Abstract
Purpose
Non-selective beta blockers remain the pharmacotherapy of choice for prevention of the first episode of variceal bleeding (primary prevention) and for prevention of its recurrence after initial hemostasis (secondary prophylaxis). This review will update the current and emerging pharmacological therapies for portal hypertension.
Recent Findings
Data have emerged on carvedilol in preventing hepatic decompensation and improving patient survival among patients with clinically significant portal hypertension. Because measurement of WHVP is invasive and not feasible in routine practice, non-invasive tests with liver stiffness measurement in combination with platelet count may be accurate in identifying clinically significant portal hypertension.
Summary
Carvedilol is more effective in reducing portal pressure compared to nadolol or propranolol. Its use has expanded to reduce risk of hepatic decompensation among patients with CSPH, which can be identified non-invasively using liver stiffness and platelet count. Studies are needed on non-invasive biomarkers to guide and optimize pharmacological treatment of portal hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
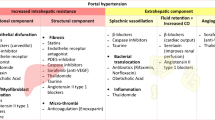
Portal hypertension is a key driver of hepatic decompensation and complications of cirrhosis. Portal pressure is a product of portal blood flow and resistance to flow (Fig. 1), derived by the equation: P = Q × R where P is portal pressure, Q is portal flow, and R is intrahepatic resistance [1, 2]. In cirrhosis, portal hypertension initially results from increased hepatic resistance to portal venous inflow, driven by distortion of the liver microvascular architecture by fibrosis (mechanical or fixed component) and vasoactive factors (dynamic component). A decrease in endogenous vasodilators mainly nitric oxide and increased release of vasoconstrictors (endothelin-1, prostacyclin, and angiotensin) leads to increased intrahepatic vascular tone [1,2,3,4]. At more advanced stages, splanchnic vasodilation ensues, resulting in increased portal venous inflow and portal pressure. Additionally, splanchnic vasodilation leads to a decrease in the effective arterial blood volume which triggers a neurohormonal pathophysiological response. The sympathetic nervous system and renin angiotensin aldosterone system are activated resulting in salt and water retention with plasma volume expansion and increased cardiac output, ultimately creating a state of hyperdynamic circulation that amplifies portal venous inflow and portal pressure [4]. Non-selective beta blockers (NSBB) have been used for the last four decades following the seminal study by Lebrec et al. which demonstrated that propranolol significantly reduced portal hypertension in patients with cirrhosis [5]. The major mechanism of action of currently used pharmacotherapies is reduction in portal flow with use of NSBB, the somatostatin analogue octreotide, and the vasopressin analogue, terlipressin. Intrahepatic resistance can be reduced with use of nitrates. The current review focuses on the established and expanding indications of NSBB in the management of portal hypertension. Emerging drugs targeting nitric oxide, vascular tone, and bacterial translocation across gut-liver axis, angiogenesis, and fibrosis are discussed briefly. We also highlight clinical unmet needs in the pharmacotherapy of portal hypertension.
Non-Selective Beta Blockers
NSBB block β1 receptors in the cardiac muscle, resulting in a decrease in cardiac output. These drugs also block the β2 receptors in the splanchnic vessels, resulting in splanchnic vasoconstriction. The net result is a decrease in portal inflow with a consequent lowering of portal pressure. Propranolol and nadolol are the two main NSBB used in practice. Carvedilol is another NSBB with an additional property of blocking the α1 adrenergic receptors in the hepatic vessels, resulting in a decrease in the intrahepatic vascular tone. This adjuvant action of carvedilol results in better efficacy with more pronounced reduction in portal pressure [6]. Furthermore, α1 blockade effect contributes to anti-inflammatory activity via suppressing cytokine-mediated inflammation [7].
