Abstract
Purpose of Review
While most patients with chronic myeloid leukemia (CML) present in a chronic phase and are expected to have a normal life expectancy, some patients present with or progress to a more aggressive accelerated phase (AP) or blast phase (BP) of CML. Herein, we discuss the diagnostic considerations of advanced phase CML and review its contemporary management.
Recent Findings
Later-generation, more potent BCR::ABL1 tyrosine kinase inhibitors (TKIs) such as ponatinib may result in superior outcomes in patients with advanced phase CML. For CML-BP, combination approaches directed against the blast immunophenotype appear superior to TKI monotherapy. The role of allogeneic stem cell transplantation is controversial in CML-AP but has consistently been shown to improve outcomes for patients with CML-BP.
Summary
Advanced phase CML, particularly CML-BP, remains a poor risk subtype of CML. However, novel combination approaches using later-generation TKIs are being explored in clinical trials and may lead to improved outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The outcomes of patients with chronic myeloid leukemia (CML) dramatically improved with the development of tyrosine kinase inhibitors (TKIs) targeting the BCR::ABL1 oncoprotein. Over 95% of patients with CML present with chronic phase disease (CML-CP), and in the current TKI era, most of these patients experience a normal life expectancy [1,2,3]. However, a minority of patients present with or eventually progress to more advanced phase disease (i.e., accelerated or blast phases) despite TKI therapy [4,5,6,7]. The outcomes of these patients with advanced phase CML are significantly worse than their counterparts with chronic phase CML, with survival outcomes that are akin to acute leukemias for patients with blast phase CML [8•]. However, the outcomes of patients with accelerated phase CML are more heterogeneous, and some patients may have relatively good outcomes with TKI monotherapy. Given the aggressive nature of advanced phase CML, more intensive, combination strategies are recommended for many of these patients, including strong consideration of allogeneic hematopoietic stem cell transplantation (HSCT), although the optimal therapy remains uncertain [9]. In this article, we will review the current classifications of advanced phase CML, risk factors for transformation, and contemporary management of both accelerated and blast phase CML, including the role of combination therapies, allogeneic HSCT, and novel treatment strategies that are being explored in ongoing clinical trials.
Diagnostic Considerations
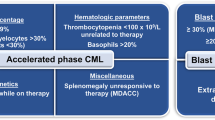
There are three commonly used consensus guidelines that provide definitions for advanced phase CML, including the MD Anderson Cancer Center (MDACC), European LeukemiaNet (ELN), and World Health Organization (WHO) criteria [10, 11••, 12••]. Historically, all 3 of these groups divided advanced phase CML into either accelerated phase CML (CML-AP) or blast phase CML (CML-BP); however, in a 2022 update, the WHO replaced CML-AP with the concept of “high-risk” CML, reflecting that some patients not meeting formal criteria for CML-AP using the previous definition can still have high-risk disease that may have poor prognosis and require more aggressive therapy.
Table 1 shows the definitions of CML-AP and CML-BP in these 3 consensus guidelines. Both MDACC and ELN define CML-CP as blasts ≥ 30%, while the WHO uses a cutoff of ≥ 20% [10, 11••]. Notably, all groups consider extramedullary leukemic involvement to be diagnostic of CML-BP. MDACC and ELN are unified in several criteria for CML-AP, including blasts 15–29%, clonal evolution occurring while on therapy, thrombocytopenia < 100 × 109/L unrelated to therapy, and/or basophils > 20%; in addition, the MDACC model considers splenomegaly unresponsive to therapy to be a criterion for CML-AP. While most patients considered to have “high-risk” CML by the WHO would fall within the above definitions, this updated terminology accounts for patients with other high-risk features, for example, a patient with a low blast percentage but a 3q26.2 rearrangement [12••]. It should be noted that the optimal definitions of advanced phase CML are in flux. While we acknowledge that the term “high-risk” CML may eventually supplant that of “CML-AP,” we refer to the historical definitions of CML-AP when discussing this entity in the present manuscript, as these definitions are most consistent with prior literature.
