Abstract
Purpose of Review
Largely, treatment advances in relapsed and/or refractory acute lymphoblastic leukemia (ALL) have been made in B cell disease leaving T cell ALL reliant upon high-intensity chemotherapy. Recent advances in the understanding of the biology of T-ALL and the improvement in immunotherapies have led to new therapeutic pathways to target and exploit. Here, we review the more promising pathways that are able to be targeted and other therapeutic possibilities for T-ALL.
Recent Findings
Preclinical models and early-phase clinical trials have shown promising results in some case in the treatment of T-ALL. Targeting many different pathways could lead to the next advancement in the treatment of relapsed and/or refractory disease. Recent advances in cellular therapies have also shown promise in this space.
Summary
When reviewing the literature as a whole, targeting important pathways and antigens likely will lead to the next advancement in T-ALL survival since intensifying chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
T cell acute lymphoblastic leukemia (T-ALL) has traditionally been thought of as a higher risk leukemia that is more resistant to therapy than its B cell counterpart. Recent advances in therapy, including intensification of combination chemotherapy in the adolescent and young adult (AYA) population, have improved overall survival (OS), and T-ALL is no longer an independent risk factor for worse outcomes [1••, 2]. Outside of combination chemotherapy, which is associated with significant morbidity and late effects, there have been few advances specific to the treatment of T-ALL. Recently, the Children’s Oncology Group (COG) demonstrated improved event-free survival (EFS) with the addition of nelarabine to an intensive pediatric chemotherapy backbone, but an improvement in OS was not demonstrated at the time of publication [3••]. With the addition of nelarabine to frontline therapy because of these data, there are now even fewer options for the treatment of relapsed or refractory (R/R) T-ALL. New agents and targets are desperately needed for T-ALL, similar to the influx of options now available for B-ALL. In this review, we describe potential novel agents and pathways for T-ALL and drugs currently in development that could impact the future of T-ALL therapy.
Signal Transduction Inhibitors
Signal transduction pathways regulate normal cell growth and homeostasis. They are frequently dysregulated in malignant cells, leading to molecular and cellular survival advantages. As important parts of malignant transformation, perturbation of these pathways offers potential opportunities for targeted therapy. We review in this section select novel pathways that may be important in future targeted therapy for T-ALL.
Interleukin-7 Receptor Pathway
Steroids are a foundation in the treatment of ALL, but steroid resistance is common in R/R T-ALL. Synthetic glucocorticoids appear to act through modulating the apoptotic pathway involving protein B cell lymphoma-2 (BCL-2) [4]. Resistance to glucocorticoid therapy has previously been tied to the Janus kinase/signal transducer and activator of transcription (JAK-STAT) and the phosphatidylinositol 3-kinase/protein kinase B (PI3K-AKT) pathways [5, 6]. The JAK-STAT pathway is linked to interleukin-7 receptor (IL7R) signaling, and its effect on cell growth and survival is potentially mediated through upregulation of BCL-2 activity [5, 6]. Recent work has demonstrated the importance of the canonical mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK-ERK) signal transduction pathway in IL7R-mediated steroid resistance [7]. Different upstream pathways have been shown to activate MAPK/ERK kinase (MEK) within this cascade and are oncogenic drivers for many malignancies [8]. Targeted inhibitors have now been developed to disrupt activation of this pathway and are clinically efficacious in other cancers, making MAPK-ERK an attractive target in T-ALL [8]. In T-ALL cell lines and patient-derived xenograft (PDX) models, mutant IL7R signaling was associated with steroid resistance and increased downstream activation of MAPK-ERK signaling, in both models whose growth was dependent on increased exogenous IL7 signaling and in models who were independent of increase exogenous IL7 signaling [7]. One downstream target of the MAPK-ERK pathway is MEK which phosphorylates BCL-2-like protein 11 (BIM). BIM is a pro-apoptotic protein that binds anti-apoptotic proteins, such as BCL-2 and others, and is inactivated by phosphorylation by MEK [5, 7, 8]. The combination of glucocorticoids and MEK inhibition resulted in reversal of steroid resistance in cell lines, PDX models, and patient samples, suggesting the promising potential of combination therapy with MEK inhibitors [5, 7]. There is a phase I/II clinical trial currently enrolling in the UK examining the safety and early efficacy of the MEK inhibitor selumetinib in combination with dexamethasone (NCT03705507).