Secondary Prophylaxis (Prevention of Recurrent Variceal Bleeding)
Among patients with acute variceal bleeding, the primary goal of treatment after initial hemostasis and control of bleeding is to prevent recurrent episodes of variceal bleeding. In the absence of prophylactic therapy, recurrent bleeding is common, with 30% mortality risk within 6 months, increasing to 70% within 1 year [8]. Several randomized controlled trials (RCT) have shown benefit of NSBB in achieving this goal. A meta-analysis of 13 RCT in 689 patients with cirrhosis and acute variceal bleeding showed that NSBB as compared to placebo prevented recurrent variceal bleeding (42 vs. 63%) with reduced overall (20 vs. 27%) and bleeding-related mortality (9 vs. 17%) [9]. Isosorbide nitrate, given its different mechanism of action, has been evaluated in combination with NSBB in 289 patients. Although isosorbide nitrate was more effective in reducing variceal bleeding by 29%, lack of improvement in patient survival and 2.5-fold increased odds of drug discontinuation (15 vs. 6%) limit the routine clinical use of isosorbide nitrate in combination with NSBB for secondary prophylaxis of variceal bleeding [10]. BAVENO VII consensus recommends combination of EVL surveillance and NSBB as first-line therapy for prevention of recurrent variceal bleeding [11••].
In a recent RCT by Dunne et al. [12], carvedilol use compared to EVL for secondary prophylaxis was associated with survival benefit, fewer liver-related deaths, and fewer hospitalizations with decompensated liver disease. However, alcohol was the predominant cause of liver disease in this study limiting generalizability to other etiologies, and retrospective long-term data collection introduces the risk of selection bias. In another retrospective cohort study on 87 patients [13], use of carvedilol induced more profound reduction in HVPG as compared to propranolol. More importantly, the higher rate of sustained HVPG response to carvedilol was associated with lower rates of recurrent variceal bleeding, liver-related death, and decompensation of cirrhosis unrelated to variceal bleeding. However, the small sample size with few clinical events and retrospective design did not allow for a definite conclusion regarding the safety profile of the two NSBB agents examined in this study. Findings from these two studies [12, 13] warrant prospective validation.
Primary Prophylaxis (Prevention of First Episode of Variceal Bleeding)
Currently, either NSBB or endoscopic variceal ligation (EVL) is recommended for prevention of first episode of variceal hemorrhage in patients with medium to large varices and in those with small varices with high-risk features (CTP Class C cirrhosis, or presence of red wale signs on varices). In a network meta-analysis of 32 RCT including 3362 adults with cirrhosis and large esophageal varices without prior history of bleeding, both NSBB monotherapy and EVL were effective in reducing risk of variceal hemorrhage. However, NSBB was associated with lower risk of serious adverse effects as compared to EVL [14]. Of the NSBB, carvedilol was most effective with a 79% risk reduction of variceal hemorrhage, 0.21 (0.08–0.56). However, for patients who have contraindications or intolerance to NSBBs, EVL remains a valid first-line option for prevention of first variceal bleeding [11••]. Hence, the American Association for Study of Liver Disease (AASLD) recommends NSBB monotherapy as the first choice for primary prophylaxis of variceal hemorrhage, and EVL reserved for those who have contraindications for or cannot tolerate NSBB [15]. The CALIBRE trial (ISRCTN number 73887615) [16], an adequately powered multicenter open-label RCT ongoing since 2019, aims to compare carvedilol vs. EVL in primary prevention of variceal bleeding in patients with cirrhosis and medium to large EV. The secondary outcomes of the study are patient survival, development of further decompensation, and safety profile. Results of this trial are expected in 2024 and will determine whether using carvedilol is effective as a first-line option in preventing the first episode of variceal bleeding and hepatic decompensation, and improving survival.
Isosorbide alone is ineffective as primary prophylaxis and in fact in one study, the overall mortality was higher, likely due to perpetuation of the vasodilatory pathophysiology of cirrhosis [17]. In a meta-analysis of 4 RCT on 552 patients (279 receiving combination), there was lack of benefit in reducing risk of variceal hemorrhage, and higher drug discontinuation (15 vs. 6%). These factors limit the use of isosorbide nitrate as an adjuvant to NSBB for prevention of the first variceal hemorrhage [10].