It is important to note that criteria for CML-AP (or “high-risk” CML) vary in their prognostic importance. Some additional chromosomal abnormalities (ACAs) such as alterations of 3q26.2, monosomy 7, and/or a complex karyotype are associated with particularly poor outcomes. This is in contrast with non-high-risk ACAs such as isolated trisomy 8 or -Y which have limited prognostic value [13,14,15]. Importantly, patients with these high-risk ACAs (particularly when they develop while on TKI therapy) may have significantly worse outcomes than patients with elevated blast percentage but without one of these high-risk ACAs [16].
Prevalence and Risk Factors for Advanced Phase CML
Presentation with advanced phase CML is relatively uncommon, observed in < 5% of patients at the time of CML diagnosis [6]. While some patients do progress to advanced phase CML despite adequate TKI therapy, the rate of transformation is less than was observed historically in the pre-TKI era, where transformation rates > 20% were reported [6]. For example, in the IRIS study that compared interferon alfa plus cytarabine to imatinib in patients with newly diagnosed CML-CP, patients in the non-TKI arm had a 12.8% rate of transformation to advanced phase CML, compared with 6.9% in the imatinib arm [17]. Notably, this study allowed crossover between the 2 arms, and thus, the transformation rate in the absence of access to BCR::ABL1 TKI therapy is expected to have been even higher. With the development of even more potent later-generation TKIs and better guidance for monitoring of adequate molecular response and criteria to switch to alternative TKIs, the transformation rate in the modern era is < 5% by most estimates [9, 18].
There are several variables that may impact a patient’s risk for developing advanced phase CML. These include both disease-related factors, such as baseline high-risk additional chromosomal abnormalities (e.g., − 7/7p, 3q26.2 rearrangements, and/or complex karyotype) [16, 19,20,21], high-risk mutations (e.g., ASXL1, RUNX1, IKZF1, TP53, or resistant ABL1 kinase domain (KD) mutations) [22,23,24,25,26,27,28,29,30], or the rare BCR::ABL1 e1a2 transcript (coding for the p190 BCR::ABL1 protein product) [31, 32], and patient-related factors, particularly adherence to daily TKI therapy [33]. Issues with proper gastrointestinal absorption of TKIs may also lead to subtherapeutic drug levels, increasing the chance for treatment failure and risk for transformation to advanced phase CML [34, 35]. Independent of the above factors, failure to achieve the recommended molecular response milestones during TKI therapy is predictive for a higher risk of transformation.
Treatment of CML-AP or High-Risk CML
De Novo CML-AP
TKI monotherapy is appropriate for many patients with de novo CML-AP as long as the expected response milestones are achieved, and most of these patients with de novo CML-AP have similar outcomes to their counterparts with CML-CP [36, 37]. In one report of imatinib therapy in 42 patients with newly diagnosed CML-AP, the 2-year progression-free survival (PFS) was 100% for patients with CML-AP by hematologic criteria alone, 93% for those with CML-AP due to the presence of ACAs alone (formally a criterion for CML-AP by the WHO), and 58% for those meeting both hematologic and ACA criteria for CML-AP, suggesting that imatinib monotherapy may be adequate for the former 2 groups but not for the latter group [36]. Notably, these differences in outcomes provide support for the newer WHO definition of “high-risk” CML rather than CML-AP. In another retrospective analysis of 51 patients with de novo CML-AP, second-generation TKIs resulted in a slightly higher but not statistically significant improvement in 3-year overall survival (OS) compared with imatinib (95% versus 87%, respectively), with survival in patients receiving a second-generation TKI that was similar to CML-CP [37]. Second-generation TKIs generally result in more rapid and deeper responses in CML and may be associated with lower rates of transformation from CML-CP to advanced phase disease, although a convincing OS benefit has not been observed in most studies [4, 5, 38, 39]. For these reasons, National Comprehensive Cancer Network (NCCN) guidelines generally recommend a second-generation TKI as first-line therapy for patients with de novo CML-AP, with the caveat that some patients may be safely treated with imatinib as long as appropriate response milestones are achieved [40].
