Abstract
Purpose of review
Spontaneous bacterial peritonitis is a life-threatening infection that is a leading cause of mortality among patients with decompensated cirrhosis. In this article, we present current trends in the management of spontaneous bacterial peritonitis (SBP), highlighting treatment failure rates of traditional regimens and ways in which to address such challenges.
Recent findings
Despite available management guidelines, there are data to support that these guidelines are often not adhered to, e.g., performing a diagnostic paracentesis at the time of hospital admission in a patient with ascites. Management of SBP is now further complicated by resistant organisms and an increasing prevalence of nosocomial and healthcare-associated infections.
Summary
Effective treatment of SBP requires careful consideration of patient risk factors, local antibiotic resistance patterns, and clinical presentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous bacterial peritonitis (SBP) is classically defined as a primary infection of ascites with a positive ascitic fluid culture and an absolute polymorphonuclear leukocyte (PMN) count ≥250 cells/mm3 in the absence of an intra-abdominal source of infection [1]. SBP occurs almost exclusively in the cirrhotic patient with ascites.
The need to recognize and treat SBP in a timely fashion is paramount to the patient’s clinical outcome. SBP is the most frequent bacterial infection in cirrhosis and carries a mortality rate between 31 and 93% after its first episode [2]. The in-hospital mortality rate for SBP approaches 32% [3] with a reported range between 10 and 50% [2, 4•].
A diagnosis of SBP can only be made via a diagnostic paracentesis where culture and PMN count are assessed; a clinical diagnosis of SBP is insufficient [2]. If present, classic symptoms include fever, abdominal pain, and worsening ascites; however, symptoms may be clinically silent in approximately a third of cases [5]. A diagnostic paracentesis, therefore, should be performed in all cirrhotic patients with ascites upon hospital admission or in the setting of gastrointestinal (GI) bleeding, hepatic encephalopathy, or hepatic decompensation [2, 6, 7]. Ideally, paracentesis should be performed prior to the administration of antibiotics. Once SBP is diagnosed, empiric antibiotic treatment should be promptly started as culture and sensitivity results are not immediately available.
There are defined variants of SBP based upon culture and PMN count [8]. Classic SBP is culture positive with PMN ≥250/mm3. Culture-negative neutrocytic ascites (CNNA) refers to ascites with a PMN ≥250 cells/mm3 without a positive bacterial culture where other causes have been ruled out (i.e., tuberculosis, pancreatitis, hemorrhagic ascites, peritoneal carcinomatosis) [9]. In monomicrobial bacterascites, the ascitic fluid bacterial culture is positive but the PMN is <250 cells/mm3. Both culture positive SBP and CNNA should be treated immediately, whereas monomicrobial bacterascites may represent either a precursor to SBP or simply a transient bacterial translocation without clinical sequelae [6, 8, 10]. In this setting, recent guidelines recommend treating patients with clinical signs or symptoms of infection. Those without symptoms or among those with a low degree of suspicion, presence of monomicrobial bacterascites on a diagnostic paracentesis should be followed by a repeat paracentesis and should be treated if the PMN is ≥250 cells/mm3 [6].
Approximately 5–10% of the cirrhotic patients with ascites with peritonitis have secondary bacterial peritonitis [3]. Secondary bacterial peritonitis stems from an intra-abdominal source (e.g., intestinal perforation or abscess). As in SBP, the PMN is ≥250 cells/mm3 but is usually polymicrobial with at least two of the following criteria: total protein >1 g/dl, LDH >upper limit of normal, and glucose <50 mg/dl [7].
Given the poor long-term survival of patients with SBP, those who recover should be referred for consideration of liver transplantation evaluation [6].
Pathogenesis
The pathogenesis of SBP is multifactorial. Bacterial translocation in a cirrhotic patient whose immune defense is impaired is thought to be the inciting event [2, 11]. Changes in the cirrhotic microbiome, increased intestinal permeability, and an impaired immune system play a central role in the pathogenesis of bacterial translocation [2]. Alterations in toll-like receptor (TLR) and nucleotide-binding oligomerization domain 2 (NOD2) have also been implicated in the pathogenesis of bacterial translocation [11]. Because of portosystemic shunting in cirrhosis, fewer gut-derived bacteria traverse the portal circulation and therefore bypass clearance by the reticuloendothelial system. Decreased phagocytic activity, excessive pro-inflammatory cytokines, decreased complement, and protein C activity all contributed to immune dysfunction in the cirrhotic patient [11].
