration of the “side door” and other care cascade dynamics will be needed to understand their implications for universal ART coverage.
Logistics of Healthcare Delivery
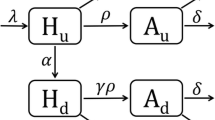
In 2011, the UNAIDS established a target to treat 15 million HIV-positive persons by 2015 [62]. As the target deadline approaches, many high-prevalence countries remain short of the target [62]. The leaky care cascade is a major demand-side determinant of this shortage, but supply-side factors exist as well, in particular, ensuring a continuous flow of medication through the supply chain, which is the process of physically transporting a product from manufacturer to consumer, involving various tiers of government and stages of delivery (Fig. 2).
Along the supply chain, poor storage facilities, lack of accurate forecasting data, and inefficient delivery have led to drug stock-outs and wasted drugs in many areas with the greatest need [63, 64, 65•]. To achieve the recent UNAIDS targets of diagnosing 90 % of HIV-positive persons, treating 90 % of diagnosed persons, and achieving viral suppression in 90 % of those on ART by the year 2020 [66], the logistical hurdles will need to be overcome. Innovative models of public-private partnerships have been developed to improve supply chain management [67], but more rigorous evaluations will be needed to produce results on the large scale. Furthermore, models of supply chain management need to be directly tied to health outcomes to ensure that the priority is improving health rather than reducing costs. Mathematical models can play an important role in bridging this gap and providing insight into optimal logistics structures [68, 69].
Current Adaptation of Delivery Costs
One way to merge supply chains with health outcomes is to aggregate the costs of ARV medication and delivery. Current estimates by the Clinton Health Access Initiative [38] and the Gates Foundation [70] include these costs as static cost accounting identities, and the USAID | DELIVER PROJECT [71] estimated the additional cost added due to final delivery of supplies to end users. However, incorporating the cost dynamically so that it varies with the HIV epidemic allows models to create cost functions that more accurately capture the contribution of logistics to the total cost of ART delivery [72••]. Previous studies have found economies of scale in HIV prevention programs [73–75], suggesting declines in per-client cost as programs are scaled up. In a country such as South Africa, where personnel costs represent nearly 50 % of ART delivery costs and only 32 % of HIV-positive people are on ART [41], economies of scale may result in substantial declines in overall costs. At this most basic level, cost functions relating program scale and ART cost can be implemented without restructuring HIV transmission models and can provide more accurate estimates of the overall cost of medication delivery.
Mathematical Models for Supply Chains
Models have been developed to assess how entire supply chains affect cost, but they fail to incorporate health outcomes. In Benin, Brown et al. [76] used a discrete-event, agent-based model to assess how the structure of Benin’s vaccine supply chain would impact the introduction of a new vaccine. They compared scenarios in which the numbers of tiers and departments within each tier were reduced and in which transportation relied on point-to-point delivery by motorcycles or on loops by delivery trucks. They found a synergistic effect when third tier “Communes” were replaced by existing “Health Zones” and motorcycle deliveries were replaced by truck loops. These changes reduced the logistics cost from $0.26 to $0.19 per vial delivered, saving $500,000 by 2017, and suggest that HIV programs may also benefit from similar optimization. The model was used in Niger by Assi et al. [77], who assessed the impact of reducing the number of tiers in the vaccine supply chain on operational efficiency. They found that by removing the regional-level stores and allowing district stores to collect from central stores rather than receive deliveries from the central store, vaccine availability increased from 70 to over 95 %, with the cost per dose administered falling from $0.14 to $0.13. Other models such as OneHealth [78] and Supply Chain Guru [79] have also been used in previous studies [71, 80], but have also only linked supply chains with costs without incorporating the impact on health. Previous models of other diseases have explicitly linked supply chains with health [81], and doing so for HIV would take advantage of the many rigorous models that exist for HIV transmission. Similar to the HIV care cascade, accurately modeling supply chains will require better clinic-level data that encompasses ART supply, ART delivery, and health outcomes [82, 83].