Transformed CML-AP
Patients who progress to CML-AP while on TKI therapy have significantly worse outcomes than those who present with de novo CML-AP at the time of diagnosis [9]. In patients previously treated with imatinib, all the second-generation TKIs (i.e., dasatinib, nilotinib, and bosutinib) appear to result in similar rates of major cytogenetic response (MCyR) (30–50%) and complete cytogenetic response (CCyR) (20–40%), with better response rates observed in patients who were intolerant rather than resistant to imatinib [41,42,43,44]. Across studies, this has translated to OS rates of > 90% [45••].
For patients with CML-AP after failure of one or more second-generation TKIs, the outcomes are relatively poor with use of other second-generation TKIs. Ponatinib is a third-generation TKI that has broader activity against ABL1 KD mutations, including T315I, which is a common mechanism of resistance to first- and second-generation TKIs [46]. In the PACE study, 83 patients with CML-AP with resistance or intolerance to dasatinib or nilotinib and/or harboring a T315I mutation received ponatinib monotherapy at a dose of 45 mg daily [47, 48]. Ponatinib resulted in a MCyR rate of 49%, a CCyR rate of 31%, and a major molecular response (MMR) rate of 22%, which translated to an estimated 5-year PFS of 22% and OS of 59% [48]. For patients with CML-AP experiencing treatment failure with a second-generation TKI, our practice is to generally use ponatinib-based therapy (or an appropriate clinical trial of a novel BCR::ABL1 TKI), as rotating through other second-generation TKIs results in suboptimal outcomes. However, several factors should be considered when selecting the appropriate TKI therapy in this scenario, including comorbidities, specific prior TKI therapies, and ABL1 KD mutations. Given the relatively poor outcomes of patients with transformed CML-AP, TKI-based combination therapies can be considered (e.g., with a hypomethylating agent and/or venetoclax), although presently there are only scant data to support this approach in CML-AP.
Treatment of CML-BP
The outcomes of CML-BP are particularly poor, with a median survival generally less than 12 months.9 Several factors may also influence the prognosis of patients with CML-BP. As with CML-AP, patients presenting with de novo CML-BP have superior outcomes to those who transformed while on TKI therapy.8 The presence of high-risk cytomolecular features also impacts the clinical outcomes. Immunophenotype of the blast compartment is both prognostic and therapeutically important. CML in myeloid blast phase (CML-MBP) is approximately twice as common as CML in lymphoid blast phase (CML-LBP), and some patients can present with a biphenotypic blast phase or other rarer subtypes. Of note, CML-LBP generally has superior outcomes to CML-MBP. Immunophenotypic classification of the blast phase disease is crucial when selecting appropriate combination therapies for patients with CML-BP (i.e., an acute myeloid leukemia (AML)–like backbone for patients with CML-MBP and an acute lymphoblastic leukemia (ALL)–like backbone for patients with CML-LBP)9.
TKI Monotherapy
Several studies have evaluated TKI monotherapy in patients with CML-BP, regardless of the immunophenotype. Outcomes with first- or second-generation TKI monotherapy are poor with relatively low rates of transient responses and median OS of 10 months or less across studies of imatinib, nilotinib, and dasatinib [49,50,51, 52•]. In the PACE study, ponatinib at a dose of 45 mg daily was evaluated in 62 patients with CML-BP (38 of whom were intolerant/resistant to imatinib, 24 with a T315I mutation) [47, 48]. The CCyR rate was only 18%, and the median PFS and OS were 3 months and 7 months, respectively, suggesting that outcomes are poor with ponatinib monotherapy for CML-BP, despite the relative potency of ponatinib compared with other commercially available TKIs. It should also be noted that while the OPTIC study showed that lower doses of ponatinib may offer the optimal risk–benefit profile for patients with CML-CP without a T315I mutation, there are no data to support lower doses of ponatinib monotherapy in CML-BP, regardless of T315I status [53•]. Newer TKIs are being studied in CML across different stages of disease, including asciminib and olverembatinib, although there are limited efficacy data in CML-BP. Overall, results are suboptimal with TKI monotherapy in CML-BP, and therefore, combination approaches should be strongly considered for most patients.