Treatment
Antibiotics
Cefotaxime, a first third-generation cephalosporin, has historically been the treatment of choice for SBP [7]. However, the increasing prevalence of nosocomial and healthcare-associated infections and drug-resistant organisms has called this recommendation into question. Given this shifting landscape, we outline management recommendations based on either community-acquired SBP or nosocomial SBP [2, 6].
Community-Acquired SBP
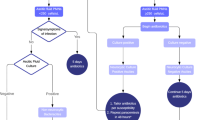
For community-acquired SBP, cefotaxime covers 95% of the bacterial flora [7]. The most common organisms isolated include Escherichia coli, streptococci, and Klebsiella pneumoniae [7]. Cefotaxime is administered 2 g intravenously every 8–12 h. Once culture susceptibility is resulted, then antibiotic therapy can be de-escalated. Antibiotics are given for a duration of 5 days; a shorter duration is equally efficacious to 10 days of therapy in uncomplicated SBP [12]. Other treatment options include other third-generation cephalosporins (ceftriaxone), amoxicillin-clavulanate, or quinolones [11]. Quinolones should be avoided in patients who are already on one for SBP prophylaxis given higher rate of quinolone-resistant gram-negative bacteria [13]. Uncomplicated SBP, defined as cases without shock, ileus, gastrointestinal bleeding, Grade 2 or greater hepatic encephalopathy, or renal impairment, can be managed with oral antibiotics such as oral quinolones [6, 11]. Switch therapy (switching from IV to PO antibiotics) is also commonly practiced and has been shown to be efficacious [6]. A repeat paracentesis 2 days after antibiotic treatment is recommended [6]. If the PMN count does not fall greater than 25% of the pretreatment value, treatment failure is suspected and the patient should be switched to another agent based on culture susceptibility results [14•], especially in patients at high risk for multidrug resistant infections (see “Nosocomial and Healthcare-Associated SBP”) [7].
Nosocomial and Healthcare-Associated SBP
The increasing prevalence of multidrug resistance organisms poses a high risk of morbidity and mortality in the patients with healthcare-associated and nosocomial SBP. Treatment failure is a known risk factor for increased mortality among patients with cirrhosis [14•]. Healthcare-associated infections have been defined as those diagnosed within 48 h of hospitalization in patients who have had exposure or contact to a healthcare system, whereas nosocomial infections are those diagnosed >48 h after hospitalization [15]. Factors giving rise to this phenomena include widespread use of quinolones for SBP prophylaxis, emergence of multidrug resistant organisms, and varying degrees of exposure to healthcare settings. Approximately 30% of the gram-negative bacteria are resistant to quinolones [6], and up to 75% resistance has been shown for third-generation cephalosporins in patients with nosocomial SBP [16]. In fact, gram-positive bacteria and multidrug resistant bacteria have become more prevalent in nosocomial settings [6, 17]. The most common multiresistant bacteria include extended-spectrum beta-lactamase (ESBL) Enterobacteriaceae, Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-sensitive and -resistant enterococcus (VSE, VRE), and non-fermentable gram-negative bacilli including Pseudomonas aeruginosa, Stenotrophomonas maltophilia, or Acinetobacter baumannii [18•].
Two small studies suggest the use of broad-spectrum antibiotics as a first line drug in this patient population until culture results return [14•, 17]. Piano et al. performed a randomized study of 32 patients comparing ceftazidime vs. meropenem/daptomycin (MER/DAPTO). Patients who did not respond to ceftazidime (based on repeat paracentesis) were switched to MER/DAPTO. While there was no difference in 30- or 90-day transplant-free survival, patients in the MER/DAPTO group had a significantly higher rate of treatment success (86.7 vs. 25%, p < 0.001). Similarly another trial that randomized cirrhotic patients with healthcare-associated infections (including SBP) to imipenem vs. cefepime showed a lower rate of treatment failure (18 vs. 51%; p = 0.001), shorter length of stay (12.3 ± 7 vs. 18 ± 15 days; p = 0.03), and a reduction in mortality with the former regimen (6 vs. 25%; p = 0.01) [17]. These two studies suggest that starting with broad-spectrum antibiotics may improve treatment outcome, including patient survival. Inadequate treatment of this high-risk patient population confers a poor prognosis. Of significant concern is the exacerbation of multidrug resistance. While there has not been any documented resistance to carbapenems, controversy exists on how best to approach this problem.