Either from mutations leading to increased activation or through overexpression of wild-type IL7R, stimulation of IL7R also leads to JAK-STAT signaling through a pathway separate from MAPK-ERK, which seems to coalesce in phosphorylation of signal transducer and activator of transcription 5 (STAT5) and induces the upregulation of proviral integration site for Moloney murine leukemia virus-1 (PIM1), a protein kinase [9, 10]. PIM1 is involved in cell cycle regulation and apoptosis and is susceptible to small-molecule inhibitors [11, 12]. Preclinical studies have demonstrated the importance of PIM1 in IL7R signaling for the survival of T-ALL cells, both in cell lines and from patient-derived samples [9, 13, 14]. PIM1 inhibition reversed cell cycle progression and cell growth in T-ALL cells in vitro and led to lower leukemic burden and longer survival in PDX models [9, 14]. PIM1 inhibition also synergizes with standard T-ALL therapy, such as corticosteroids, making it a potential addition to standard chemotherapy in the future [9]. Multiple PIM1 inhibitors are in early-phase clinical trials for both solid tumors and hematologic malignancies [15].
NOTCH Pathway Inhibitors
NOTCH receptors, specifically NOTCH1, play an important role in thymocyte development, and activating mutations are found in well over half of all T-ALL cases [16, 17]. NOTCH mutations resulting in increased activity exert at least part of their oncogenesis through upregulation of MYC transcription [18]. Significant work has been done to target this pathway with some early successes, but nothing has yet resulted in a Food and Drug Administration (FDA)-approved therapy. NOTCH1 is activated through cleavage, and gamma secretase inhibitors (GSIs) can inhibit NOTCH1 activation by preventing this cleavage [19]. GSIs were originally developed for the treatment of Alzheimer’s disease. Rather than directly cause apoptosis, GSIs arrest the cell cycle and may reintroduce steroid sensitivity in steroid-resistant T-ALL [19]. In combination with steroids, GSIs work synergistically to induce T-ALL cell death partly through transcriptional upregulation of the nuclear receptor subfamily 3 group C member 1 (NR3C1) gene and increased expression of pro-apoptotic regulators such BIM and BCL2 [19]. Unfortunately, GSIs cause considerable gastrointestinal (GI) toxicity, including diarrhea. Severe diarrhea may arise from disrupted NOTCH regulation of transcription of the gene KLF4 (Krüppel-like factor 4), which is involved in goblet cell differentiation in the GI tract [20]. While steroids seem to abrogate this side effect in vivo, diarrhea was the dose-limiting toxicity in early-phase studies [19, 21, 22]. A multicenter, nonrandomized, open-label, dose escalation phase I study of adult patients with R/R T-ALL was performed combining crenigacestat, a GSI, with dexamethasone, but the dose escalation of the GSI was limited by grade 3 GI toxicity despite steroid treatment [23]. This combination did result in stable disease in 17% of patients and one complete response (CR), which lasted 10.5 months, and established a dose to be investigated in further trials [23]. In a separate multicenter phase I trial, another GSI, BMS-906024, demonstrated similar toxicity patterns and some early evidence of response with a decrease in percentage of blasts in the bone marrow, one CR, and one partial response (PR) [21]. The patient who obtained a CR had an early T cell precursor ALL (ETP-ALL) and subsequently achieved a deep molecular response, underwent an allogeneic hematopoietic cell transplant (HCT), and remains without disease [22]. This patient’s ETP-ALL also carried mutations in protein tyrosine phosphatase non-receptor type 11 (PTPN11), DNA methyltransferase 3 alpha (DNMT3A), and colony-stimulating factor 3 receptor (CSF3R) in addition to a mutation in NOTCH1, possibly explaining this exceptional response, but these findings require further investigation [22]. Preclinical studies have demonstrated that resistance mutations will commonly emerge in the setting of GSI treatment in part through alternative activation of MYC [24, 25]. Two of these pathways identified through preclinical testing involve upregulation of BCL-2 and BRD4, which are targetable and therefore lend themselves to future clinical testing [25].
Cyclin-Dependent Kinase (CDK) Inhibitors
CDKs are a heterogenous group of proteins that play a critical role in cell cycle regulation and act as transcriptional cofactors. Within T-ALL, cyclin D3 is integral to NOTCH-driven tumorigenesis [26•]. Mouse cyclin D3 knockout models and mice treated with a CDK4/6 inhibitor both demonstrated NOTCH-driven T-ALL regression [27]. In other mouse models, CDK4/6 inhibitors with mammalian target of rapamycin (mTOR) inhibitors or steroids demonstrated synergism and disease response [28, 29]. Preclinical work has also targeted other CDKs, such as through a novel agent THZ1, which irreversibly covalently binds CDK7 outside of its kinase domain but still prevents its kinase activity [30]. Since THZ1 irreversibly binds to CDK7, on-target toxicity similar to GSIs would be expected, but THZ1 preferentially impacts oncogenes driven by super-enhancers, which suggests the possibility of a clinically relevant therapeutic window that could be further explored [30, 31]. Finally, targeting CDK9 has been attractive as it is involved in promoting transcription of myeloid cell leukemia-1 (MCL1). AZD4573 targets CDK9 through an alternative PROteolysis TArgeting Chimeras (PROTAC) approach [32, 33]. Tested on MOLT-4 T-lymphoblasts, the PROTAC approach induced apoptosis even after drug washout, suggesting a prolonged effect in vitro [33]. This preclinical evidence has led to multiple clinical trials, including one sponsored by the COG that combines palbociclib with reinduction chemotherapy (NCT03792256) and an institutional trial examining the combination of ribociclib, dexamethasone, and everolimus (NCT03740334).