Combination Approaches for CML-MBP
Some studies suggest that the outcomes of patients with CML-MBP can be improved with combination therapies. In a retrospective analysis of 104 patients with CML-MBP, patients were stratified by the initial therapy received for BP disease (intensive chemotherapy alone [n = 8], TKI alone [n = 56], intensive chemotherapy plus a TKI [n = 20], and hypomethylating agent plus a TKI [n = 20]) [54]. Combination approaches resulted in higher rates of complete remission (CR) or complete remission with incomplete hematologic recovery (CRi) (57% versus 34%, P < 0.05) and CCyR (45% versus 11%, P < 0.001) as compared with TKI monotherapy. Driven in part by the higher rates of response, more patients who received combination therapy were able to be bridged to allogeneic HSCT (32% versus 11%, P < 0.01). Combination therapy using a second- or third-generation TKI also resulted in higher rates of 5-year event-free survival (28% versus 0%, P < 0.05) and 5-year OS (34% versus 8%, P = 0.23), as compared with second- or third-generation TKI monotherapy. This study strongly supports the use of combination TKI-based therapies for patients with CML-MBP rather than TKI monotherapy.
In the prospective phase I/II MATCHPOINT study, 17 patients with CML-BP (MBP [n = 9], LBP [n = 4], mixed phenotype acute leukemia [n = 4]) received FLAG-Ida (fludarabine, cytarabine, idarubicin, and G-CSF) plus ponatinib 30 mg daily [55]. The complete hematologic response (CHR) rate was 29%, CCyR rate was 47%, and MMR rate was 29%. With a median follow-up of 36 months, the median OS was 12 months and was not reached in patients who were bridged to allogeneic HSCT. The combination of decitabine and dasatinib was also explored in a prospective study of 19 patients with CML-BP (18 of whom had MBP) [56]. Seven of 17 evaluable patients (41%) achieved CHR, 5 of whom were consolidated with allogeneic SCT.
Combination therapies using a TKI and venetoclax are potentially promising future options for patients with CML-MBP. In a retrospective analysis of 9 patients with CML-MBP (all of whom had transformed on TKI therapy), 5 (56%) achieved CR/CRi, and 3 (33%) achieved CCyR, which translated to a median OS of 10.9 months. [57] In an ongoing phase II study of decitabine, venetoclax, and ponatinib for patients with advanced phase CML, 15 patients have been treated (MBP [n = 10], transformed AP [n = 4], Philadelphia chromosome–positive [Ph +] AML [n = 1]) [58]. Eleven patients (73%) responded (including CR, CRi, and morphologic leukemia-free state [MLFS]), including 6 patients (40%) with CR/CRi. Four patients were bridged to allogeneic HSCT, and the median OS was 11 months. This study continues to accrue patients (ClinicalTrials.gov NCT04188405), and this triplet combination may represent an effective option for patients with advanced phase CML, including CML-MBP, including those who are not candidates for intensive chemotherapy.
Combination Approaches for CML-LBP
The treatment approach for patients with CML-LBP is largely modeled after clinical experience with Ph + ALL. Most studies have used a hyper-CVAD (hyper-fractioned cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose methotrexate and cytarabine) backbone in combination with a TKI. In a retrospective study, 42 patients with CML-LBP (38 of whom transformed on prior TKI therapy) received either hyper-CVAD plus imatinib (n = 27) or dasatinib (n = 15) [59]. The complete hematological response rate (CHR) was 85% with imatinib and 100% with dasatinib, and the CCyR rates were 41% and 87%, respectively. Outcomes were superior in those who underwent subsequent allogeneic HSCT. In a later update with 23 patients with CML-LBP who received hyper-CVAD plus dasatinib, a 5-year OS of 59% was achieved, a survival outcome substantially better than reported in CML-MBP, highlighting the superior prognosis of CML-LBP [60].
