Abstract
Purpose of Review
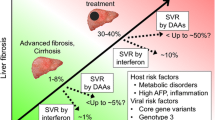
The hepatitis C virus (HCV) is a major cause of liver-related morbidity and mortality, and a major risk factor of hepatocellular carcinoma (HCC) around the world. Early detection, continued prevention of transmission, and antiviral treatment of chronically infected persons are the pillars to decrease incidence and mortality of HCV. The widespread access to safe and effective direct acting anti-viral agents (DAA) has allowed the elimination of the infection possible in almost all treated patients, thus leading to a significant reduction of liver-related and overall mortality due to HCV in the cured population. Treatment of HCV does not completely eradicate HCC risk in populations with advanced liver disease and those with cofactors known to promote liver carcinogenesis such as diabetes, obesity, and excessive alcohol consumption.
Recent Findings
Molecular-based biomarkers are expected to overcome the limits of liver disease severity, thus improving the identification of screening candidates.
Summary
The implementation of risk-stratified surveillance programs coupled with the identification of biomarkers to predict HCC in HCV cured patents is deemed necessary for implementing the cost-effective management of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hepatitis C virus (HCV), a single-stranded RNA virus belonging to the Flaviviridae family, is globally responsible for more than 150,000 deaths from hepatocellular carcinoma (HCC), every year [1]. This is the inevitable consequence of 58.5 million people living lifelong with this virus, a huge reservoir of chronic hepatitis C that is refuelled by more than 1.5 million new infections every year. The fact that the number of new infections with HCV surpasses the number of people being treated leads to a restless accumulation of chronically infected persons who remain at lifelong risk of developing end-stage liver disease and HCC [2, 3]. From 1990 to 2019, the prevalence of HCV-related HCCs rose from 22 to 28% [4], reflecting not only a reshuffle of the global incidence of the various risk factors for HCC but also the increased longevity of the exposed population which allows full expression of the carcinogenetic potential of HCC risk factors. In 2020, liver cancer in persons chronically infected by HCV rose to more than 50% of all liver cancers in countries with the highest burdens, such as the USA, Pakistan, and Egypt. Compared to hepatitis B virus, the global leading risk factor of HCC and the dominant factor of HCC mortality in males, HCV is the first and second leading cause of liver cancer deaths for females and males, respectively [1]. As a matter of fact, the incidence of HCC is on the raise globally, with an expected increase from 841,000 cases in 2018 to 1.4 million cases in 2040 that fuels increasing trends of mortality rates in less economically developed countries [5]. Currently, despite the availability of such highly performant antiviral therapy to treat hepatitis C as direct antiviral agents (DAA), only one-fifth of all viraemic individuals have been identified, making therefore the WHO target of the elimination of HCV as a global health threat by 2030 unlikely to be reached [6]. To strengthen the commitment of the stakeholders engaged in the fight against HCV, the European Association for the Study of the Liver (EASL) has suggested the positioning of hepatitis C in the context of Europe’s efforts to prevent cancer, with the aim of including HCC among lifesaving screening programs that are recommended by the Commission [7]. Owing to the fact that the risk of neoplastic transformation of the liver stays lifelong even after pharmacological eradication of HCV, but it is magnified in patients with advanced liver disease [8,9,10], identification and treatment of early HCV infection become imperative. This is even more so in patients with additional liver injuries like those with overweight, alcohol abuse, tobacco smoking, diabetes, hepatitis B, or HIV, co-morbidities that add on to the carcinogenic potential of HCV that is expressed both as genetic and epigenetic changes in the liver cells [11]. At the same time, the accumulation of cured patients with any stage of hepatitis C calls for the implementation of cost-effective, risk-stratified screening aimed at optimizing both early diagnosis and cure of liver cancer [12]. Unfortunately, working against this goal is the histological and molecular heterogeneity of the tumour, not to speak of the time dependence of the outcomes in patients with HCC, the lack of calibration studies of the currently available biomarkers and their nonlinear trajectory over time, which conflicts with the linearity of the models utilized for assessing the predictive power of such biomarkers.