A recent single center study found that 22% of the patients did not respond to initial antibiotic treatment [4•]. Furthermore, 21% of those with culture positive SBP grew out gram-positive organisms with third-generation cephalosporin resistance. Even in patients thought to have low risk or “easiest to treat” SBP, treatment failure rates were 23% (similar to the overall cohort) [4•]. Low-risk patients in the study met the following inclusion criteria: no recent beta-lactam exposure, typical organism culture (if culture positive), community-acquired SBP, and clinical improvement or stability 72 h after SBP diagnosis [4•].
Given the high prevalence of treatment failure in real-world settings and the ongoing challenges of treating nosocomial SBP, a repeat paracentesis may be advisable in most patients [4•] and is supported by European guidelines [6]. The AASLD guidelines recommend repeat paracentesis in the following patient population: recent B-lactam exposure, culture with an atypical organism, an atypical response to treatment, or ascites in a nosocomial setting [7]. We present a SBP checklist in Table 1.
Albumin and the Prevention of Hepatorenal Syndrome
In addition to antibiotic therapy, albumin infusion is recommended to prevent renal impairment, namely hepatorenal syndrome [8, 19]. Renal failure is a major cause of death among cirrhotic patients and develops in 30–40% of the patients with SBP [20] Patients who received albumin infusion (1.5 g/kg albumin was infused on Day 1 and 1 g/kg was given on Day 3) in addition to cefotaxime (vs. cefotaxime alone) had a decreased risk of developing renal impairment (10 to 33%) and had a lower risk of morality (10 to 29%) as compared to antibiotics alone [21]. Albumin is recommended in patients with Cr >1 mg/dl, BUN >30 mg/dl, or if the total bilirubin is >4 mg/dl. This recommendation is further supported by a meta-analysis of four randomized controlled trials examining the effect of albumin on renal impairment and mortality in patients with SBP. The pooled odds ratio for the decrease in mortality after receiving albumin was 0.34 (95% CI: 0.19–0.60) [22]. Albumin provides benefit not only by volume expansion but also by improving cardiac function as well as by mitigating arterial vasoconstriction [19]. Albumin is also known to have anti-oxidant and anti-inflammatory properties [23]. While hypoalbuminemia is a known feature of cirrhosis, our understanding of albumin dysfunction in cirrhosis is still not completely understood [23].
Primary Prophylaxis
Primary prophylaxis is recommended in patients without prior SBP who have low protein ascites (<1.5 g/dl) alongside impaired renal and liver dysfunction [2]. Norfloxacin (400 mg/day) in patients with advanced liver disease (ascitic total protein <1.5 mg/dl, creatinine ≥1.2 mg/dl, BUN ≥25 mg/dl, Na ≤130 mEq/l, Child-Pugh score ≥9, and bilirubin ≥3 mg/dl) was associated with a reduction in the 1-year probability of SBP (7 vs. 61%, p < 0.001), hepatorenal syndrome (28 vs. 41%, p = 0.02), and 1 year mortality (60 vs. 48%, p = 0.05) [24].
A more recent randomized trial using the same inclusion criteria for renal and liver dysfunction compared alternating rifaximin with norfloxacin vs. either rifaximin or norfloxacin alone for a 6-month period. Patients alternated taking rifaximin with norfloxacin on a monthly basis for 6 months. Rates of prophylaxis were superior in those who alternated vs. either norfloxacin or rifaximin alone (74.7 vs. 56.4 vs. 68.3%, p < 0.048) [25]. Rifaximin as primary prophylaxis in retrospective studies may confer a transplant-free survival benefit in cirrhotic patients with ascites [26]. Resistance to rifaximin has not been found in patients with cirrhosis and ascites followed for up to 5 years [27]. Rifaximin may offer many potential advantages including low resistance profile, broader range of antimicrobial activity, and mechanism of action in the small intestine, the site of bacterial translocation occurrence [2]. Further studies are needed prior to broad use of rifaximin in patients with SBP. Of note, antibiotic cycling has also been proposed as another solution to avoid continuously using the same antibiotic [2]. However, there have been a lack of studies in this regard.