Most patients with CML-LBP have a B cell immunophenotype, raising the potential for incorporating effective antibody therapies such as the anti-CD22 antibody–drug conjugate inotuzumab ozogamicin or the CD3-CD19 bispecific T cell engaging antibody blinatumomab that have shown efficacy in B cell ALL, including in some small studies of Ph + ALL [61, 62]. In an ongoing prospective study of blinatumomab plus ponatinib, 6 patients with CML-LBP were treated, 5 of whom (83%) achieved CR/CRi, including 3 with MMR and 2 with a complete molecular response (CMR) [63]. In a study of mini-hyper-CVD (dose-attenuated hyper-fractioned cyclophosphamide, vincristine, and dexamethasone, alternating with methotrexate and cytarabine), ponatinib, and blinatumomab, all 3 patients with CML-LBP achieved CR, including 2 with CMR [64].
The Role of Allogeneic HSCT in Advanced Phase CML
In the modern era, allogeneic HSCT is rarely needed for patients with CML-CP, although it still has a role for many—but not all—patients with advanced phase CML. An algorithm for the treatment of advanced phase CML and the role of allogeneic HSCT is shown in Fig. 1. For patients with de novo CML-AP, HSCT is not recommended for patients who meet appropriate molecular response milestones, as these patients can have excellent survival outcomes with TKI therapy [36, 37]. The role of allogeneic HSCT in patients with transformed CML-AP is more controversial. Some analyses have suggested no benefit with HSCT for patients with transformed CML-AP, especially then they received a ponatinib-based regimen; however, some patients with very high-risk CML-AP or with suboptimal response to TKI therapy still have poor outcomes and should be considered for HSCT [65, 66•]. While there are limited data to support HSCT decisions for CML-AP based on specific cytomolecular abnormalities, the presence of a 3q26.2 rearrangement is associated with a particularly poor prognosis, and its presence should prompt expeditious HSCT referral [20].
Proposed treatment algorithm for patients with advanced phase CML. Abbreviations: CML, chronic myeloid leukemia; AP, accelerated phase; BP, blast phase; TKI, tyrosine kinase inhibitor; KD, kinase domain; HSCT, hematopoietic stem cell transplantation; AML, acute myeloid leukemia; HMA, hypomethylating agent; ALL, acute lymphoblastic leukemia; MRD, measurable residual disease
Most patients with CML-BP should be recommended for allogeneic HSCT if adequate response is achieved. Ideally, patients should proceed to HSCT after reversion to CML-CP, as this pre-HSCT response has been shown to be the strongest predictor of post-HSCT outcomes in patients with CML-BP [66•, 67]. In a retrospective analysis of patients with CML-MBP, allogeneic HSCT was associated with superior 5-year OS (58% versus 22% with no HSCT) [54]. In another analysis limited specifically to patients with T315I-mutated CML-BP who received ponatinib, HSCT was also associated with superior survival (4-year OS 26% versus 2% for no HSCT, P = 0.026) [68]. Allogeneic HSCT is also associated with superior outcomes in patients with CML-LBP, with a 5-year OS of 88% for patients who received hyper-CVAD plus dasatinib followed by HSCT versus 57% for those who did not proceed to HSCT (P = 0.04) [60]. However, it is possible that universal HSCT may not be required for patients with CML-LBP if adequate molecular response is achieved and with clearance of the malignant lymphoid clone using high-sensitivity next-generation sequencing measurable residual disease assays, as are commonly used to guide treatment decisions in ALL [69,70,71]. For patients with advanced phase CML who do undergo HSCT, the role of TKI maintenance is controversial. There was no benefit to this practice in a CIBMTR analysis, although it is still routinely used at many centers [72].
Conclusions
While the availability of more effective TKIs and guidelines to appropriately monitor therapeutic response have fortunately decreased the incidence of advanced phase CML, some patients still present with or develop CML-AP or CML-BP. Patients with de novo CML-AP can have excellent outcomes, but transformed CML-AP and CML-BP are still significant therapeutic challenges. These are relatively rare diseases, and therefore, there is a paucity of robust data to guide therapeutic decisions for these entities. Given their aggressive nature, more potent TKIs such as ponatinib are generally preferred for patients without a contraindication, and combination therapies using an AML-like or ALL-like backbone have been shown to be more effective than TKI monotherapy for patients with CML-BP. Consolidation with allogeneic HSCT is recommended for most patients with CML-BP and can lead to long-term survival in a majority of patients, although the role of HSCT in patients with CML-AP is less clear. Ongoing studies with venetoclax-based combinations have shown preliminary efficacy in CML-MBP, and the use of inotuzumab ozogamicin and/or blinatumomab may also play an important role in the management of CML-LBP. Future studies understanding the pathobiology of advanced phase CML and mechanisms of resistance to BCR::ABL1 TKIs are needed to develop more effective therapies for this disease and hopefully further improve outcomes for these patients.