Mechanisms of HCV-Related Liver Carcinogenesis
The neoplastic transformation of an HCV-infected liver is a multifactorial process driven by persisting liver cell inflammation associated with unrested virus replication as documented by the clear epidemiological association that exists between HCC and cirrhosis. The starting point is the persistence of an immune cell-mediated attack to the infected liver cells which causes the release of reactive oxygen species (ROS) and pro-inflammatory cytokines by liver, natural killer, and T cells. Liver inflammation, however, should be regarded as a double-edged sword as, in certain contexts, it may be a favourable histologic predictor of HCC outcome, owing to the fact that immune cell infiltrates may circumvent cancer-dependent immunotolerance and destroy transformed liver cells [13]. On the other hand, persistent necro-inflammation of the liver cells is harmful to it fuels the process of oxidative stress resulting in the induction of epigenetic and oncogenic alterations, and telomere shortening, altogether well-known drivers of genomic instability [13,14,15,16]. The fibrotic remodelling of the liver consequent to the chronic inflammation that is elicited by HCV is an add on complication as it fuels the process of liver carcinogenesis driven by the core and the non-structural NS5A proteins of the virus, a mechanism that impairs liver cell homeostasis [17,18,19,20]. All in all, the virus ability of evading the virus-neutralizing response of the host immunity is the turning point allowing HCV to hijack the homeostatic mechanisms of the liver cells and promote liver carcinogenesis [14,15,16]. In this context, gut microbiota seems to play a significant role as suggested by the increased incidence of liver cancer that has been observed in mice transplanted with a microbiota from patients with HCC as compared with mice with transplanted microbiota from healthy donors [21].
Which HCV Infected Individuals Are at Risk of HCC?
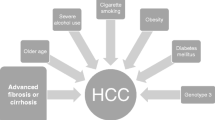
Any longstanding infection with HCV builds on the risk of liver carcinogenesis, which, however, appears to be accelerated by the hepatic accumulation of fibrosis, co-occurrence of metabolic morbidities, and such lifestyle factors as alcohol abuse, tobacco smoking, and sedentary life. For the sake of cost effectiveness, surveillance with abdominal ultrasound is recommended whenever the risk of developing HCC approaches the threshold of 1.5% per year, a recommendation that holds true for both viraemic and DAA-cured individuals with chronic hepatitis C [8,9,10, 13]. Not surprisingly, the annual risk of HCC in DAA-cured patients with advanced hepatitis C is much lower than that in untreated or patients who failed to respond to anti-HCV therapy (1.8% vs. 2.8%), as reported in a large cohorts of veterans in the USA [22]. HCC risk in individuals achieving the pharmacological eradication of HCV tends to be more profoundly attenuated in the absence of advanced fibrosis compared to what happens when liver shows either pre-cirrhotic or cirrhotic changes. At the same time, HCC risk is exacerbated by the coexistence of co-morbidities like diabetes, overweight/obesity, tobacco smoking, and heavy alcohol use, probably because they fuel liver cell inflammation, and the combinations of risk factors were often shown to be synergistic rather than additive [23,24,25]. According to a simulation model (HEP-SIM) devised to predict the burden of US population with HCV in need of HCC surveillance, HCC incidence was predicted to shrink from 30,000 people in 2012 to 13,000 in 2040, whereas the percentage of candidates with a cured hepatitis C in need of surveillance was estimated to rise from 8.5% in 2012 to 64.6% in 2040, totalling 6000 per 100 thousand/year [26]. In the era of hepatitis C elimination, the exponential growth of HCV-cured individuals who may benefit from HCC surveillance has made impellent the development of cost-effective screening programs based on HCC risk stratification, as seen in Table 1, mainly focused on the identification of patients with advanced liver disease. To assess liver disease severity, a widely adopted approach is the simplified algorithm FIB-4 score, which combines the routine chemistries transaminases and platelet count with patient age. The > 3.25 cut-off, which identifies patients with cirrhosis, is also able to separate non-cirrhotic patients with a > 2% annual risk of HCC from similar patients with < 0.5% annual risk of malignant transformation of the liver, for whom surveillance does not appear to be cost effective [30,31,32,33, 30, 31]. All attempts to reinforce clinical algorithms for HCC prediction through the incorporation of genetic scores failed to convincingly serve the purpose. One recent example is the incorporation of polygenic scores in cirrhotic patients with a cured HCV or alcoholic disease, where the aMAP score built on age, sex, albumin, bilirubin, and platelet count was potentiated by the incorporation of six single-nucleotide polymorphisms (SNIPS) for the lipid metabolism and one SNIP for Wnt-beta-catenin into a. This model failed to predict HCC with respect to aMAP alone [34]. Alarmingly enough, screening of the general population for other cancers like prostate, breast, and colon cancer did not provide survival benefits when the standard screening tests were coupled with a polygenic score compared to the standard screening tests alone [35]. Though the race towards risk-stratified screening for HCC remains a priority as it should to provide less-intensive screening to low-risk individuals and reduce the unnecessary harms and costs of over-screening, yet the implementation of risk-stratified surveillance for HCC has to cope with numerous hurdles. These include the histological and molecular heterogeneity of the tumour, the lack of external validation, and calibration of most of the current biomarkers and the time dependence of the outcomes.