Gastrointestinal Bleeding
Patients with cirrhosis and gastrointestinal bleeding are at high risk for infections [3]. Norfloxacin has also been studied in this setting; however, one study demonstrated that ceftriaxone is more effective than oral norfloxacin in the prevention of infections [28]. For this reason, Ceftriaxone 1 g IV daily × 7 days is often given to hospitalized cirrhotic patients (Child-Pugh Class B or C) with GI bleeding. Once the patient has stabilized and resumed oral intake, IV antibiotics are often switched to PO [7].
Secondary Prophylaxis
Prior Episode of SBP
All patients with a previous episode of SBP should receive secondary prophylaxis. Patients who have had an episode of SBP are at high risk for recurrence and reduced survival. The risk of recurrence is up to 70% in the first year with a survival rate of 30–50% in the first year [3]. These patients should be considered for suppressive antibiotic therapy as well as be considered for LT evaluation given the increased mortality rate. Norfloxacin (400 mg/day) has been shown to reduce the risk of recurrence to approximately 20% in the first year [29]. Trimethoprim-sulfamethoxazole (one double-strength tablet daily) or ciprofloxacin (400 mg/day) are often used in the USA. Rifaximin has also been studied in secondary prophylaxis, but further data are needed before it is widely used in this regard. In a randomized controlled trial of 262 cirrhotic patients, patients received either 1200 mg rifaximin or 400 mg norfloxacin. SBP recurrence (3.9 vs. 14.1%) and mortality (13.7 vs. 24.4%) were significantly lower in the rifaximin group [30]. The rifaximin group also experienced decreased side effects and fewer encephalopathy-related deaths [30]. While long-term prophylaxis has shown to be beneficial in reducing SBP recurrence and mortality, one significant consequence has been the emergence of gram-negative bacteria resistant to both quinolones and trimethoprim-sulfamethoxazole and increased risk of gram-positive infection in this population as previously outlined [6].
Role of Non-Selective Beta-Blockers in SBP
Non-selective beta-blockers have been used primarily in cirrhotics to prevent against variceal bleeding [31, 32]; however, the role of beta-blockers in the patient with cirrhosis has been a subject of debate in recent years. Within the last decade, a few studies have suggested that beta-blockers may cause potential harm for patients with advanced liver disease [33,34,35]. One current hypothesis, known as the “window hypothesis,” posits that beta-blockade is only useful within a certain clinical window [36]. Beta-blockers are thought at one end of the spectrum to be ineffective in early cirrhosis [37] and at the other end of the spectrum harmful in patients with end-stage liver disease complicated by refractory ascites. Such patients have poor cardiac reserve, and beta-blockade may lead to decreased organ perfusion and even hepatorenal syndrome [35, 38]. There is only a brief window, during a period of decompensated cirrhosis when a patient’s cardiac reserve is still intact where beta-blockers have been shown to improve survival, primarily in the context of secondary prophylaxis of gastrointestinal bleeding [33]. This poses a dilemma for the patient with SBP who already has decompensated liver disease with risk of progression to acute on chronic liver failure or refractory ascites.
On the other hand, beta-blockers play a potential role in decreasing bacterial translocation, a key step in the pathogenesis of SBP [39]. One animal study showed that propranolol in cirrhotic rats compared to those without propranolol resulted in lower portal pressure, faster intestinal transit, and lower rates of bacterial overgrowth and translocation [40]. One clinical study with 50 patients with cirrhosis demonstrated a correlation between increased portal pressure (HVPG measurements were performed) and increased intestinal permeability. Patients given a non-selective beta-blocker (NSBB) had decreased intestinal permeability and a decrease in bacterial translocation [41]. Further studies should be performed to further investigate the role of portal hypertension, NSBB, and bacterial translocation.
