Abstract
Purpose of Review
Recent advances have been made in circulating tumor DNA (ctDNA), the method to minimally invasive detect lymphoma sensitively with tumor-derived DNA in the blood of patients with lymphomas. This article discusses these various methods of ctDNA detection and the clinical context in which they have been applied to for a variety of lymphoma subtypes.
Recent Findings
ctDNA has been applied to a variety of subtypes of lymphoma and has been used in the context of genotyping somatic mutations and classification of disease, monitoring of response during treatment, detecting minimal residual disease even with radiographic remission, and predicting relapse and long-term survival outcomes. There are a variety of techniques used to measure ctDNA including digital polymerase chain reaction and next-generation sequencing techniques including high-throughput variable-diversity-joining rearrangement sequencing, high-throughput sequencing of somatic mutations, and Cancer Personalized Profiling by deep sequencing. While the greatest data has been generated in diffuse large B cell lymphoma, there have been studies utilizing application of ctDNA in follicular lymphoma, mantle cell lymphoma, Hodgkin’s lymphoma, peripheral T cell lymphoma, and primary CNS lymphoma among others.
Summary
ctDNA is an emerging biomarker in lymphoma that can minimally invasively provide further genotypic information, diagnostic clarification, and treatment prognostication by detection of minimal residual disease even without radiographic evidence of disease. Future studies are needed to standardize the use of ctDNA and translate its use clinically for the management of lymphoma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Precision medicine approaches in non-Hodgkin’s lymphoma (NHL) have really revolutionized the field and has enabled the selection of therapies tailored to the molecular profile of each patient. The traditional method of determining tumor genetic profiles has been through tissue biopsies which contains many challenges including procedural risks, sampling error, and spatial tumor heterogeneity. This has led to a lot of interest in liquid biopsies, which involves sampling blood to analyze tumor cells or tumor cell products. The idea of liquid biopsy was developed initially among solid tumors and has more recently been adapted to malignant hematology including NHL.
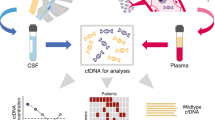
Cell-free DNA (cfDNA) is the circulating double-stranded DNA fragment that is released from tumor tissue into the peripheral blood after normal processes of cell proliferation and cell death. Circulating tumor DNA (ctDNA) is the proportion of tumor-derived cfDNA. A variety of recent studies have investigated the role of ctDNA as a sensitive biomarker for detection of disease, monitoring of treatment response, and detection of early relapse in a variety of lymphoma subtypes. There are a variety of technologies currently available for ctDNA detection including polymerase chain reaction (PCR)–based methods, next-generation sequencing (NGS)–based techniques, and cancer personalized profiling by deep sequencing (CAPP-seq) techniques.
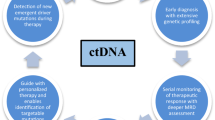
ctDNA has a variety of applications: pre-treatment ctDNA levels can correlate with tumor burden and prognosticate outcomes; ctDNA during treatment can be used to monitor response and resistance; lastly, ctDNA can also be used post-treatment for surveillance, monitoring, and detection of early relapse.
Methodologies of Measuring ctDNA
Both PCR-based and NGS-based ctDNA methods have been developed and used in lymphomas. PCR-based methods include digital PCR (dPCR) which allows for the evaluation of individual clonal genomic variants. Given that only limited and known variants can be screened with this method, NGS-based methods are favored for detecting and monitoring ctDNA in lymphoma [1••]. The three types of NGS-based techniques include Ig sequencing, high-throughput sequencing of somatic mutations such as lymphopanel, and deep sequencing with CAPP-seq [1••].