BCL-2 Family Inhibitors
BCL-2 is an anti-apoptotic protein that was first discovered in B cell lymphoma. Along with B cell lymphoma-extra-large (BCL-XL) and MCL1, BCL-2 is a member of an anti-apoptotic family of proteins that share BCL-2 homology (BH) domains including BH3. These proteins bind to pro-apoptotic proteins such as BCL-2 associated X (BAX) and BCL-2 homologous antagonist/killer (BAK), sequestering them and thereby inhibiting apoptosis [34]. This family of proteins is important in the pathogenesis of T-ALL as evidenced by their involvement in other targetable pathways discussed above (section “Interleukin-7 Receptor Pathway”), but also by BH3 profiling of T-ALL cell lines, which have demonstrated different levels of dependence on BCL-2 or BCL-XL based on their level of cellular differentiation [35].
There is already significant experience targeting BH3 proteins in clinical practice. A retrospective case series descried the potential utility of venetoclax, a BCL-2 inhibitor, in combination with various chemotherapy regimens, including hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone (hyper-CVAD), asparaginase, nelarabine, decitabine, and others, for the treatment of 13 patients with R/R T-ALL (5 of whom had ETP-ALL) [36]. Of the 10 evaluable patients, 60% achieved a composite CR (3 with a CR, 1 with a CR with incomplete hematologic recovery (CRi), and 2 with a morphologic leukemia-free state (MLFS)) [36]. The median venetoclax dose was 200 mg daily for 21 days, and prolonged cytopenias were seen with daily doses of 400 mg or durations of 14 days or more per cycle [36]. A phase I study of venetoclax in combination with a modified hyper-CVAD protocol, mini-hyper-CVD, in older adults with untreated ALL and adult patients with R/R ALL demonstrated promising results with manageable toxicities and recommended a phase II venetoclax dose of 600 mg daily [37••]. All newly diagnosed patients in this study achieved a response with 9 of the 10 (90%) achieving a CR with undetectable minimal residual disease (MRD) based on multicolor flow cytometry, while one achieved a PR [37••]. Of these patients, 3 had T-ALL (2 had ETP-ALL) [37••]. In the R/R ALL cohort, 3 out of 8 (38%) achieved a CR/CRi with 2 of the 3 becoming MRD negative [37••]. A different phase I study evaluated the use of venetoclax in combination with low-dose navitoclax, a BCL-XL inhibitor, and chemotherapy to treat patients with both B-ALL and T-ALL with the hypothesis that the combination would limit the thrombocytopenia from navitoclax while still achieving clinically significant BCL-2 family inhibition [38•]. A total of 44 patients with R/R ALL were treated (57% B-ALL and 43% T-ALL), and the CR rate was 64% for B-ALL and 53% for T-ALL [38•]. The study recommended phase II doses of navitoclax 50 mg daily (reduced to 25mg if less than 45 kg) and venetoclax 400 mg daily continuously, while response is maintained [38•]. Protocols are now being designed to incorporate these BH3 mimetics (venetoclax and navitoclax) into frontline treatment regimens.
Epigenetic Modifiers
There are two main classes of epigenetic modifying agents: histone deacetylase inhibitors (HDACi) and hypomethylating agents (HMAs). Histone deacetylases regulate chromatin structure, and HDACi have been approved for the treatment of lymphomas and myeloma. One HDACi, panobinostat, has shown some efficacy in the MOLT-4 T-ALL cell line in vitro and in xenografts in vivo, in contrast to little or no efficacy in B-ALL cell lines [39]. In PDX models, panobinostat in combination with chemotherapy prolonged mouse survival over either chemotherapy or HDACi alone [39]. This has led to several small clinical trials including a phase I trial in both older, newly diagnosed ALL patients, and those with R/R ALL that combined entinostat (a HDACi) with clofarabine [40]. In this study, 5 of 28 patients had T-ALL, but responses were not reported separately for this subset; CR or CRi was achieved in 4 patients in total [40].
HMAs play an important role in the modern treatment of acute myeloid leukemia (AML) but are not routinely used in ALL. Preclinical work on adult T-ALL samples has identified subgroups of T-ALL based on DNA methylation profiles [41]. A hypermethylated T-ALL subgroup had a worse outcome than other subgroups [41]. Using PDX models of this hypermethylated subgroup, treatment with azacitidine, a HMA, prolonged survival and delayed leukemia progression [41]. Case reports show clinical response to decitabine, another HMA, in combination with venetoclax [40,41,42]. Two cases of T-ALL achieved MRD negative CR, and one case of T cell lymphoblastic lymphoma also achieved a CR [42,43,44].