While beta-blockers can ameliorate a key step in the pathogenesis of bacterial translocation, controversy regarding safety of beta-blockers in decompensated cirrhotics remains. One meta-analysis published in 2009 looking at three randomized controlled trials and three retrospective studies showed that beta-blockers may play a role in protecting against spontaneous bacterial peritonitis in cirrhotic patients [42] (Table 2(a)). Subsequently, Mandorfer et al. found in a retrospective study that beta-blockers were associated with an increased risk of HRS and death in patients with cirrhosis and SBP [43]. However, multiple recent studies demonstrated that beta-blockers were not associated with increased mortality and in fact had a reduced mortality compared to those not on beta-blockade [44,45,46,47,48]. Mookerjee et al. demonstrated benefit from beta-blockade even in patients thought to have the most advanced liver disease, acute on chronic liver failure [49••]. The patient sample included patients enrolled in the CANONIC study, a prospective observational investigation of patients with acute decompensation. They found a relative risk reduction for death of 0.596 in the beta-blocker group [49••]. Given the controversy with beta-blocker use, one may postulate continuing with current practice of care but consider cessation of beta-blockade if other reasons for discontinuation, such as low blood pressure, exist.
Proton Pump Inhibitors and SBP
Proton pump inhibitors (PPIs) are among the one of the most widely prescribed drugs in both the USA and abroad and have come under scrutiny given overutilization [50] and linkage to infections, including C. difficile and pneumonia [51, 52].
Proton pump inhibitors are often prescribed in patients with cirrhosis given bleeding complications from peptic ulcer disease [53]. Like beta-blockers, the use of proton pump inhibitors in cirrhosis has also been controversial [54]. Proton pump inhibitors may be associated with an increased risk of developing SBP [55,56,57] (Table 2(b)). The proposed pathogenesis is that PPIs can lead to small intestinal bacterial overgrowth which can in turn lead to bacterial translocation and infection [58••, 59]. Data from a recent multicenter trial concluded that PPIs continue to be a risk factor for SBP [59]. Cox regression analysis was used to compare rates of SBP between users and non-users of PPIs. Among PPI users, the adjusted HR of SBP was 1.72 (95% CI, 1.10–2.69). This study, however, was retrospective in nature, and the dataset used was from another trial. A small but statistically significant association between PPI use and SBP was shown in a recent meta-analysis examining 14 observational studies (6 case control, 8 cohort). The pooled odds ratio was 2.32 (95% CI, 1.57–3.42) with a lower OR (1.49; 95% CI, 1.19–1.88) when a sensitivity analysis was performed for high quality studies [60].
Two larger prospective studies, however, have offered different results. One study specifically looking at this question among hospitalized patients with decompensated cirrhosis found no association between PPI use and the incidence of SBP [58••]. In this multicenter prospective study, there was no difference in PPI use among the patients with SBP (n = 95) and patients with ascites without SBP (n = 289) (46 vs. 42%) [58••]. Another study among outpatient cirrhotics also failed to find any significant association between PPI use and SBP [61]. In multivariate analyses, Child-Pugh score was the only predictor of SBP [61].
Given the conflicting nature of reports on the association between SBP and infection, PPI use in decompensated cirrhotics needs further clarification and whether duration of PPI use and degree of decompensation may provide a tipping point beyond which PPI use should be discontinued or prescribed at a lower dose.
Conclusion
The changing landscape of organisms responsible for spontaneous bacterial peritonitis presents many treatment challenges. Increasing multidrug-resistant organisms and the shift of culprit organisms from gram-negative to gram-positive bacteria have led to suboptimal treatment success rates. Early diagnosis and prompt initiation of antibiotics and albumin remain the cornerstone of therapy; however, vigilance is necessary to identify the increasing subset of patients that do not respond to standard therapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology(Baltimore, Md). 1982;2(4):399–407.
Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61(2):297–310.
de Mattos AA, Costabeber AM, Lionco LC, Tovo CV. Multi-resistant bacteria in spontaneous bacterial peritonitis: a new step in management? World J Gastroenterol. 2014;20(39):14079–86.