Strategies of HCC Surveillance
Ultrasound is the standard of care imaging modality recommended for HCC surveillance by all liver societies for both viraemic and HCV-cured patients. Semi-annual abdominal ultrasound exam is recommended by all societies for patients with cirrhosis, whereas surveillance of those with metavir F3 stage is advised by EASL and APASL only. Indeed, AASLD recommends against surveillance of patients with advanced fibrosis but without cirrhosis. Alpha fetoprotein (AFP) is insufficient as a standard alone biomarker for HCC screening, but it has a role in conjunction with other tests for the early detection of HCC [8, 31, 36]. Risk-stratified screening with ultrasound and high scores of GALAD, a phase III validated biomarker that includes gender, age, AFP-L3, AFP and des-carboxy prothrombin (DCP) level, is recommended by the International Liver Cancer Association ILCA for patients with advanced fibrosis [37]. However, in a prospective cohort phase III study of patients with HCV, alcohol abuse, or NAFLD, GALAD was associated with an increase in false-positive results that caused the score performance to be modest and not different from the standard of care US screening [38].
Surveillance with abdominal ultrasound confers significant clinical benefits in patients with cirrhosis in virtue of its ability to identify liver cancer at a curable stage. This is the convincing message of a systemic review of 59 studies comparing 41,052 patients with HCC detected by surveillance and 104,344 patients with an incident HCC, where patients under surveillance had 1.86 (95% CI 1.73–1.98) gain of early detected tumours, 1.83 (95% CI 1.69–1.97) gain of receiving curative therapies, and 0.67 (95% CI 0.61–0.72) reduction of mortality. Interestingly, all those clinical benefits came at the expenses of mild in severity harms that affected 8.8%–27.8% of the individuals [39]. In a previous meta-analysis of 32 studies with 13,367 patients, the same group reported quite a low diagnostic sensitivity of ultrasound of 47% (95% CI 33%–61%), that however could be raised to 63% (95% CI 48%–75%) by combined determination of serum AFP [40]. Though semi-annual surveillance with ultrasound is associated with superior survival compared to annual surveillance (40.3 vs. 30 months, p = 0.03), yet overall survival cannot be further improved with quarterly surveillance [41, 42]. As seen in Table 2, the recommendations of the international societies do not perfectly match with each other with respect to the recommendations for liver biopsy and second level imaging techniques that are employed to achieve the final diagnosis of HCC.