Abbreviations
- CML:
-
Chronic myeloid leukemia
- ELN:
-
European LeukemiaNet
- WHO:
-
World Health Organization
- BM:
-
Bone marrow
- WBC:
-
White blood cells
- ACA:
-
Additional cytogenetic abnormality
- PB:
-
Peripheral blood
- CNS:
-
Central nervous system
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7.
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95:691–709.
Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186–93.
Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34:2333–40.
Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Hoffmann VS, Baccarani M, Hasford J, et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia. 2017;31:593–601.
O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
• Jain P, Kantarjian HM, Ghorab A, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: cohort study of 477 patients. Cancer. 2017;123:4391–402. Retrospective analysis identifying prognostic factors in patients with CML-BP.
Senapati J, Jabbour E, Kantarjian H, Short NJ. Pathogenesis and management of accelerated and blast phases of chronic myeloid leukemia. Leukemia. 2023;37:5–17.
Giles FJ, Cortes JE, Kantarjian HM, O’Brien SM. Accelerated and blastic phases of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:753–74.
•• Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84. The European LeukemiaNet recommendations on CML, which include definitions of CML-AP and CML-BP.
•• Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19. The WHO 2020 recommendations that removed the category of CML-AP and replaced it with “high-risk CML.
Wang W, Chen Z, Hu Z, et al. Clinical significance of trisomy 8 that emerges during therapy in chronic myeloid leukemia. Blood Cancer J. 2016;6:e490-e.
Issa GC, Kantarjian HM, Gonzalez GN, et al. Clonal chromosomal abnormalities appearing in Philadelphia chromosome–negative metaphases during CML treatment. Blood. 2017;130:2084–91.
Senapati J, Sasaki K. Chromosomal instability in chronic myeloid leukemia: mechanistic insights and effects. Cancers. 2022;14:2533.
Clark RE, Apperley JF, Copland M, Cicconi S. Additional chromosomal abnormalities at chronic myeloid leukemia diagnosis predict an increased risk of progression. Blood Adv. 2021;5:1102–9.
Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–27.
Bataller A, Sasaki K, Jabbour E, et al. Prognostic model of transformation to blast phase in patients with chronic myeloid leukemia. Blood. 2022;140:3895–6.
Hehlmann R, Voskanyan A, Lauseker M, et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia. 2020;34:2074–86.
Wang W, Cortes JE, Lin P, et al. Clinical and prognostic significance of 3q26.2 and other chromosome 3 abnormalities in CML in the era of tyrosine kinase inhibitors. Blood. 2015;126:1699–706.
Wang W, Cortes JE, Tang G, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016;127:2742–50.
Braun TP, Eide CA, Druker BJ. Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell. 2020;37:530–42.
Carella AM, Garuti A, Cirmena G, et al. Kinase domain mutations of BCR-ABL identified at diagnosis before imatinib-based therapy are associated with progression in patients with high Sokal risk chronic phase chronic myeloid leukemia. Leuk Lymphoma. 2010;51:275–8.
Loy K, Zenger M, Meggendorfer M, et al. Analysis of mechanisms of blast crisis in chronic myeloid leukemia by whole genome sequencing. Blood. 2020;136:19.
Machnicki MM, Pepek M, Solarska I, et al. ASXL1 mutations detectable at diagnosis may predict response to imatinib in patients with chronic myeloid leukemia. Blood. 2019;134:4148.
Marum JE, Yeung DT, Purins L, et al. ASXL1 and BIM germ line variants predict response and identify CML patients with the greatest risk of imatinib failure. Blood Adv. 2017;1:1369–81.