Other aspects of the HIV supply chain also warrant in-depth studies based on mathematical models. The delivery of laboratory tests is similar to medication delivery in that samples must be temperature controlled, deliveries must be timely, and a limited number of sites have the capacity to conduct and store laboratory tests [84, 85]. Spatial analyses, which have previously been used to study risk distribution for polio in Nigeria [86] and HIV in Kenya [87], may also contribute to optimizing laboratory test delivery [88]. Medication development will also need to expand capacity to treat the 34 million HIV patients, requiring large-scale changes to manufacturing processes [89]. Once manufacturing expands, strategies to pool purchases will be needed to reduce the cost of product purchasing [90]. Finally, the lack of accurate demand forecasting requires more accurate predictive modeling to guide the production of medications and reduce the volatility of funding cycles [91].
Conclusions
Universal access to ART for treatment as prevention is an ambitious goal that will require substantial logistical planning across all sectors of the HIV field. Mathematical models, in particular, can help to critically assess potential strategies to overcome barriers to universal ART access. Although many mathematical models have been developed in recent years, they have been similar in the scope of their questions. Changing the focus of modeling from the impact of ART scale-up to program implementation will utilize modeling’s strengths to guide future health policy. One pressing issue is the leaky HIV care cascade, which results in HIV transmission and loss of life that could be prevented by ART provision. Models studying the care cascade will help policymakers decide where to focus their efforts to identify, treat, and retain HIV patients. Another pressing issue is the inefficiency of the existing HIV supply chain, which results in delays, medication stock-outs, and overpriced medication. Modeling studies can evaluate how to optimize supply chain characteristics, such as the location of delivery centers, frequency of deliveries and pickups, and number of distribution tiers. Finally, it is also critical to connect these models to health outcomes in order to achieve the ultimate goal of preventing HIV infections and improving quality-of-life for HIV-positive persons. Mathematical models have increasingly been used to guide health policy around the world, but to reach the recent UNAIDS targets, new questions relevant to ART scale-up must be addressed. Expanding ART to millions of people has been proven feasible, but reaching all HIV-infected persons will require innovative service delivery. Mathematical models can drive this innovation by exploring key challenges and opportunities in developing efficient systems that maximize the potential of treatment as prevention.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–84.
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. This paper describes the results of the first randomized study of early ART treatment for preventing HIV transmission (HPTN 052) which demonstrated a 96% reduction in the risk of HIV transmission with early ART provision.
Delva W, Eaton JW, Meng F, Fraser C, White RG, Vickerman P, et al. HIV treatment as prevention: optimising the impact of expanded HIV treatment programmes. PLoS Med. 2012;9:e1001258.
Sidibé M, Zuniga JM, Montaner J. Leveraging HIV treatment to end AIDS, stop new HIV infections, and avoid the cost of inaction. Clin Infect Dis. 2014;59 Suppl 1:S3–6.
Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–71.
Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–9.
Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE. 2010;5:e11068.
Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. This mathematical modeling study is the first to suggest that HIV treatment as prevention can eliminate HIV transmission.
Cremin I, Alsallaq R, Dybul M, Piot P, Garnett G, Hallett TB. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS. 2013;27:447–58.
Eaton JW, Johnson LF, Salomon JA, Bärnighausen T, Bendavid E, Bershteyn A, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245.
Hayes R, Ayles H, Beyers N, Sabapathy K, Floyd S, Shanaube K, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment—a study protocol for a cluster randomised trial. Trials. 2014;15:57.
Easterbrook PJ, Doherty MC, Perriëns JH, Barcarolo JL, Hirnschall GO. The role of mathematical modelling in the development of recommendations in the 2013 WHO consolidated antiretroviral therapy guidelines. AIDS. 2014;28 Suppl 1:S85–92.
Wilson DP. HIV treatment as prevention: natural experiments highlight limits of antiretroviral treatment as HIV prevention. PLoS Med. 2012;9:e1001231.
Kilmarx PH, Mutasa-Apollo T. Patching a leaky pipe: the cascade of HIV care. Curr Opin HIV AIDS. 2013;8:59–64.
Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056.
van Rooyen H, Barnabas RV, Baeten JM, Phakathi Z, Joseph P, Krows M, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64:e1–8.
Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001351.
Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Uptake of community-based HIV testing during a multi-disease health campaign in rural Uganda. PLoS ONE. 2014;9:e84317.
Suthar AB, Ford N, Bachanas PJ, Wong VJ, Rajan JS, Saltzman AK, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10:e1001496. This review and meta-analysis summarizes various community-based modalities for increasing HIV testing and linkage to care.
Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS ONE. 2012;7:e43400.
Cohen MS, Smith MK, Muessig KE, Hallett TB, Powers KA, Kashuba AD. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here? Lancet. 2013;382:1515–24.
Lima VD, Thirumurthy H, Kahn JG, Saavedra J, Cárceres CF, Whiteside A. Modeling scenarios for the end of AIDS. Clin Infect Dis. 2014;59 Suppl 1:S16–20.
El-Sadr WM, Philip NM, Justman J. Letting HIV transform academia—embracing implementation science. N Engl J Med. 2014;370:1679–81.
Young B, Zuniga JM, Montaner J, Mayer KH. Controlling the HIV epidemic with antiretrovirals: moving from consensus to implementation. Clin Infect Dis. 2014;59 Suppl 1:S1–2.
Wilson D, Fraser N. Who pays and why? Costs, effectiveness, and feasibility of HIV treatment as prevention. Clin Infect Dis. 2014;59 Suppl 1:S28–31.
McNairy ML, El-Sadr WM. A paradigm shift: focus on the HIV prevention continuum. Clin Infect Dis. 2014;59 Suppl 1:S12–5.
Nachega JB, Uthman OA, Del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis. 2014;59 Suppl 1:S21–7.
Granich R, Kahn JG, Bennett R, Holmes CB, Garg N, Serenata C, et al. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost-effectiveness 2011-2050. PLoS ONE. 2012;7:e30216.
Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369:1715–25.
Cori A, Ayles H, Beyers N, Schaap A, Floyd S, Sabapathy K, et al. HPTN 071 (PopART): a cluster-randomized trial of the population impact of an HIV combination prevention intervention including universal testing and treatment: mathematical model. PLoS ONE. 2014;9:e84511.
Granich R, Gupta S, Suthar AB, Smyth C, Hoos D, Vitoria M, et al. Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res. 2011;9:446–69.
Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, Cori A, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. 2013;2:23–34.
Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56.
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410.
Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74.
Alsallaq RA, Baeten JM, Celum CL, Hughes JP, Abu-Raddad LJ, Barnabas RV, et al. Understanding the potential impact of a combination HIV prevention intervention in a hyper-endemic community. PLoS ONE. 2013;8:e54575.
Kates J, Wexler A, Lief E. Financing the response to HIV in low- and middle-income countries: international assistance from donor governments in 2013. Kaiser Family Foundation; 2014.
Facility-based unit costing for antiretroviral treatment in five Sub-Saharan African countries. The Clinton Health Access Initiative; 2011.
National AIDS and STI Control Programme (NASCOP), Kenya. Kenya AIDS indicator survey 2012: final report. Nairobi: NASCOP; 2014.
Shisana O, Rehle T, Simbayi L, Parker W, Jooste S, van Wyk VP, et al. South African national HIV prevalence, incidence, behavior and communication survey 2008: a turning tide among teenagers? Cape Town, South Africa; 2008.
Shisana O, Rehle T, Simbayi L, Zuma K, Jooste S, Zungu N, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town; 2014.
Lugada E, Millar D, Haskew J, Grabowsky M, Garg N, Vestergaard M, et al. Rapid implementation of an integrated large-scale HIV counseling and testing, malaria, and diarrhea prevention campaign in rural Kenya. PLoS ONE. 2010;5:e12435.
Lazarus JV, Safreed-Harmon K, Nicholson J, Jaffar S. Health service delivery models for the provision of antiretroviral therapy in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2014.
Rosenberg NE, Pettifor AE, De Bruyn G, Westreich D, Delany-Moretlwe S, Behets F, et al. HIV testing and counseling leads to immediate consistent condom use among South African stable HIV-discordant couples. J Acquir Immune Defic Syndr. 2013;62:226–33.
Montgomery ET, van der Straten A, Chidanyika A, Chipato T, Jaffar S, Padian N. The importance of male partner involvement for women’s acceptability and adherence to female-initiated HIV prevention methods in Zimbabwe. AIDS Behav. 2011;15:959–69.
Barnabas R, Ying R, van Rooyen H, Murnane P, Hughes J, Baeten J, et al. Use of HIV viral-load suppression to estimate the effect of community-wide home-based HIV counselling and testing and linkage to antiretroviral therapy on HIV incidence in South Africa: a mathematical modelling analysis. Lancet. 2013;382(Supplement 2):S6.
Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by ‘test and treat’ in hyperendemic settings. AIDS. 2010;24:729–35.
Birger RB, Hallett TB, Sinha A, Grenfell BT, Hodder SL. Modeling the impact of interventions along the HIV continuum of care in Newark, New Jersey. Clin Infect Dis. 2013. This mathematical model simulates the HIV care cascade in Newark, New Jersey, and is one of the first models to critically assess specific steps in the care cascade.
Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25:2177–81.
Bassett IV, Regan S, Chetty S, Giddy J, Uhler LM, Holst H, et al. Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. AIDS. 2010;24 Suppl 1:S37–44.
Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15 Suppl 1:1–15.
Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53:405–11.
Yu JK, Chen SC, Wang KY, Chang CS, Makombe SD, Schouten EJ, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–4.
Rosen S, Ketlhapile M. Cost of using a patient tracer to reduce loss to follow-up and ascertain patient status in a large antiretroviral therapy program in Johannesburg, South Africa. Trop Med Int Health. 2010;15 Suppl 1:98–104.
Rich ML, Miller AC, Niyigena P, Franke MF, Niyonzima JB, Socci A, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. J Acquir Immune Defic Syndr. 2012;59:e35–42.
Montaner JS, Lima VD, Harrigan PR, Lourenço L, Yip B, Nosyk B, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV treatment as prevention” experience in a Canadian setting. PLoS ONE. 2014;9:e87872.
Nosyk B, Montaner JS, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14:40–9.
Estill J, Tweya H, Egger M, Wandeler G, Feldacker C, Johnson LF, et al. Tracing of patients lost to follow-up and HIV transmission: mathematical modeling study based on 2 large ART programs in Malawi. J Acquir Immune Defic Syndr. 2014;65:e179–86.
Klein DJ, Bershteyn A, Eckhoff PA. Dropout and re-enrollment: implications for epidemiological projections of treatment programs. AIDS. 2014;28 Suppl 1:S47–59.
Hoffmann CJ, Lewis JJ, Dowdy DW, Fielding KL, Grant AD, Martinson NA, et al. Mortality associated with delays between clinic entry and ART initiation in resource-limited settings: results of a transition-state model. J Acquir Immune Defic Syndr. 2013;63:105–11.
Hallett TB, Eaton JW. A side door into care cascade for HIV-infected patients? J Acquir Immune Defic Syndr. 2013;63 Suppl 2:S228–32.
UNAIDS/WHO. Global report: UNAIDS report on the global AIDS epidemic, 2013. Joint United Nations Programme on HIV/AIDS; 2013.
Tolle MA, Phelps BR, Desmond C, Sugandhi N, Omeogu C, Jamieson D, et al. Delivering pediatric HIV care in resource-limited settings: cost considerations in an expanded response. AIDS. 2013;27 Suppl 2:S179–86.
Okoronkwo I, Okeke U, Chinweuba A, Iheanacho P. Nonadherence factors and sociodemographic characteristics of HIV-infected adults receiving antiretroviral therapy in Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria. ISRN AIDS. 2013;2013:843794.
Kraiselburd S, Yadav P. Supply chains and global health: an imperative for bringing operations management scholarship into action, production and operations management. Production and operations management; 2013. pp. 377–381. This paper discusses the importance of supply chains in delivering critical medications in global health, as well as current supply chain weaknesses.
Ambitious treatment targets: writing the final chapter of the AIDS epidemic. Geneva: UNAIDS; 2014.
Fernandez M. Improving access to critical medicines: the last mile. HIV treatment as prevention workshop. Vancouver; 2014.
Trochim WM, Cabrera DA, Milstein B, Gallagher RS, Leischow SJ. Practical challenges of systems thinking and modeling in public health. Am J Public Health. 2006;96:538–46.
Leischow SJ, Milstein B. Systems thinking and modeling for public health practice. Am J Public Health. 2006;96:403–5.
Bill & Melinda Gates Foundation. Oral PrEP in South Africa. Bottom-up cost model.