Surveillance is recommended in patients with clinical decompensation only when liver transplantation is an option, whereas pending is the identification of what age cut off stops surveillance to be cost-effective. In HCV-cured patients with advanced fibrosis, biannual HCC surveillance with ultrasound and AFP was considered cost-effective up to the age of 60, resulting in additional 23 quality adjusted life years and detection of 24 potentially curable tumours per 1000 patients [44]. From a public health perspective, this has important implications given the growing population of aged patients with a cured hepatitis C, in whom HCC risk is not abolished following HCV eradication, whereas virus sterilization is widely recognized to increase life expectancy in consequence of a reduction of mortality from clinical decompensation and extra hepatic complications of hepatitis C [45]. In countries that are on track of reaching the WHO goals of HCV elimination by 2030, the population with a cured hepatitis C who are in need of surveillance is expanding to a point to surpass the canonical population with HCV-related HCC, further supporting the race towards development of cost-effective strategies of HCC surveillance. However, mitigating against the effectiveness of HCC surveillance in these populations are several hurdles that span from the underuse of screening to the suboptimal accuracy of currently available screening tests. To improve patient adherence to screening, programs of mail outreach coupled with specific training of nurses and dedicated pathways to screening have proven to be of some efficacy [46]. As seen in Fig. 1, accuracy of screening tests can be improved with two-phase CT and contrast-enhanced MRI that in one study yielded superior sensitivity for early-stage HCC detection than ultrasound (86% vs. 29%, respectively). However, the use of second-level imaging is limited by cost, radiologic capacity, and potential adverse effects by contrast and/or radiation exposure [47, 48]. A user-friendly diagnostic approach like abbreviate magnetic resonance imaging (AMRI) might better serve certain patients like obese individuals, patients with metabolic associated steato-hepatitis, and those with decompensated cirrhosis. In a meta-analysis of 15 studies including 917 patients with HCC, this approach allowed detection of any-size tumour in 86% of the affected patients and in 69% of those with < 2 cm HCC [53•]. AMRI overcame ultrasound in terms of sensitivity while it paralleled contrast-enhanced AMRI in terms of specificity (94%). Mitigating the appeal of non-contrast AMRI represented by low invasiveness, cost, and repeatability are some technical constrains like the lower contrast-to-noise ratio (CNR) and dependence from diffusion-weighted imaging (DWI) that may challenge the identification of HCC nodules [54, 55]. Noticeably, patients investigated with second-level imaging techniques and, in general, low-risk patients may suffer potential harms related to overdiagnosis that offset the minimal benefits of screening [56].
The Remaining Challenges
In low- and middle-income countries, the price of medications and diagnostics has been the main obstacle to scaling up testing and treatment of hepatitis C; thus, reducing cost of antiviral therapy is a prerequisite for implementing the WHO plans of HCV elimination on a global scale. A significant step forward in this direction was taken by the Clinton Health Access Initiative and the Hepatitis Fund that recently announced pricing breakthrough to reduce cost of viral hepatitis treatment by over 90% [57]. Another pillar of the fight against the lethal consequences of hepatitis C is the optimization of secondary prevention of HCV-related HCC which concerns also individuals with a cured infection. While the lack of performant biomarkers for risk-stratified screening of individuals at risk of developing HCC stands as a major hurdle to the implementation of cost-effective programs of HCC surveillance; at present a heterogeneous array of experimental blood-based biomarkers are under scrutiny for prediction of HCC in both viraemic and cured individuals. Promising data are accumulating on the use of vehicles of small RNA clusters like the extracellular vesicles, cell-free DNA, and polygenic algorithms combined with demographic features of the patient and classical biomarkers of HCC: all in all, those studies reported > 90% specificity and 74%–100% sensitivity of the newest biomarkers [58]. While needing to be prospectively and externally validated, these biomarkers might allow for risk-stratified surveillance to become a standard of care to be employed in the secondary prophylaxis of HCV-related HCC, too. With this in mind, biannual ultrasound should be recommended for low-risk patients, whereas patients at higher risk of HCC should be more aggressively screened using AMRI or CT-scan along intensified screening intervals. In patients at intermediate risk of HCC, surveillance should be reinforced through education programs, mailed outreach, and dedicated pathways. Remaining challenges are the lack of validated cut-offs for risk stratification and difficulty of granting an increased access to AMRI or CT scan for millions of screening candidates worldwide. Owing to the fact that the future of surveillance of infectious diseases might be shaped by emerging forms of artificial intelligence, right now the latter may be of strategic importance in the educational activity. As a matter of fact, a questionnaire-based study that was conducted in two liver transplant centres in the USA to investigate the power of generative pretrained transformers (GPT) to empower patients and improve health literacy in the liver cancer domain gave a rather disappointing message. The study, in fact, highlighted better responses on basic knowledge, life style, and treatment of liver cancer (and cirrhosis) than those regarding diagnosis and preventing medicine, as highlighted by the rates of comprehensive responses (41.1%) of the 164 questions graded by two transplant hepatologists, only [59]. As expected, central to primary prevention of hepatitis C–related HCC remains the development of a protective vaccine, owing to the fact that despite any effort to prevent HCV transmission and treating the reservoir of infected individuals, new infections continue to outpace achieving SVR. Despite promising interactions with virus replication, there is limited evidence to date that currently developed vaccines are protective against HCV [60].