Proteasome Inhibitors
The proteasome degrades and processes proteins within cells and when inhibited can induce apoptosis due to the collection of unfolded or misfolded proteins [45]. Available proteasome inhibitors include bortezomib, carfilzomib, and ixazomib, and this class of drugs is FDA-approved and commonly used for the treatment of multiple myeloma. They selectively inhibit the 26S proteasome, leading to several anti-cancer mechanisms: increased pro-apoptotic proteins such as phorbol-12-myristate-13-acetate-induced protein 1 (NOXA), suppressed nuclear factor kappa B (NF-ĸB) signaling, and decreased anti-apoptotic proteins BCL-XL and BCL-2 [46]. Pediatrics has more readily embraced the potential role of proteasome inhibitors in the treatment of ALL to date. In a large study, 135 pediatric patients with relapsed ALL received reinduction chemotherapy plus bortezomib, yielding a promising CR rate of 68% for R/R T-ALL (22 of the 135 patients) [47]. This study led to a large COG study of T-ALL using bortezomib in combination with chemotherapy in the upfront setting (AALL1231) [48••]. The study randomized 824 patients to a standard pediatric chemotherapy backbone with or without bortezomib [48••]. The intervention arm had a 4-year EFS of 83.8% and 4-year OS of 88.3%, compared to 80.1% and 85.7%, respectively, in the control arm. These differences were not statistically significant, but there was also no increase in toxicity on the bortezomib arm [48••]. There was a statistically significant difference favoring bortezomib in the T lymphoblastic lymphoma subset, with a 4-year EFS of 86.4% and 4-year OS of 89.5%, compared with 76.5% and 78.3%, respectively, in the control arm (p=0.009) [48••]. The next upfront study from the COG for T-ALL may randomize a proteasome inhibitor with a different chemotherapy backbone.
Other studies, primarily involving patients with B-ALL, have evaluated the use of bortezomib and ixazomib. One study of 10 children with R/R ALL (9/10 with B-ALL) gave mitoxantrone, dexamethasone, pegaspargase, vincristine, intrathecal methotrexate, and bortezomib (1.3 mg/m2 for 4 weekly doses), and 80% achieved CR or CRi, including a CR in the one patient with T-ALL [49]. A phase I dose-finding study of 19 adults (51–75 years old) with B-ALL evaluated the use of ixazomib together with induction therapy with prednisone, vincristine, and doxorubicin (plus dasatinib if BCR-ABL1 positive); 79% had CR or CRi [50]. The recommended phase II dose for ixazomib was 2.3 mg weekly [50].
JAK/STAT Inhibitors
The JAK/STAT signal transduction pathway is an important driver in the development and propagation of T-ALL and has been discussed already in relationship to other pathways in a previous section (section “Interleukin-7 Receptor Pathway”). In addition to these associations, recurring mutations in T-ALL have been described in genes encoding different JAK and STAT proteins, including STAT5b, JAK1, and JAK3 [51,52,53]. This pathway is directly inhibited by ruxolitinib, a JAK 1/2 inhibitor. In preclinical models, the JAK/STAT pathway was hyperactive with increased IL-7 signaling that can be effectively inhibited in vitro with ruxolitinib [54]. This inhibition was more effective in ETP-ALL than non-ETP-ALL subtypes and was even seen without mutations in the IL7-JAK-STAT pathway [54]. Ruxolitinib can also decrease glucocorticoid resistance in ETP and T-ALL preclinical samples, possibly through IL-7 pathway hyperstimulation [55]. Because of this synergism, ruxolitinib is currently being investigated in R/R T-ALL in combination with glucocorticoids and chemotherapy (NCT03613428).
Tyrosine Kinase Inhibitors (TKIs)
Mutations and amplification of the ABL1 (Abelson tyrosine–protein kinase 1) gene in T-ALL lead to aberrant tyrosine kinase activation [26•, 56]. Fusions involving ABL1 also occur in T-ALL, but these fusions are more commonly with NUP214 (nucleoporin 214) than BCR (breakpoint cluster region, which when fused with ABL1 is referred to as the Philadelphia (Ph) chromosome) [57]. In NUP214::ABL1 translocated T-ALL, TKIs such as imatinib, nilotinib, and dasatinib have demonstrated efficacy both in vitro and in PDX models, leading to increases in mouse OS [57,58,59]. In some NUP214::ABl1 T-ALL, co-occurring TLX1 (T cell leukemia homeobox 1) mutations have been shown to be drivers of proliferation and survival, making these T-ALL cells sensitive to targeting with a combination of TKIs and BCL-2 inhibitors [59]. The NUP214::ABL1 translocation is not common in T-ALL; thus, the larger family of available TKIs has only limited utility in this disease [57]. Dasatinib does have significant activity outside of targeting ABL kinases and can induce cell death in 30% of T-ALL in vitro, regardless of the mutation profile, which has been confirmed in mouse models [60, 61]. Case reports have described dramatic responses to dasatinib [61, 62]. Besides the NUP214::ABL1 translocation, there are currently no predictable mutational patterns for sensitivity to TKIs. It is possible that phosphoproteomic profiling of T-ALL can help identify targetable kinases such as insulin receptor (InsR) and insulin-like growth factor-1 receptor (IGF-1R), leading to increased sensitivity to dasatinib [63]. In R/R T-ALL, novel combinations with dasatinib are appealing, and more data should be gathered about predictive patterns in T-ALL.