Whether primary prophylaxis should be used in patients who do not have medium to large or high-risk varices was addressed in the PREDESCI trial [18•], a multicenter double-blind placebo controlled randomized trial. This study recruited 201 patients with compensated cirrhosis and clinically significant portal hypertension (CSPH). Over a median follow-up period of 37 months, NSBB use was associated with a significant reduction in decompensation or death compared to placebo (16% vs. 27%) (hazard ratio (HR) 0.51, 95% CI 0.26–0.97, p = 0.041). A total of 9 patients needed to be treated to prevent hepatic decompensation in one patient over a 3-year follow-up period [18•]. The reduced decompensation with NSBB was predominantly due to reduced incidence of ascites (HR 0.42, 95% CI 0.19–0.92, p = 0.03). Recently, a systematic review and meta-analysis by Villanueva et al. using individual patient data from 4 RCT comparing carvedilol vs. control arm (no active treatment or EVL) in patients with compensated cirrhosis and CSPH showed that carvedilol significantly decreases the risk of decompensation (mainly by reducing the risk of development of ascites) with improved survival [19]. Subsequently, BAVENO VII consensus advocated for consideration of NSBB for prevention of hepatic decompensation among patient with CSPH [11••].
A recent single-center RCT randomized 96 patients on propranolol for primary variceal bleeding prophylaxis to switch to carvedilol at 12.5 mg/day (n = 64) or continue propranolol (n = 32) in a 2:1 ratio. At 12 months, the carvedilol group showed a significant improvement in systemic vascular resistance, glomerular filtration rate, and renal blood flow with significant decreases in plasma renin activity and plasma noradrenaline. After 2 years, carvedilol compared to propranolol reduced further hepatic decompensation (10.5% vs. 35.9%, p = 0.003) and improved patient survival (86% vs. 64.1%, p = 0.01). Ascites was the most common decompensation event in 7 (5 in the propranolol group) patients [20]. With the low cost of NSBB and number needed to treat of 9 to prevent one decompensation over a 3-year period [18•], the use of NSBB particularly carvedilol for primary prophylaxis of variceal bleeding and prevention of hepatic decompensation will be of great potential in reducing the healthcare burden and cost of managing patients with compensated cirrhosis. Hence, a recently concluded Baveno VII consensus conference recommended that patients with compensated cirrhosis and CSPH should be initiated on NSBB, preferably carvedilol, to prevent liver decompensation and improvement in long-term patient survival [11••].
Measurement of Portal Pressure and Identification of CSPH
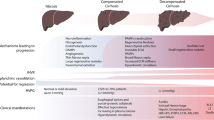
The anatomical location of the portal vein between two capillary beds (the hepatic sinusoids and splanchnic capillaries) is a challenge for obtaining a direct measurement of portal pressure. Therefore, the HVPG which represents the difference between the wedged hepatic venous pressure (wedging a catheter or occluding it with a balloon in the supra-hepatic vein) and the free hepatic venous pressure is an indirect and a safer tool to measure portal pressure. HVPG measurement has been used in clinical trials among patients with cirrhosis due to alcohol or viral hepatitis, and shows a good correlation with direct portal pressure measurements [2, 21]. With a normal HVPG of 3–5 mm Hg, a value > 5 mm Hg defines portal hypertension, and a level ≥ 10 mm Hg identifies CSPH, the strongest predictor for risk of development of varices and of decompensation in patients with compensated cirrhosis [21]. The threshold for esophageal variceal bleeding risk is 12 mm Hg. Furthermore, HVPG ≥ 20 mm Hg is predictive of refractory variceal bleeding and higher mortality [21]. However, despite being the gold standard, its invasive nature, expertise limited to a few centers, issues related to consistency of measurements, and test–retest reliability, particularly among patient with decompensated cirrhosis, limit its use in routine clinical practice [22, 23].