Rosen JE, Bancroft E, Hasselback L, Levin C, Mvundura M, Tien M. Last mile costs of public health supply chains in developing countries: recommendations for inclusion in the United Nations OneHealth Model. Arlington: USAID | DELIVER PROJECT, Task Order 4; 2012.
Meyer-Rath G, Over M. HIV treatment as prevention: modelling the cost of antiretroviral treatment—state of the art and future directions. PLoS Med. 2012;9:e1001247. The authors explain the importance of including cost functions into mathematical models to provide more accurate estimates of the programmatic costs of ART provision.
Marseille E, Dandona L, Marshall N, Gaist P, Bautista-Arredondo S, Rollins B, et al. HIV prevention costs and program scale: data from the PANCEA project in five low and middle-income countries. BMC Health Serv Res. 2007;7:108.
Guinness L, Kumaranayake L, Rajaraman B, Sankaranarayanan G, Vannela G, Raghupathi P, et al. Does scale matter? The costs of HIV-prevention interventions for commercial sex workers in India. Bull World Health Organ. 2005;83:747–55.
Guinness L, Kumaranayake L, Hanson K. A cost function for HIV prevention services: is there a ‘u’-shape? Cost Eff Resour Alloc. 2007;5:13.
Brown ST, Schreiber B, Cakouros BE, Wateska AR, Dicko HM, Connor DL, et al. The benefits of redesigning Benin’s vaccine supply chain. Vaccine. 2014;32:4097–103.
Assi TM, Brown ST, Kone S, Norman BA, Djibo A, Connor DL, et al. Removing the regional level from the Niger vaccine supply chain. Vaccine. 2013;31:2828–34.
Futures Institute. OneHealthTool. 2011.
Llamasoft. Supply Chain Guru. 2014.
Jamison DT, Summers LH, Alleyne G, Arrow KJ, Berkley S, Binagwaho A, et al. Global health 2035: a world converging within a generation. Lancet. 2013;382:1898–955.
Ekici A, Keskinocak P, Swann JL. Pandemic influenza response. Winter simulation conference. Miami, FL; 2008.
Mvundura M, Kien VD, Nga NT, Robertson J, Cuong NV, Tung HT, et al. How much does it cost to get a dose of vaccine to the service delivery location? Empirical evidence from Vietnam’s Expanded Program on Immunization. Vaccine. 2014;32:834–8.
McChord J, Tien M, Sarley D. Guide to public health supply chain costing: a basic methodology. Arlington: USAID | DELIVER PROJECT, Task Order 4; 2013.
Nkengasong JN, Nsubuga P, Nwanyanwu O, Gershy-Damet GM, Roscigno G, Bulterys M, et al. Laboratory systems and services are critical in global health: time to end the neglect? Am J Clin Pathol. 2010;134:368–73.
Alemnji G, Fonjungo P, Van Der Pol B, Peter T, Kantor R, Nkengasong J. The centrality of laboratory services in the HIV treatment and prevention cascade: the need for effective linkages and referrals in resource-limited settings. AIDS Patient Care STDS. 2014;28:268–73.
Upfill-Brown AM, Lyons HM, Pate MA, Shuaib F, Baig S, Hu H, et al. Predictive spatial risk model of poliovirus to aid prioritization and hasten eradication in Nigeria. BMC Med. 2014;12:92.
Anderson SJ, Cherutich P, Kilonzo N, Cremin I, Fecht D, Kimanga D, et al. Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. Lancet. 2014;384:249–56.
Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–61.
Reiser H. Making new drugs available to all. HIV treatment as prevention workshop. Vancouver, Canada; 2014.
Huff-Rousselle M. The logical underpinnings and benefits of pooled pharmaceutical procurement: a pragmatic role for our public institutions? Soc Sci Med. 2012;75:1572–80.
Levine R, Pickett J, Sekhri N, Yadav P. Demand forecasting for essential medical technologies. Am J Law Med. 2008;34:225–55.
Compliance with Ethics Guidelines
Conflict of Interest
Roger Ying, Ruanne V. Barnabas, and Brian G. Williams declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ying, R., Barnabas, R.V. & Williams, B.G. Modeling the Implementation of Universal Coverage for HIV Treatment as Prevention and its Impact on the HIV Epidemic. Curr HIV/AIDS Rep 11, 459–467 (2014). https://doi.org/10.1007/s11904-014-0232-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-014-0232-x