Digital-PCR
Studies have demonstrated that dPCR is more sensitive than quantification PCR for the measurement of circulating biomarkers [2]. A sensitive and specific probe-based dPCR assay was designed to detect common mutations in lymphoid malignancies: exportin-1 (XPO1) E571K mutation (primary mediastinal B cell lymphoma and Hodgkin’s lymphoma), EZH2 Y641N mutation (follicular lymphoma and diffuse large B cell lymphoma (DLBCL)), MYD88 L265P mutation (lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia, DLBCL, and primary CNS lymphoma), BRAFV600E (hairy cell leukemia), RHOA G7V (angioimmunoblastic T cell lymphoma) [3••]. This has a sensitivity of a 10.−5 detection limit and is relatively easy, quick turnaround, and inexpensive. However, the major disadvantage is that this technique can only screen and detect a limited number of mutations. The limited amount of ctDNA in blood samples of lymphoma patients also further restricts the ability of dtPCR to monitor MRD. The detection limit may also lead to some false positives [4].
High-Throughput Variable-Diversity-Joining Rearrangement Sequencing
Each B cell lymphoma contains a unique clonotypic variable-diversity-joining (VDJ) rearrangement that can be identified on the initial tumor tissue using high-throughput VDJ NGS [3••, 4]. The ClonoSeq assay (Adaptive Biotechnologies, Seattle, WA) can be used to monitor disease by tracking the unique clonotype for each patient [3••]. Clonoseq is the first and only FDA-cleared assay used for MRD monitoring in any lymphoid cancer. By identifying and tracking tumor clones, it can provide a comprehensive landscape of immunoglobulin gene rearrangements [5•]. ClonoSeq has been found to be highly sensitive in detecting a deeper disease response to therapy in a variety of lymphoma subtypes and thus may be able to aid in both therapeutic decision-making and prognostication [6]. There is rising evidence that this NGS assay may be more sensitive than standard imaging in predicting relapse in DLBCL. Limitations of this technique include the absence of VDJ rearrangements in some patients (such as immunoglobulin negative primary mediastinal B cell lymphoma and some de novo DLBCL), lack of reproducibility, and inability to tailor targeted therapy or detect the emergence of resistance clones under therapy [4].
High-Throughput Sequencing of Somatic Mutations
There have been many studies that have demonstrated the recurrence of targetable activating mutations in DLBCL and other B cell lymphomas. An NGS “Lymphopanel” has been developed to identify somatic mutations in 34 genes and was able to detect identifiable mutations in majority of patients with de novo DLBCL, including ones that may help tailor the use of targeted therapies (i.e., EZH2, MYD88, XPO1) while also serving as a MRD biomarker [7•]. Through detecting these mutations in patients’ plasma ctDNA, the lymphopanel can be used along with PET imaging to more precisely evaluate treatment response as demonstrated in a prospective series of 30 DLBCL patients treated with frontline RCHOP in which three of the four patients who had residual mutations detected via ctDNA mid-treatment had partial response seen on PET [8•]. Genotyping liquid biopsies can serve as a marker of MRD and further classify disease to personalize treatment. One study found that identification of mutations via liquid biopsies had 80% concordance with the Hans algorithm in determining cell of origin (COO) [9]. Lymphoma genotyping on ctDNA can address the need for a comprehensive and minimally invasive source of tumor genotyping. Given ctDNA provides comprehensive information regarding the entire lymphoma heterogeneity, it may overcome the sampling bias caused by tissue biopsies in reconstructing the clonal architecture of the tumor, identify resistant clones that may be difficult to access via biopsy, and allow for continuous monitoring of treatment emergent resistant mutations in real time [10]. However, further studies assessing the validity of this approach need to be performed before implementing in routine clinical practice and decision-making [11•].