In this regard, non-invasive tests especially liver stiffness measurement (LSM) have emerged an important tool in accurately identifying CSPH and optimizing the use of screening upper gastrointestinal endoscopy in patients with compensated cirrhosis (Fig. 2) [11••, 24•]. A LSM ≤ 15 kPa and platelet count ≥ 150 × 109/L has over 90% sensitivity with excellent negative predictive value in ruling out CSPH in patients with compensated advanced chronic liver disease. In contrast, a LSM value of > 25 kPa and 20–25 with platelets < 150 × 109/L is quite specific for CSPH. For patients not in these categories, further decision on endoscopic screening for varices can be optimized using the platelet count. For example, LSM 15–20 kPa and platelet count ≥ 150 × 109/L is quite accurate in excluding significant varices and can be used to avoid screening endoscopic examination. On the other hand, LSM values between 15 and 20 kPa and platelet count < 110 × 109/L are associated with a CSPH risk of at least 60% that may necessitate a screening endoscopic examination. The recently concluded BAVENO VII consensus recommends the use of this non-invasive algorithm for screening for CSPH and for optimization of screening upper endoscopic examination for decisions regarding the use of NSBB in patients with compensated cirrhosis [11••].
Portal Hypertensive Gastropathy
There is lack of data on the NSBB use for prevention of bleeding from portal hypertensive gastropathy [25]. However, the BAVENO VII guidelines recommend NSBBs as a first-line therapy for prevention of recurrent bleeding from portal hypertensive gastropathy [11••].
Gastric and Ectopic Varices
Varices in the cardio-esophageal region of the stomach are defined as gastric varices and varices at anatomical location other than esophagus and stomach are defined as ectopic varices. Gastric varices are present in about 20% of patients with portal hypertension; and approximately 25–50% of patients with gastric varices have isolated gastric varices in the absence of esophageal varices [26]. NSBB are recommended for primary prophylaxis of variceal bleeding from gastric varices with high-risk stigmata. NSBB are not very effective for secondary prophylaxis of bleeding from gastric varices. In a meta-analysis of 9 RCT in 647 patients with at least 6 weeks of follow-up after an episode of gastric variceal bleeding, balloon retrograde transvenous obliteration and cyanoacrylate injection of gastric varices were best therapies for secondary prophylaxis [27]. Interestingly, NSBB alone were associated with higher risk of bleeding compared to most other interventions and over fourfold mortality risk compared to cyanoacrylate injection, 4.12 (1.50–11.36). One of the limitations of this meta-analysis is lack of head to head studies that limit making strong recommendations for clinical practice [27]. Currently, NSBB are not recommended as a standalone treatment for secondary prophylaxis against bleeding from gastric varices [15]. The data are very scanty for any clear recommendations on primary and secondary prevention of bleeding from ectopic varices.