Conclusion
Over the past decade, the advent of DAAs has allowed for HCV cure in almost all treated patients, reducing liver-related and overall mortality in this population. However, the risk of developing HCC remains in subsets of patients that require lifelong surveillance. Today, international scientific liver societies recommend biannual HCC screening in post-SVR patients with cirrhosis/advanced liver fibrosis by US with or without AFP. However, the non-screened population, which is growing by the day, does not have a homogeneous risk of HCC occurrence, with patients characterized by cofactors such as diabetes, obesity, and excessive alcohol consumption still being at risk of developing HCC. On the other hand, the current HCC surveillance strategy has limitations in terms of compliance and ultrasound sensitivity. For these reasons, there is a strong need for reliable and widely accepted risk stratification tools that can be used to narrow the under-screened population, focus efforts on the high-risk population, and improve the cost-effectiveness of current screening programs. Ideally this could lead to personalized screening strategies based on the individual risk of HCC, reserving tools with better sensitivity, such as AMRI or low-dose CT, for the higher-risk patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Tan DJH, Setiawan VW, Ng CH, Lim WH, Muthiah MD, Tan EX, et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology. 2023;77:1150–63.
Blach S, Terrault NA, Tacke F, Gamkrelidze I, Craxi A, Tanaka J, et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415.
Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
Toh MR, Wong EYT, Wong SH, Ng AWT, Loo L-H, Chow PK-H, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. 2023;164:766–82.
Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–606.
Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. Accessed 5 June 2022.
Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL–Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61–116.
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Lockart I, Yeo MGH, Hajarizadeh B, Dore GJ, Danta M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: a meta-analysis. Hepatology. 2022;76:139–54.
Hsu CC, Gopalakrishna H, Mironova M, Lee M-H, Chen C-J, Yang H-I, et al. Risk of hepatocellular carcinoma after spontaneous clearance of hepatitis C virus and in noncirrhosis chronic hepatitis C patients with sustained virological response: a systematic review. Clin Infect Dis. 2023;77:S245–56.
Hamdane N, Jühling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, et al. HCV-induced epigenetic changes associated with liver cancer risk persist after sustained virologic response. Gastroenterology. 2019;156:2313–2329.e7.
Nahon P, Vo Quang E, Ganne-Carrié N. Stratification of hepatocellular carcinoma risk following HCV eradication or HBV control. J Clin Med. 2021;10:353.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6.
Lemon SM, McGivern DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274–8.
Bandiera S, Billie Bian C, Hoshida Y, Baumert TF, Zeisel MB. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr Opin Virol. 2016;20:99–105.
D’souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020;26:5759–83.
Wölfl M, Rutebemberwa A, Mosbruger T, Mao Q, Li H, Netski D, et al. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. J Immunol. 2008;181:6435–46.
Li J-T, Liao Z-X, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J Gastroenterol. 2008;43:419–28.
Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int J Mol Sci. 2019;20:1358.
Quan H, Zhou F, Nie D, Chen Q, Cai X, Shan X, et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial–mesenchymal transition. Oncogene. 2014;33:2826–35.
Iida N, Mizukoshi E, Yamashita T, Yutani M, Seishima J, Wang Z, et al. Chronic liver disease enables gut Enterococcus faecalis colonization to promote liver carcinogenesis. Nat Can. 2021;2:1039–54.
Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005.e1.
Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology. 2019;157:1264–1278.e4.
Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155:411–421.e4.
Degasperi E, D’Ambrosio R, Iavarone M, Sangiovanni A, Aghemo A, Soffredini R, et al. Factors associated with increased risk of de novo or recurrent hepatocellular carcinoma in patients with cirrhosis treated with direct-acting antivirals for HCV infection. Clin Gastroenterol Hepatol. 2019;17:1183–1191.e7.
Chen Q, Ayer T, Adee MG, Wang X, Kanwal F, Chhatwal J. Assessment of incidence of and surveillance burden for hepatocellular carcinoma among patients with hepatitis C in the era of direct-acting antiviral agents. JAMA Netw Open. 2020;3:e2021173.
Ciancio A, Ribaldone DG, Spertino M, Risso A, Ferrarotti D, Caviglia GP, et al. Who should not be surveilled for HCC development after successful therapy with DAAS in advanced chronic hepatitis C? Results of a long-term prospective study. Biomedicines. 2023;11:166.
Pons M, Rodríguez-Tajes S, Esteban JI, Mariño Z, Vargas V, Lens S, et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020;72:472–80.
Tahata Y, Sakamori R, Yamada R, Kodama T, Hikita H, Nozaki Y, et al. Risk of hepatocellular carcinoma after sustained virologic response in hepatitis C virus patients without advanced liver fibrosis. Hepatol Res. 2022;52:824–32.
Pawlotsky J-M, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511.
Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922–65.
Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2021;74:458–65.
Kim NJ, Vutien P, Berry K, Ioannou GN. Hepatocellular carcinoma risk declines but remains high enough for screening in the first 7 years after hepatitis C virus cure with direct-acting antivirals in patients with cirrhosis or high fibrosis-4 score. Gastroenterology. 2022;163:1104–1106.e3. Findings from this study suggest that all patients following SVR should undergo HCC screening, except those with low Fib-4 and no pre-treatment cirrhosis.
Nahon P, Bamba-Funck J, Layese R, Trépo E, Zucman-Rossi J, Cagnot C, et al. Integrating genetic variants into clinical models for hepatocellular carcinoma risk stratification in cirrhosis. J Hepatol. 2023;78:584–95.
Huntley C, Torr B, Sud A, Rowlands CF, Way R, Snape K, et al. Utility of polygenic risk scores in UK cancer screening: a modelling analysis. Lancet Oncol. 2023;24:658–68.
Omata M, Cheng A-L, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–70.
Allaire M, Bruix J, Korenjak M, Manes S, Maravic Z, Reeves H, et al. What to do about hepatocellular carcinoma: recommendations for health authorities from the International Liver Cancer Association. JHEP Rep. 2022;4:100578.
Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The Performance of AFP, AFP-3, DCP as Biomarkers For Detection Of Hepatocellular Carcinoma (HCC): A Phase 3 Biomarker Study in the United States. Clin Gastroenterol Hepatol. 2023;21:415–423.e4.
Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;77:128–39.
Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance Imaging And Alpha Fetoprotein For Early Detection Of Hepatocellular Carcinoma In Patients With Cirrhosis: A Meta-Analysis. Gastroenterology. 2018;154:1706–1718.e1.
Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del PP, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–7.
Trinchet J-C, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: A randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–97.
Omata M, Kanda T, Wei L, Yu M-L, Chuang W-L, Ibrahim A, et al. APASL consensus statements and recommendations for hepatitis C prevention, epidemiology, and laboratory testing. Hepatol Int. 2016;10:681–701.
Mueller PP, Chen Q, Ayer T, Nemutlu GS, Hajjar A, Bethea ED, et al. Duration and cost-effectiveness of hepatocellular carcinoma surveillance in hepatitis C patients after viral eradication. J Hepatol. 2022;77:55–62.
Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265–73.
Singal AG, Reddy S, Radadiya aka Patel H, Villarreal D, Khan A, Liu Y, et al. Multicenter Randomized Clinical Trial Of A Mailed Outreach Strategy For Hepatocellular Carcinoma Surveillance. Clin Gastroenterol Hepatol. 2022;20:2818–2825.e1.