PI3K/AKT/mTOR Inhibitors
Phosphoinositide-3 kinase (PI3K) plays an important role in normal T cell development and in T-ALL leukemogenesis. PI3K is activated by multiple different growth factors and G protein-coupled receptors and then activates the protein kinase B (AKT), which increases mTOR activity and drives cell proliferation and survival [64]. Nearly half of T-ALL have alterations in this pathway, including mutations in PTEN (phosphatase and tensin homolog; PI3K inhibition), USP7 (ubiquitin specific protease 7; PTEN inhibition), PIK3R1 (phosphoinostidide-3-kinase regulatory subunit 1), and PIK3CD (phosphatidylinositol-4, 5-bisphosphonated 3-kinase catalytic subunit delta) [26•]. These mutations seem to cluster in T-ALL that also carry TAL1 (T cell acute lymphocytic leukemia protein 1) mutations, possibly because of interaction with AKT within the PI3K pathway [26•, 65]. Mutations in this pathway contribute to resistance to chemotherapy and corticosteroids [66]. Numerous studies have demonstrated the efficacy of PI3K and AKT inhibition in various T-ALL models, which resensitizes leukemic blasts to corticosteroids [5, 13, 67]. Perhaps the most attractive target in this pathway is mTOR, for which multiple FDA-approved inhibitors exist for other diseases, including sirolimus/rapamycin, everolimus, and temsirolimus. T-ALL cell death is induced in vitro and in vivo through either mTOR inhibition alone or both mTOR and PI3K inhibition [13, 68]. In a phase I/II study of everolimus combined with hyper-CVAD for R/R ALL, a response rate of 50% was noted in a very heavily pretreated population of T-ALL (median of 4 prior lines of therapy) [69]. A number of clinical trials that are either actively recruiting or recently closed include an mTOR inhibitor in combination with other chemotherapy and targeted agents for R/R ALL.
Anti-CD38 Therapy
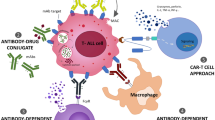
CD38 (cluster of differentiation 38) is typically expressed on the surfaces of immune cells as a signal transducer. It is also expressed on the surface of lymphoblasts from patients with T-ALL, making it a potential target for therapeutic intervention [70•, 71]. CD38 expression persisted on T-ALL cells despite 1 month of exposure to induction chemotherapy of dexamethasone, vincristine, daunorubicin, and PEGylated asparaginase with or without bortezomib [70•, 71]. This was not true for B-ALL, which downregulated CD38 after exposure to induction chemotherapy [70•]. Similar to proteasome inhibitors, the anti-CD38 monoclonal antibodies daratumumab and isatuximab are more commonly used in multiple myeloma than in leukemia, likely because plasma cells have the highest expression of CD38 [71]. However, PDX models of T-ALL showed reduction of leukemia burden after daratumumab [70•, 71]. A phase II study evaluating daratumumab combined with chemotherapy for the treatment of pediatric and young adult patients with R/R ALL, including those with T-ALL, has enrolled 47 participants to date (NCT03384654). A phase II study of isatuximab monotherapy in 11 patients with T-ALL was not encouraging as no patients had CR or CRi, most had progressive disease, and over half had an infusion reaction [72]. A novel approach using a bispecific T cell-engaging antibody targeting CD38 and CD3 (XmAb 18968 CD3-CD38) has preclinical evidence of efficacy in multiple myeloma and is currently undergoing clinical trials in T-ALL (NCT05038644) [73].