Cancer Personalized Profiling by Deep Sequencing
CAPP-seq is a highly sensitive, high-performing, and high-throughput sequencing technique for ctDNA measurement that allows for the rapid quantification of rare circulating somatic mutations in tumors. Establishing the COO via immunohistochemistry or via ctDNA using CAPP-seq method was found to have a high 88% concordance [9]. CtDNA via CAPP-seq has also demonstrated the ability to identify clonal evolution that may distinguish indolent follicular lymphoma from subsequent transformed DLBCL [9]. CAPP-seq also correlates with DLBCL disease volume on PET scan during treatment, thus highlighting its potential application as a marker of treatment response and relapse. This technique also allows for identification of structural variations including translocations and copy number variations [12]. CAPP-seq can measure disease burden molecularly, determine lymphoma genotype, determine treatment resistance by expanding somatic mutations, detect MRD, and predict pre-clinical relapse. However, further studies need to be done to test its reproducibility prior to incorporating into real-time clinical practice and use in clinical trials for detection of MRD [4].
Clinical Applications of ctDNA Measurement
Diffuse Large B Cell Lymphoma
Multiple prior studies have explored the role of ctDNA in DLBCL and demonstrated its potential to serve as a prognostic biomarker. The studies have spanned a variety of methods of ctdNA detection focused on various lymphoma-specific targets used at a variety of time points through the course of treatment.
ctDNA as a Baseline Prognostic Marker in DLBCL
The baseline tumor burden in DLBCL can help risk-stratify patients. Baseline ctDNA levels have been demonstrated to correlate with overall tumor burden in DLBCL, thus allowing ctDNA to serve as a prognostic marker at the time of diagnosis. A study of 126 patients demonstrated that baseline ctDNA level using NGS VDJ rearrangement sequencing was associated with baseline international prognostic index (IPI) scores, lactate dehydrogenase levels, and radiographic staging of DLBCL patients [13•]. A separate study utilizing similar ctDNA assay demonstrated that ctDNA correlated with total metabolic tumor volume (TMTV) on baseline FDG-PET scan [14•]. A large study evaluating pre-treatment ctDNA levels in 267 patients with DLBCL using CAPP-seq found that pre-treatment ctDNA levels were significantly associated with stage, IPI, and TMTVs. The study also found a direct correlation between shorter diagnosis-to-treatment interval (DTI) and higher pretreatment ctDNA levels. Furthermore, ctDNA level was prognostic of event-free survival (EFS) independent of DTI and IPI in multivariable Cox regression [15•]. Another large study of 217 patients also evaluating the prognostic role of ctDNA using CAPP-seq found that baseline ctDNA was associated with survival outcomes (EFS and overall survival (OS)) in patients receiving frontline or salvage therapy [16•]. On multivariable analysis, baseline ctDNA concentration was found to be a better predictive marker of EFS compared to other prognostic markers such as IPI score, tumor burden on baseline scan, or COO [16•]. Correlatives from the polatuzumab-bendamustine rituximab study demonstrated that after adjusting for a variety of factors including number of prior therapies, IPI score, and LDH, high baseline ctDNA was independently prognostic for shorter progression-free survival and OS [17]. A prospective study that evaluated ctDNA via VDJ high-throughput sequencing found that pre-axi-cel chimeric antigen receptor therapy (CAR T) treatment ctDNA concentration was associated with PFS after axi-cel and was also associated with the development of toxicities of special interest, namely cytokine-release syndrome and immune effector cell–associated neurotoxicity syndrome [18•].
ctDNA can also identify and integrate genetic information from DLBCL patients in a non-invasive manner and identify somatic mutations that establish distinct molecular signatures in DLBCL. Studies have shown high concordance (nearly 90%) of ctDNA with tumor tissue IHC in classifying DLBCL according to COO subtype at the time of diagnosis [9]. ctDNA has also been used to identify key somatic mutations and lymphoma-relevant genetic aberrations at the time of diagnosis to help tailor targeted treatments [19]. Repetitive genotyping using ctDNA can also help identify resistance mutations [19]. In transformed DLBCL, ctDNA can be used to identify distinct models of clonal evolution thus suggesting its potential use for noninvasive prediction of histologic transformation.