NSBB Use in Clinical Practice
Of the four available NSBB, timolol is not available in oral formulation leaving propranolol, nadolol, and carvedilol available to be used in clinical practice. Nadolol and carvedilol have an advantage of better compliance with a one-time dose as compared to propranolol which is used in two to three divided doses. However, extended-release propranolol overcomes this limitation and can be used once daily as with the other two NSBB. NSBB should not be used and are contraindicated in patients with second- or third-degree heart block, bronchial asthma, baseline systolic blood pressure below 90 mm Hg or mean arterial blood pressure below 65 mm Hg, peripheral arterial disease, and known allergy to NSBB. Caution should be exercised in using NSBB in patients with poorly controlled diabetes mellitus and Raynaud’s phenomenon. After initiation, the dose of NSBB is titrated to maximum tolerated dose (Table 1). In several studies measuring HVPG in response to administration of NSBB, a decrease of HVPG by 20% or more or to an absolute level < 12 mm Hg is considered adequate response for variceal bleeding prophylaxis [28,29,30]. As measuring HVPG is not feasible in clinical practice, NSBB dose is titrated and optimized on follow-up with monitoring hemodynamics, with an aim to achieve a goal heart rate of 55–60 bpm or maximum tolerated dose as defined by development of side effects or systolic blood pressure below 90 mm Hg [31]. In a meta-analysis of 23 NSBB trials, adequate beta blockade as evaluated by achieving heart rate below 60 bpm was associated with adequate decrease in portal pressure, simultaneous improvement in myocardial oxygen demand and coronary perfusion, and improved patient survival [32]. Use of NSBB in patients with decompensated cirrhosis, acute on chronic liver failure, and infections including spontaneous bacterial peritonitis is outside the scope of this review. In general, a lower dose is recommended in patient with refractory ascites to prevent acute kidney injury and adopting these blood pressure thresholds has been associated with improved survival [33]. NSBB are generally safe, but rarely may be associated with deleterious impact on patient’s quality of life due to adverse effects which are not class specific. These include fatigue, reduced exercise tolerance, erectile dysfunction, nausea, constipation, hypotension, dizziness, and lightheadedness [11••, 15, 34, 35]. However, these non-specific adverse effects may also occur due to underlying cirrhosis and/or concomitant depression. Therefore, a reasonable approach is to reduce the dose or switch to different NSBB. Using NSBB as a nighttime dose helps reduce daytime adverse effects.
Emerging Pharmacologic Targets
Portal vein thrombosis, a common complication in patients with cirrhosis and portal hypertension, can result in hepatic decompensation and worsening of portal hypertension. Anticoagulants are effective in the management and/or prevention of portal vein thrombosis and reduction in hepatic decompensation and portal pressure [36, 37]. One of the most attractive and potentially useful group of drugs in portal hypertension, which is currently being extensively evaluated, is statins due to their beneficial effects of hepatocyte protection by inhibiting inflammation, proliferation, and fibrosis [38, 39]. These benefits result in improvement of both the dynamic as well as fixed components in the pathophysiology of portal hypertension (Fig. 1). The benefit of statins has been observed in several observational studies as well as RCTs. In a meta-analysis of 13 studies including 3 small RCTs in CTP-A cirrhosis, use of statins was beneficial in reducing hepatic decompensation by 46%, variceal bleeding and progression of portal hypertension by 27%, and patient mortality by 46% [40]. Large RCT are needed before routinely recommending statin use in clinical practice for the sole purpose of improving liver elated outcomes. However, their use is encouraged in patients with cirrhosis and/or chronic liver disease for their standard indication of dyslipidemia or coronary heart disease.
Several other targets are being examined targeting the increased intrahepatic resistance (34). These are stratified based on the mechanism to decrease intrahepatic resistance by (a) increasing intrahepatic nitric oxide (statins, obeticholic acid, phosphodiesterase-5 inhibitors, recombinant human relaxin-2, flavonoids including resveratol, and modulators of superoxide dismutase); (b) modulating vasoactive molecules (renin–angiotensin–aldosterone inhibitors such as angiotensin converting enzyme inhibitors and aldosterone antagonists or receptor blockers, thromboxane or prostaglandin E antagonists, leukotriene inhibitors, endothelin antagonists, urotensin II receptor antagonists); (c) inhibiting angiogenesis (sorafenib and other tyrosine kinase inhibitors to antagonize VEGF and PDGF); (d) modifying gut microbiome and inhibition of bacterial translocation (antibiotics including rifaximin, probiotics); (e) modulation of metabolism (metformin, GLP-1 analogue liraglutide, PPAR-α agonism by fenofibrate); (f) inhibiting cell death (pan-caspase inhibitor emricasan); and (g) miscellaneous (ascorbic acid, caffeine, curcumin).