Menezes J, Salgado RN, Acquadro F, et al. ASXL1, TP53 and IKZF3 mutations are present in the chronic phase and blast crisis of chronic myeloid leukemia. Blood Cancer J. 2013;3:e157-e.
Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–9.
Branford S, Wang P, Yeung DT, et al. Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood. 2018;132:948–61.
Lee KL, Ko TK, Saw NYL, et al. Validation and refinement of a RUNX1 mutation-associated gene expression signature in blast crisis chronic myeloid leukemia. Leukemia. 2022;36:892–6.
Adnan-Awad S, Kim D, Hohtari H, et al. Characterization of p190-Bcr-Abl chronic myeloid leukemia reveals specific signaling pathways and therapeutic targets. Leukemia. 2021;35:1964–75.
Verma D, Kantarjian HM, Jones D, et al. Chronic myeloid leukemia (CML) with P190BCR-ABL: analysis of characteristics, outcomes, and prognostic significance. Blood. 2009;114:2232–5.
Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117:3733–6.
Hehlmann R, Lauseker M, Saußele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406.
Lauseker M, Hasford J, Saussele S, et al. Smokers with chronic myeloid leukemia are at a higher risk of disease progression and premature death. Cancer. 2017;123:2467–71.
Ohanian M, Kantarjian HM, Shoukier M, et al. The clinical impact of time to response in de novo accelerated-phase chronic myeloid leukemia. Am J Hematol. 2020;95:1127–34.
Ohanian M, Kantarjian HM, Quintas-Cardama A, et al. Tyrosine kinase inhibitors as initial therapy for patients with chronic myeloid leukemia in accelerated phase. Clin Lymphoma Myeloma Leuk. 2014;14:155-62.e1.
Cortes JE. A second-generation TKI should always be used as initial therapy for CML. Blood Adv. 2018;2:3653–5.
Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231–7.
NCCN. Chronic Myeloid leukemia NCCN website: NCCN Version 3.2022. https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. 2022. Accessed 1 May 2023.
Apperley JF, Cortes JE, Kim D-W, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START A trial. J Clin Oncol. 2009;27:3472–9.
Francesca P, Fausto C, Giuliana A, et al. The long-term durability of cytogenetic responses in patients with accelerated phase chronic myeloid leukemia treated with imatinib 600 mg: the GIMEMA CML Working Party experience after a 7-year follow-up. Haematologica. 2009;94:205–12.
le Coutre PD, Giles FJ, Hochhaus A, et al. Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 2012;26:1189–94.
Ottmann O, Saglio G, Apperley JF, et al. Long-term efficacy and safety of dasatinib in patients with chronic myeloid leukemia in accelerated phase who are resistant to or intolerant of imatinib. Blood Cancer J. 2018;8:88.
•• Senapati J, Sasaki K, Issa GC, et al. Management of chronic myeloid leukemia in 2023 – common ground and common sense. Blood Cancer J. 2023;13:58. Prospective study of ponatinib monotherapy across different CML subgroups, including CML-AP and CML-BP.
O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12.
Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med. 2013;369:1783–96.
Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome–positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404.
Cortes J, Rousselot P, Kim D-W, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2006;109:3207–13.
Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–42.
Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26:959–62.
• Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study. Cancer. 2010;116:3852–61. Retrospective analysis of patients with CML-MBP, showing the benefit of combination therapies with chemotherapy or a hypomethylating agent plus a TKI, and also the benefit of HSCT.
• Cortes J, Apperley J, Lomaia E, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138:2042–50. Prospective study evaluating FLAG-Ida plus ponatinib in patients with CML-BP, most of whom had myeloid blast phase.
Saxena K, Jabbour E, Issa G, et al. Impact of frontline treatment approach on outcomes of myeloid blast phase CML. J Hematol Oncol. 2021;14:94.
Copland M, Slade D, McIlroy G, et al. Ponatinib with fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor chemotherapy for patients with blast-phase chronic myeloid leukaemia (MATCHPOINT): a single-arm, multicentre, phase 1/2 trial. Lancet Haematol. 2022;9:e121–32.