• Goel A, Biewald M, Huprikar S, Schiano T, Im GY. A real-world evaluation of repeat paracentesis-guided management of spontaneous bacterial peritonitis. J Clin Gastroenterol. 2017;51(3):278–84. This study highlights real-world outcomes in SBP at a US center, including high rates of treatment failure and resistant infections. This study supports repeat paracentesis at 48 hours.
Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268–73.
EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53(3):397–417.
Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology(Baltimore, Md). 2013;57(4):1651–3.
Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis—bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41(11):1116–31.
Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology(Baltimore, Md). 1984;4(6):1209–11.
de Vos M, De Vroey B, Garcia BG, Roy C, Kidd F, Henrion J, et al. Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int. 2013;33(9):1316–23.
Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: a critical review and practical guidance. World J Hepatol. 2016;8(6):307–21.
Baskol M, Gursoy S, Baskol G, Ozbakir O, Guven K, Yucesoy M. Five days of ceftriaxone to treat culture negative neutrocytic ascites in cirrhotic patients. J Clin Gastroenterol. 2003;37(5):403–5.
Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology(Baltimore, Md). 2002;35(1):140–8.
• Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatology (Baltimore, Md). 2016;63(4):1299–309. This is the first randomized controlled clinical trial to compare broad spectrum antibiotics against 3rd generation cephalopsorins in the treatment of nosocomial SBP. Given increasing prevalence of nosocomial pathogens, this study established improved survival in broader spectrum antibiotic group.
Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, et al. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56(4):825–32.
Fernandez J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology(Baltimore, Md). 2012;55(5):1551–61.
Merli M, Lucidi C, Di Gregorio V, Lattanzi B, Giannelli V, Giusto M, et al. An empirical broad spectrum antibiotic therapy in health-care-associated infections improves survival in patients with cirrhosis: a randomized trial. Hepatology(Baltimore, Md). 2016;63(5):1632–9.
• Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60(6):1310–24. This is a position paper based on the EASL Special Conference 2013 on bacterial infections in cirrhosis. This is an important adjunct to the EASL clinical practice guidelines on the management of SBP (published 2010) and the AASLD clinical practice guidelines (last updated in 2012).
Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310–8.
Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology(Baltimore, Md). 1994;20(6):1495–501.
Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403–9.
Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clinical Gastroenterol Hepatol. 2013;11(2):123–30. e1
Valerio C, Theocharidou E, Davenport A, Agarwal B. Human albumin solution for patients with cirrhosis and acute on chronic liver failure: beyond simple volume expansion. World J Hepatol. 2016;8(7):345–54.
Fernandez J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133(3):818–24.
Assem M, Elsabaawy M, Abdelrashed M, Elemam S, Khodeer S, Hamed W, et al. Efficacy and safety of alternating norfloxacin and rifaximin as primary prophylaxis for spontaneous bacterial peritonitis in cirrhotic ascites: a prospective randomized open-label comparative multicenter study. Hepatol Int. 2016;10(2):377–85.
Hanouneh MA, Hanouneh IA, Hashash JG, Law R, Esfeh JM, Lopez R, et al. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol. 2012;46(8):709–15.
Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28(3):450–5.
Fernandez J, Ruiz del Arbol L, Gomez C, Durandez R, Serradilla R, Guarner C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131(4):1049–56. quiz 285
Soriano G, Guarner C, Tomas A, Villanueva C, Torras X, Gonzalez D, et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology. 1992;103(4):1267–72.
Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2016;28(12):1450–4.
Garcia-Tsao G, Bosch J. Varices and variceal hemorrhage in cirrhosis: anew view of an old problem. Clin Gastroenterol Hepatol. 2015;13(12):2109–17.
Lebrec D, Nouel O, Corbic M, Benhamou JP. Propranolol—a medical treatment for portal hypertension? Lancet(London, England). 1980;2(8187):180–2.
Ge PS, Runyon BA. The changing role of beta-blocker therapy in patients with cirrhosis. J Hepatol. 2014;60(3):643–53.
Serste T, Francoz C, Durand F, Rautou PE, Melot C, Valla D, et al. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a cross-over study. J Hepatol. 2011;55(4):794–9.
Serste T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology(Baltimore, Md). 2010;52(3):1017–22.
Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61(7):967–9.
Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353(21):2254–61.
Krag A, Bendtsen F, Henriksen JH, Moller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59(1):105–10.