Yoon JH, Lee JM, Lee DH, Joo I, Jeon JH, Ahn SJ, et al. A Comparison Of Biannual Two-Phase Low-Dose Liver CT and US for HCC Surveillance In A Group At High Risk of HCC Development. Liver Cancer. 2020;9:503–17.
Kim SY, An J, Lim Y-S, Han S, Lee J-Y, Byun JH, et al. MRI With Liver-Specific Contrast For Surveillance Of Patients With Cirrhosis At High Risk Of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456.
Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol. 2016;14:875–886.e6.
Kim DH, Choi SH, Shim JH, Kim SY, Lee SS, Byun JH, et al. Meta-analysis of the accuracy of abbreviated magnetic resonance imaging for hepatocellular carcinoma surveillance: non-contrast versus hepatobiliary phase-abbreviated magnetic resonance imaging. Cancers (Basel). 2021;13:2975.
Lin N, Lin Y, Xu J, Liu D, Li D, Meng H, et al. A multi-analyte cell-free DNA–based blood test for early detection of hepatocellular carcinoma. Hepatol Commun. 2022;6:1753–63.
Kim AK, Hamilton JP, Lin SY, Chang T-T, Hann H-W, Hu C-T, et al. Urine DNA biomarkers for hepatocellular carcinoma screening. Br J Cancer. 2022;126:1432–8.
Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: A systematic review and meta-analysis. J Hepatol. 2021;75:108–19. Findings from this study suggest that AMRI might be superior than US in certain subgroups of patients, including obese individuals, patients with nonalcoholic steatohepatitis, and those with Child Pugh B or C cirrhosis.
Lee JY, Huo EJ, Weinstein S, Santos C, Monto A, Corvera CU, et al. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol. 2018;43:1627–33.
Yokoo T, Masaki N, Parikh ND, Lane BF, Feng Z, Mendiratta-Lala M, et al. Multicenter validation of abbreviated MRI for detecting early-stage hepatocellular carcinoma. Radiology. 2023;307. https://doi.org/10.1148/radiol.220917.
Nguyen MH, Roberts LR, Engel-Nitz NM, Bancroft T, Ozbay AB, Singal AG. Gaps in hepatocellular carcinoma surveillance in a United States cohort of insured patients with cirrhosis. Curr Med Res Opin. 2022;38:2163–73.
CHAI and The Hepatitis Fund announce pricing breakthrough to reduce cost of viral hepatitis treatment by over 90 percent. The Clinton Health Access Initiative (CHAI). 2023 [Cited 2023 Dec 19]. [Internet]. Available from: https://www.clintonhealthaccess.org/news/chai-and-the-hepatitis-fund-announce-pricing-breakthrough-to-reduce-cost-of-viral-hepatitis-treatment-by-over-90-percent/
Parikh ND, Tayob N, Singal AG. Blood-based biomarkers for hepatocellular carcinoma screening: approaching the end of the ultrasound era? J Hepatol. 2023;78:207–16.
Yeo YH, Samaan JS, Ng WH, Ting P-S, Trivedi H, Vipani A, et al. Assessing the performance of ChatGPT in answering questions regarding cirrhosis and hepatocellular carcinoma. Clin Mol Hepatol. 2023;29:721–32.
Page K, Melia MT, Veenhuis RT, Winter M, Rousseau KE, Massaccesi G, et al. Randomized trial of a vaccine regimen to prevent chronic HCV infection. N Engl J Med. 2021;384:541–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author AA is part of the advisory board of Abbvie, Gilead, MSD, and Intercept. Author AA has received speaker honorarium from Abbvie, Gilead, and Mylan. Authors MC and DP declare that have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aghemo, A., Polverini, D. & Colombo, M. Hepatocellular Carcinoma in the Era of Direct Antiviral Agents Against Hepatitis C Virus. Curr Hepatology Rep 23, 364–372 (2024). https://doi.org/10.1007/s11901-024-00664-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-024-00664-5