Summary and Conclusion
Non-selective beta blockers are the frontline treatment for prevention of variceal bleeding in patients with high-risk varices (primary prevention) and for prevention of recurrent bleeding after controlling the acute variceal episode (secondary prophylaxis). Carvedilol due to its additional effect on intrahepatic resistance apart from decreasing the portal blood flow is more effective in reducing portal pressure compared to nadolol or propranolol. Recently, the indication for non-selective beta blocker use has expanded with the evolving data on carvedilol in reducing hepatic decompensation and improved patient survival among patients with CSPH. HVPG measurement being invasive, non-invasive measurement of liver stiffness in combination with platelet count may be recommended to identify CSPH as per recently concluded BAVENO VII conference. There remains clinical unmet need for studies on non-invasive biomarkers to guide therapeutic approach and help follow-up and optimization of NSBB in the management of portal hypertension.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Singal AK, Kamath PS. Variceal hemorrhage. In: Sahni P, Nundy S, editors. New Delhi, India, 2012:1.
Qi X, Berzigotti A, Cardenas A, Sarin SK. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol. 2018;3:708–19.
Engelmann C, Claria J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75(Suppl 1):S49–66.
Iwakiri Y, Trebicka J. Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep. 2021;3:100316.
Lebrec D, Nouel O, Corbic M, Benhamou JP. Propranolol–a medical treatment for portal hypertension? Lancet. 1980;2:180–2.
Rabiee A, Garcia-Tsao G, Tapper EB. Nonselective beta-blockers in portal hypertension: why, when, and how? Clin Liver Dis (Hoboken). 2022;19:118–23.
Sharma D, Farrar JD. Adrenergic regulation of immune cell function and inflammation. Semin Immunopathol. 2020;42:709–17.
D’Amico G, Luca A. Natural history. Clinical-haemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol. 1997;11:243–56.
Miao Z, Lu J, Yan J, Lu L, Ye B, Gu M. Comparison of therapies for secondary prophylaxis of esophageal variceal bleeding in cirrhosis: a network meta-analysis of randomized controlled trials. Clin Ther. 2020;42(1246–1275):e1243.
Gluud LL, Langholz E, Krag A. Meta-analysis: isosorbide-mononitrate alone or with either beta-blockers or endoscopic therapy for the management of oesophageal varices. Aliment Pharmacol Ther. 2010;32:859–71.
•• de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VIIF. Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. Recent Baveno VII conference recommendations for the management of portal hypertension.
Dunne PDJ, Young D, Chuah CS, Hayes PC, Tripathi D, Leithead J, Smith LA, et al. Carvedilol versus endoscopic band ligation for secondary prophylaxis of variceal bleeding-long-term follow-up of a randomised control trial. Aliment Pharmacol Ther. 2022;55:1581–7.
Jachs M, Hartl L, Simbrunner B, Bauer D, Paternostro R, Balcar L, Hofer B, et al. Carvedilol achieves higher hemodynamic response and lower rebleeding rates than propranolol in secondary prophylaxis. Clin Gastroenterol Hepatol 2022;S1542-3565(22)00642-5.
Sharma M, Singh S, Desai V, Shah VH, Kamath PS, Murad MH, Simonetto DA. Comparison of therapies for primary prevention of esophageal variceal bleeding: a systematic review and network meta-analysis. Hepatol. 2019;69:1657–75.
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatol. 2017;65:310–35.
Tripathi D, Hayes PC, Richardson P, Rowe I, Ferguson J, Devine P, Mathers J, et al. Study protocol for a randomised controlled trial of carvedilol versus variceal band ligation in primary prevention of variceal bleeding in liver cirrhosis (CALIBRE trial). BMJ Open Gastroenterol. 2019;6:e000290.
Angelico M, Carli L, Piat C, Gentile S, Capocaccia L. Effects of isosorbide-5-mononitrate compared with propranolol on first bleeding and long-term survival in cirrhosis. Gastroenterol. 1997;113:1632–9.
• Villanueva C, Albillos A, Genesca J, Garcia-Pagan JC, Calleja JL, Aracil C, Banares R, et al. beta blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–608. The seminal randomized controlled trial to demonstrate the benefit of carvedilol in preventing hepatic decompensation and improving patient survival in patients with clinically significant portal hypertension.