CAR T Cell Therapy
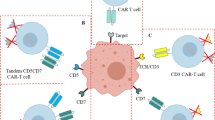
Chimeric antigen receptor (CAR) T cell therapy has been used successfully for patients with B-ALL, but there is now early evidence for its use in T-ALL [74, 75]. The CARs typically consist of an extracellular antigen binding domain, a transmembrane domain, costimulatory molecules, and immunoreceptor tyrosine-based activation motifs [76]. Currently, designs with modified costimulatory domains are in their fourth generation, but a second generation CAR T cell with CD3ζ and 4-1BB domains (tisagenlecleucel) was the first-in-class FDA-approved CAR T cell therapy for R/R CD19-positive B-ALL in children and young adults up to 25 years of age [77]. Additional CAR T cell products have been approved in adult R/R B-ALL, large B cell lymphoma, follicular lymphoma, mantle cell lymphoma, and, most recently, multiple myeloma. CAR T cell therapy is much more at its infancy in the treatment of T-ALL because of the difficulty of a target that also does not lead to untenable toxicity and lifelong late effects [78]. Treatment of T cell malignancies with CAR T cell therapy is also complicated by fratricide, which is the destruction of CAR T cells by other CAR T cells [78]. In a phase I trial, 20 patients with R/R T-ALL received anti-CD7 CAR T cell therapy with 90% (18/20) achieving CR and 35% (7/20) going on for an allogenic HCT [75]. CD1a is another CAR T cell target being explored in T-ALL [79]. Methods to address fratricide and future studies to evaluate effective CAR T cell targets in T-ALL will likely lead to further advancements in this therapy line.
Conclusion
A variety of targets are under investigation to improve the treatment and clinical outcomes for patients with T-ALL. We have summarized important medications in Table 1 that may reshape the way we approach T-ALL treatment and reduce disparities in health outcomes between B-ALL and T-ALL. Current and future studies will help better delineate which promising treatments are most advantageous for patients depending on their disease biology and genetics.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stock W, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–59. Practice changing publication on AYA ALL from an adult cooperative group.
Schrappe M, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8):2077–84.
Dunsmore KP, et al. Children's Oncology Group AALL0434: a phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol. 2020;38(28):3282–93. Practice changing trial for young people with T ALL from the pediatric collaborative group.
Jing D, et al. Opposing regulation of BIM and BCL2 controls glucocorticoid-induced apoptosis of pediatric acute lymphoblastic leukemia cells. Blood. 2015;125(2):273–83.
Li Y, et al. IL-7 receptor mutations and steroid resistance in pediatric T cell acute lymphoblastic leukemia: a genome sequencing study. PLOS Med. 2016;13(12):e1002200.
Barata JT, et al. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200(5):659–69.
van der Zwet JCG, et al. MAPK-ERK is a central pathway in T-cell acute lymphoblastic leukemia that drives steroid resistance. Leukemia. 2021;35(12):3394–405.
Dillon M, et al. Progress on Ras/MAPK signaling research and targeting in blood and solid cancers. Cancers (Basel). 2021;13(20).
Ribeiro D, et al. STAT5 is essential for IL-7-mediated viability, growth, and proliferation of T-cell acute lymphoblastic leukemia cells. Blood Adv. 2018;2(17):2199–213.
de Bock CE, et al. HOXA9 cooperates with activated JAK/STAT signaling to drive leukemia development. Cancer Discov. 2018;8(5):616–31.
Blanco-Aparicio C, Carnero A. Pim kinases in cancer: diagnostic, prognostic and treatment opportunities. Biochem Pharmacol. 2013;85(5):629–43.
Bachmann M, et al. The oncogenic serine/threonine kinase Pim-1 directly phosphorylates and activates the G2/M specific phosphatase Cdc25C. Int J Biochem Cell Biol. 2006;38(3):430–43.
Silva A, et al. Overexpression of wild-type IL-7Rα promotes T-cell acute lymphoblastic leukemia/lymphoma. Blood. 2021;138(12):1040–52.
De Smedt R, et al. Targeting cytokine- and therapy-induced PIM1 activation in preclinical models of T-cell acute lymphoblastic leukemia and lymphoma. Blood. 2020;135(19):1685–95.
Luszczak S, et al. PIM kinase inhibition: co-targeted therapeutic approaches in prostate cancer. Signal Transduct Targeted Ther. 2020;5(1):7.
Mansour MR, et al. High incidence of Notch-1 mutations in adult patients with T-cell acute lymphoblastic leukemia. Leukemia. 2006;20(3):537–9.
Weng AP, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20(15):2096–109.
Moellering RE, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462(7270):182–8.
Real PJ, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15(1):50–8.
Zheng H, et al. KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G490–8.
Zweidler-McKay PA, et al. The safety and activity of BMS-906024, a gamma secretase inhibitor (GSI) with anti-Notch activity, in patients with relapsed T-cell acute lymphoblastic leukemia (T-ALL): initial results of a phase 1 trial. Blood. 2014;124(21):968–8.
Knoechel B, et al. Complete hematologic response of early T-cell progenitor acute lymphoblastic leukemia to the γ-secretase inhibitor BMS-906024: genetic and epigenetic findings in an outlier case. Cold Spring Harb Mol Case Stud. 2015;1(1):a000539.