Madsen BS, Havelund T, Krag A. Targeting the gut-liver axis in cirrhosis: antibiotics and non-selective beta-blockers. Adv Ther. 2013;30(7):659–70.
Perez-Paramo M, Munoz J, Albillos A, Freile I, Portero F, Santos M, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology(Baltimore, Md). 2000;31(1):43–8.
Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58(5):911–21.
Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, et al. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int. 2009;29(8):1189–93.
Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146(7):1680–90. e1
Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Nonselective beta-blockers do not affect mortality in cirrhosis patients with ascites: post hoc analysis of three randomized controlled trials with 1198 patients. Hepatology(Baltimore, Md). 2016;63(6):1968–76.
Leithead JA, Rajoriya N, Tehami N, Hodson J, Gunson BK, Tripathi D, et al. Non-selective beta-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut. 2015;64(7):1111–9.
OnaliS, KalafateliM, MajumdarA, WestbrookR, O'BeirneJ, LeandroG, et al. Non-selective beta-blockers are not associated with increased mortality in cirrhotic patients with ascites. Liver Int 2017.
SinhaR, LockmanKA, MallawaarachchiN, RobertsonM, PlevrisJN, HayesPC. Carvedilol use is associated with improved survival in patients with liver cirrhosis and ascites. J Hepatol 2017.
Bang UC, Benfield T, Hyldstrup L, Jensen JE, Bendtsen F. Effect of propranolol on survival in patients with decompensated cirrhosis: a nationwide study based Danish patient registers. Liver Int. 2016;36(9):1304–12.
•• Mookerjee RP, Pavesi M, Thomsen KL, Mehta G, Macnaughtan J, Bendtsen F, et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64(3):574–82. This is the first study to show that nonselective beta blockers (NSBBs) are safe in the sickest of cirrhotic patients, those presenting with acute on chronic liver failure. This is one of many studies examining the relationship between NSBB and mortality in cirrhotic patients.
Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Ther Adv Gastroenterol. 2012;5(4):219–32.
Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308–13.
Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955–60.
Venkatesh PG, Parasa S, Njei B, Sanaka MR, Navaneethan U. Increased mortality with peptic ulcer bleeding in patients with both compensated and decompensated cirrhosis. Gastrointest Endosc. 2014;79(4):605–14. e3
deMattosAZ, MiozzoSA, TovoCV, deMattosAA. Risks of proton pump inhibitors for cirrhotic patients—the controversy remains. Hepatology (Baltimore, Md). 2017.
Bajaj JS, Zadvornova Y, Heuman DM, Hafeezullah M, Hoffmann RG, Sanyal AJ, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol. 2009;104(5):1130–4.
Goel GA, Deshpande A, Lopez R, Hall GS, van Duin D, Carey WD. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol. 2012;10(4):422–7.
O’Leary JG, Reddy KR, Wong F, Kamath PS, Patton HM, Biggins SW, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clinical Gastroenterol Hepatol. 2015;13(4):753–9. e1-2
•• Terg R, Casciato P, Garbe C, Cartier M, Stieben T, Mendizabal M, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. 2015;62(5):1056–60. This was the first multicenter prospective study examining the association between PPI and the development of SBP. Given the ongoing controversy between PPI and SBP, this is an important study. Many of the studies reporting a negative association between PPI and SBP have been retrospective in nature.
Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology(Baltimore, Md). 2016;64(4):1265–72.
Khan MA, Kamal S, Khan S, Lee WM, Howden CW. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2015;27(11):1327–36.
Miozzo SA, Tovo CV, John JA, de Mattos AA. Proton pump inhibitor use and spontaneous bacterial peritonitis in cirrhosis: an undesirable association? J Hepatol. 2015;63(2):529–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
This work was partly supported by the Baylor Foundation Grant.
Additional information
This article is part of the Topical Collection on Management of the Cirrhotic Patient
Rights and permissions
About this article
Cite this article
Ho, C.K., Asrani, S.K. Current Trends in the Management of Spontaneous Bacterial Peritonitis. Curr Hepatology Rep 16, 212–219 (2017). https://doi.org/10.1007/s11901-017-0355-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-017-0355-9