Villanueva C, Torres F, Sarin SK, Shah HA, Tripathi D, Brujats A, Rodrigues SG, et al. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J Hepatol 2022; 77(4):1014–25.
Kalambokis GN, Christaki M, Tsiakas I, Despotis G, Fillipas-Ntekouan S, Fotopoulos A, Tsiouris S, et al. Conversion of propranolol to carvedilol improves renal perfusion and outcome in patients with cirrhosis and ascites. J Clin Gastroenterol. 2021;55:721–9.
Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–82.
Bai W, Al-Karaghouli M, Stach J, Sung S, Matheson GJ, Abraldes JG. Test-retest reliability and consistency of HVPG and impact on trial design: a study in 289 patients from 20 randomized controlled trials. Hepatol. 2021;74:3301–15.
Garcia-Tsao G. Can we rely on changes in HVPG in patients with cirrhosis? Hepatol. 2021;74:2945–7.
• Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA. 2021;326:165–76. A review and update on diagnosis and management of alcohol-associated liver disease including non-invasive assessment in high-risk patients.
Cubillas R, Rockey DC. Portal hypertensive gastropathy: a review. Liver Int. 2010;30:1094–102.
Sarin SK, Kumar A. Gastric varices: profile, classification, and management. Am J Gastroenterol. 1989;84:1244–9.
Osman KT, Nayfeh T, Abdelfattah AM, Alabdallah K, Hasan B, Firwana M, Alabaji H, et al. Secondary prophylaxis of gastric variceal bleeding: a systematic review and network meta-analysis. Liver Transpl. 2022;28:945–58.
Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodes J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatol. 2003;37:902–8.
Albillos A, Banares R, Gonzalez M, Ripoll C, Gonzalez R, Catalina MV, Molinero LM. Value of the hepatic venous pressure gradient to monitor drug therapy for portal hypertension: a meta-analysis. Am J Gastroenterol. 2007;102:1116–26.
Kerbert AJ, Chiang FW, van der Werf M, Stijnen T, Slingerland H, Verspaget HW, van Hoek B, et al. Hemodynamic response to primary prophylactic therapy with nonselective beta-blockers is related to a reduction of first variceal bleeding risk in liver cirrhosis: a meta-analysis. Eur J Gastroenterol Hepatol. 2017;29:380–7.
Bosch J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatol. 2010;51:2214–8.
McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–94.
Alvarado-Tapias E, Ardevol A, Garcia-Guix M, Montanes R, Pavel O, Cuyas B, Graupera I, et al. Short-term hemodynamic effects of beta-blockers influence survival of patients with decompensated cirrhosis. J Hepatol. 2020;73:829–41.
Bunchorntavakul C, Reddy KR. Pharmacologic management of portal hypertension. Clin Liver Dis. 2019;23:713–36.
Kulkarni AV, Rabiee A, Mohanty A. Management of portal hypertension. J Clin Exp Hepatol. 2022;12:1184–99.
Simonetto DA, Singal AK, Garcia-Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG clinical guideline: disorders of the hepatic and mesenteric circulation. Am J Gastroenterol. 2020;115:18–40.
Villa E, Camma C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterol. 2012;143(1253–1260):e1254.
Pose E, Trebicka J, Mookerjee RP, Angeli P, Gines P. Statins: old drugs as new therapy for liver diseases? J Hepatol. 2019;70:194–202.
Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118.
Kim RG, Loomba R, Prokop LJ, Singh S. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15(1521–1530):e1528.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Portal Hypertension and Liver Transplantation
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elfeki, M.A., Singal, A.K. & Kamath, P.S. Pharmacotherapies for Portal Hypertension: Current Status and Expanding Indications. Curr Hepatology Rep 22, 44–50 (2023). https://doi.org/10.1007/s11901-023-00600-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-023-00600-z