Borthakur G, et al. Phase 1 study to evaluate Crenigacestat (LY3039478) in combination with dexamethasone in patients with T-cell acute lymphoblastic leukemia and lymphoma. Cancer. 2021;127(3):372–80.
O'Neil J, et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007;204(8):1813–24.
Knoechel B, et al. An epigenetic mechanism of resistance to targeted therapy in T cell acute lymphoblastic leukemia. Nat Genet. 2014;46(4):364–70.
Liu Y, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49(8):1211–8. Article that includes a detailed description of genetic changes seen in T ALL.
Sicinska E, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4(6):451–61.
Choi YJ, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22(4):438–51.
Pikman Y, et al. Synergistic drug combinations with a CDK4/6 inhibitor in T-cell acute lymphoblastic leukemia. Clin Cancer Res. 2017;23(4):1012–24.
Kwiatkowski N, et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature. 2014;511(7511):616–20.
Mansour MR, et al. Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science. 2014;346(6215):1373–7.
Cidado J, et al. AZD4573 is a highly selective CDK9 inhibitor that suppresses MCL-1 and induces apoptosis in hematologic cancer cells. Clin Cancer Res. 2020;26(4):922–34.
Olson CM, et al. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat Chem Biol. 2018;14(2):163–70.
Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17(3):393–403.
Chonghaile TN, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4(9):1074–87.
Richard-Carpentier G, et al. Clinical experience with venetoclax combined with chemotherapy for relapsed or refractory T-cell acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(4):212–8.
Jain N, et al. A multicenter phase I study combining venetoclax with mini-hyper-CVD in older adults with untreated and relapsed/refractory acute lymphoblastic leukemia. Blood. 2019;134(Supplement_1):3867–7. An excellent preliminary study in older adults with combination chemotherapy that will likely drive future randomized trials.
Pullarkat VA, et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021;11(6):1440–1453. Early phase study demonstrating effectiveness of BCL-2/BCL-XL combination in relapsed ALL.
Vilas-Zornoza A, et al. Preclinical activity of LBH589 alone or in combination with chemotherapy in a xenogeneic mouse model of human acute lymphoblastic leukemia. Leukemia. 2012;26(7):1517–26.
Carraway HE, et al. Phase 1 study of the histone deacetylase inhibitor entinostat plus clofarabine for poor-risk Philadelphia chromosome-negative (newly diagnosed older adults or adults with relapsed refractory disease) acute lymphoblastic leukemia or biphenotypic leukemia. Leuk Res. 2021;110:106707.
Touzart A, et al. Epigenetic analysis of patients with T-ALL identifies poor outcomes and a hypomethylating agent-responsive subgroup. Sci Transl Med. 2021;13(595).
Baig MU, et al. Venetoclax and decitabine in pediatric refractory T-cell lymphoblastic lymphoma. J Pediatr Hematol Oncol. 2021;43(7):e991–6.
Farhadfar N, et al. Venetoclax and decitabine for treatment of relapsed T-cell acute lymphoblastic leukemia: a case report and review of literature. Hematol Oncol Stem Cell Ther. 2021;14(3):246–51.
Rahmat LT, et al. Venetoclax in combination with decitabine for relapsed T-cell acute lymphoblastic leukemia after allogeneic hematopoietic cell transplant. Case Rep Hematol. 2018;2018:6092646.
Ito S. Proteasome inhibitors for the treatment of multiple myeloma. Cancers (Basel). 2020;12(2).
Lato MW, et al. The new therapeutic strategies in pediatric T-cell acute lymphoblastic leukemia. Int J Mol Sci. 2021;22(9).
Horton TM, et al. Bortezomib reinduction chemotherapy in high-risk ALL in first relapse: a report from the Children's Oncology Group. Br J Haematol. 2019;186(2):274–85.
Teachey DT, et al. Children's Oncology Group Trial AALL1231: a phase III clinical trial testing bortezomib in newly diagnosed T-cell acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2022:Jco2102678. Initial evidence of potential utility of proteasome inhibition in T ALL.
August KJ, et al. Treatment of children with relapsed and refractory acute lymphoblastic leukemia with mitoxantrone, vincristine, pegaspargase, dexamethasone, and bortezomib. Pediatr Blood Cancer. 2020;67(3):e28062.
Amrein P, et al. Ixazomib in addition to chemotherapy for the treatment of acute lymphoblastic leukemia in older adults. Leuk Lymphoma. 2022;63:1–8.
Vicente C, et al. Targeted sequencing identifies associations between IL7R-JAK mutations and epigenetic modulators in T-cell acute lymphoblastic leukemia. Haematologica. 2015;100(10):1301–10.
Girardi T, et al. The genetics and molecular biology of T-ALL. Blood. 2017;129(9):1113–23.
Zhang J, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–63.
Maude SL, et al. Efficacy of JAK/STAT pathway inhibition in murine xenograft models of early T-cell precursor (ETP) acute lymphoblastic leukemia. Blood. 2015;125(11):1759–67.