Abaza Y, Kantarjian H, Alwash Y, et al. Phase I/II study of dasatinib in combination with decitabine in patients with accelerated or blast phase chronic myeloid leukemia. Am J Hematol. 2020;95:1288–95.
Maiti A, Franquiz Miguel J, Ravandi F, et al. Venetoclax and BCR-ABL tyrosine kinase inhibitor combinations: outcome in patients with Philadelphia chromosome-positive advanced myeloid leukemias. Acta Haematol. 2021;143:567–73.
Senapati J, Ravandi F, Dinardo CD, et al. A phase 2 study of the combination of decitabine (DAC), venetoclax (VEN), and ponatinib in patients (Pts) with chronic myeloid leukemia (CML) in accelerated phase (AP)/myeloid blast phase (MBP) or Philadelphia-chromosome positive (Ph+) acute myeloid leukemia (AML). Journal of Clinical Oncology 2023;41.
Strati P, Kantarjian H, Thomas D, et al. HCVAD plus imatinib or dasatinib in lymphoid blastic phase chronic myeloid leukemia. Cancer. 2014;120:373–80.
Morita K, Kantarjian HM, Sasaki K, et al. Outcome of patients with chronic myeloid leukemia in lymphoid blastic phase and Philadelphia chromosome–positive acute lymphoblastic leukemia treated with hyper-CVAD and dasatinib. Cancer. 2021;127:2641–7.
Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–47.
Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740–53.
Jabbour E, Short NJ, Jain N, et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial. Lancet Haematol. 2023;10:e24–34.
Nguyen D, Jabbour E, Short N, et al. A phase II study of the sequential combination of low-intensity chemotherapy (mini-hyper-CVD) and ponatinib followed by blinatumomab and ponatinib in patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Blood. 2022;140:6127–9.
Hu B, Lin X, Lee HC, et al. Timing of allogeneic hematopoietic cell transplantation (alloHCT) for chronic myeloid leukemia (CML) patients. Leuk Lymphoma. 2020;61:2811–20.
• Radujkovic A, Dietrich S, Blok H-J, et al. Allogeneic stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: a retrospective study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2019;25:2008–16. Study showing the benefit of HSCT in patients with T315I-mutated CML-BP, even when treated with ponatinib.
Barrett AJ, Ito S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood. 2015;125:3230–5.
Nicolini FE, Basak GW, Kim D-W, et al. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer. 2017;123:2875–80.
Kotrová M, Koopmann J, Trautmann H, et al. Prognostic value of low-level MRD in adult acute lymphoblastic leukemia detected by low- and high-throughput methods. Blood Adv. 2022;6:3006–10.
Short NJ, Jabbour E, Macaron W, et al. Ultrasensitive NGS MRD assessment in Ph+ ALL: prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol. 2023;98(8):1196–203.
Short NJ, Kantarjian H, Ravandi F, et al. High-sensitivity next-generation sequencing MRD assessment in ALL identifies patients at very low risk of relapse. Blood Adv. 2022;6:4006–14.
DeFilipp Z, Ancheta R, Liu Y, et al. Maintenance tyrosine kinase inhibitors following allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2020;26:472–9.
Funding
Supported by an MD Anderson Cancer Center Support Grant (CA016672) and SPORE. N. J. S. is supported by the American Society of Hematology Junior Faculty Scholar Award in Clinical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
N. J. S. has served as consultant for Pfizer Inc., GSK, NKARTA, and Sanofi, reports receiving research grants from Takeda Oncology, Astellas Pharma Inc., Xencor, Stemline Therapeutics, and NextCure, and has received honoraria from Novartis, Amgen, Pfizer Inc., Astellas Pharma Inc., Sanofi, and BeiGene. J. S. has been on the Advisory Board of Kite. E. J.: AbbVie: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Short, N.J., Senapati, J. & Jabbour, E. An Update on the Management of Advanced Phase Chronic Myeloid Leukemia. Curr Hematol Malig Rep 18, 234–242 (2023). https://doi.org/10.1007/s11899-023-00709-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-023-00709-4