Delgado-Martin C, et al. JAK/STAT pathway inhibition overcomes IL7-induced glucocorticoid resistance in a subset of human T-cell acute lymphoblastic leukemias. Leukemia. 2017;31(12):2568–76.
Barber KE, et al. Amplification of the ABL gene in T-cell acute lymphoblastic leukemia. Leukemia. 2004;18(6):1153–6.
Graux C, et al. Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nat Genet. 2004;36(10):1084–9.
Quintás-Cardama A, et al. Activity of tyrosine kinase inhibitors against human NUP214-ABL1-positive T cell malignancies. Leukemia. 2008;22(6):1117–24.
Vanden Bempt M, et al. Cooperative enhancer activation by TLX1 and STAT5 drives development of NUP214-ABL1/TLX1-positive T cell acute lymphoblastic leukemia. Cancer Cell. 2018;34(2):271–285.e7.
Laukkanen S, et al. In silico and preclinical drug screening identifies dasatinib as a targeted therapy for T-ALL. Blood Cancer J. 2017;7(9):e604.
Frismantas V, et al. Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood. 2017;129(11):e26–37.
Deenik W, et al. Rapid complete cytogenetic remission after upfront dasatinib monotherapy in a patient with a NUP214-ABL1-positive T-cell acute lymphoblastic leukemia. Leukemia. 2009;23(3):627–9.
Cordo’ V, et al. Phosphoproteomic profiling of T cell acute lymphoblastic leukemia reveals targetable kinases and combination treatment strategies. Nat Comm. 2022;13(1):1048.
Pocock R, et al. Current and emerging therapeutic approaches for T-cell acute lymphoblastic leukaemia. Br J Haematol. 2021;194(1):28–43.
Palamarchuk A, et al. Akt phosphorylates Tal1 oncoprotein and inhibits its repressor activity. Cancer Res. 2005;65(11):4515–9.
Gutierrez A, et al. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114(3):647–50.
Piovan E, et al. Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell. 2013;24(6):766–76.
Kong D, et al. Growth inhibition and suppression of the mTOR and Wnt/β-catenin pathways in T-acute lymphoblastic leukemia by rapamycin and MYCN depletion. Hematol Oncol. 2021;39(2):222–30.
Daver N, et al. A phase I/II study of the mTOR inhibitor everolimus in combination with HyperCVAD chemotherapy in patients with relapsed/refractory acute lymphoblastic leukemia. Clin Cancer Res. 2015;21(12):2704–14.
Bride KL, et al. Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood. 2018;131(9):995–9. Potential preclinical evidence to incorporate daratumumab in future T ALL clinical trials.
Naik J, et al. CD38 as a therapeutic target for adult acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Haematologica. 2019;104(3):e100–3.
Boissel N, et al. Isatuximab monotherapy in patients with refractory T-acute lymphoblastic leukemia or T-lymphoblastic lymphoma: phase 2 study. Cancer Med. 2022;11(5):1292–8.
Zuch de Zafra CL, et al. Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cell-recruiting antibody optimized for cytotoxicity and cytokine release. Clin Cancer Res. 2019;25(13):3921–33.
Teachey DT, Hunger SP. Anti-CD7 CAR T cells for T-ALL: impressive early-stage efficacy. Nat Rev Clin Oncol. 2021;18(11):677–8.
Pan J, et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J Clin Oncol. 2021;39(30):3340–51.
Zhang C, et al. Engineering CAR-T cells. Biomark Res. 2017;5(1):22.
Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48.
Safarzadeh Kozani P, Safarzadeh Kozani P, Rahbarizadeh F. CAR-T cell therapy in T-cell malignancies: is success a low-hanging fruit? Stem Cell Res Ther. 2021;12(1):527.
Sánchez-Martínez D, et al. Fratricide-resistant CD1a-specific CAR T cells for the treatment of cortical T-cell acute lymphoblastic leukemia. Blood. 2019;133(21):2291–304.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
DuVall: speaker for CE Concepts; Wesevich: no conflicts of interests or competing interests; Larson: consultant or advisor to AbbVie, Amgen, Ariad/Takeda, Astellas, Celgene/BMS, Curis, CVS/Caremark, Epizyme, Immunogen, Jazz Pharmaceuticals, Kling Biotherapeutics, MedPace, MorphoSys, Novartis, and Servier and has received clinical research support to his institution from Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven/Gilead, Novartis, and Rafael Pharmaceuticals and royalties from UpToDate.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
DuVall, A.S., Wesevich, A. & Larson, R.A. Developing Targeted Therapies for T Cell Acute Lymphoblastic Leukemia/Lymphoma. Curr Hematol Malig Rep 18, 217–225 (2023). https://doi.org/10.1007/s11899-023-00706-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-023-00706-7