ctDNA as a Marker of Treatment Response During Therapy in DLBCL
Quantitative ctDNA can serve as a dynamic measure of treatment efficacy. A landmark prospective study evaluated 30 newly diagnosed DLBCL patients with serial ctDNA measurement while being treated with frontline R-CHOP [20•]. This study demonstrated that responding patients had rapid clearance of ctDNA suggesting that ctDNA can serve as a tool that allows for real-time evaluation of response and detection of new treatment-resistant clones during therapy [20•, 21••]. Another study found that DLBCL patients who had undetectable ctDNA, measured by NGS immunoglobulin receptor gene sequencing, after two cycles of RCHOP therapy had superior PFS compared with patients who remained ctDNA positive [13•]. A larger study using CAPP-seq for ctDNA demonstrated that a 2-log reduction in ctDNA after one cycle of RCHOP therapy as well as 2.5 log reductions after 2 cycles of RCHOP for DLBCL were more predictive of EFS and OS compared with baseline ctDNA levels, IPI score, or interim PET results [16•]. Thus, dynamic changes in ctDNA can not only measure response in a more sensitive way than radiographic imaging but can also serve as a prognostic marker for survival outcomes. ctDNA correlatives from polatuzumab and bendamustine + rituximab phase II study in 23 r/r DLBCL patients with baseline and end of treatment (EOT) samples demonstrated that patients who achieved complete response had significantly greater decrease in ctDNA levels [17].
ctDNA After Therapy as a Marker of Early Disease Relapse and Clonal Evolution in DLBCL
Serial monitoring of ctDNA after completion of therapy can also serve as a minimally invasive surveillance strategy to identify asymptomatic relapse even before radiographic detection and to characterize treatment resistance and clonal evolution. One study evaluated 107 DLBCL patients prospectively after completion of induction therapy with radiographic scans surveillance paired with blood samples [13•]. Seventeen of these patients had relapsed after initial remission and 88% of these patients had detectable ctDNA at the time of or prior to clinical or radiographic relapse with a median lead time of ctDNA detection prior to clinical relapse of 3.5 months. In the remaining 90 patients who did not relapse after remission, ctDNA was persistently negative at almost all time points [13•]. Another study measuring ctDNA via immunoglobulin high-throughput sequencing found that ctDNA correlated with radiographic disease burden and often preceded radiographic detection of relapse in patients achieving remission [14•]. A study measuring ctDNA via CAPP-Seq demonstrated that ctDNA was detectable in all patients at time of relapse and in 73% of patients prior to radiographic relapse with a median lead time of over 2 months [9]. A prospective study that evaluated 401 patients with DLBCL who achieved a radiographic complete response and had, as part of post-treatment surveillance strategy, measurement of ctDNA via an NGS-based assay every 3 months for 2 years found that 91% of patients who relapsed had detectable ctDNA. In 56% of patients, the ctDNA was detectable before at the time of relapse and in 26% of patients ctDNA was detectable with a lead time of over 3 months prior to clinical detection [22]. Another prospective study that evaluated ctDNA via VDJ high-throughput sequencing found that at 1 month post axi-cel infusion, patients with detectable ctDNA had a statistically significant lower median PFS (3 months vs not reached) and OS (19 months vs not reached) when compared to those with undetectable ctDNA. In 94% of patients, ctDNA was detected at or before radiographic relapse. All durably responding patients had undetectable ctDNA at or before 3 months after axi-cel infusion. This suggests the role of ctDNA as a prognostic marker of survival outcomes and early predictor of relapse post CAR T [18•].
Evaluation of ctDNA after completion of therapy as part of surveillance can also be used to understand clonal evolution and characterize the development of new clones at the time of resistance. A study using CAPP-seq for ctDNA detected new mutations at the time of relapse that were not present at the time of original diagnosis [20•]. Thus, these studies demonstrate ctDNA can be used as a surveillance tool to identify patients not in molecular remission despite radiographic remission and thus detect disease prior to clinical or radiographic relapse and to also track genomic clonal evolution and identify new clones of resistance that can be used to tailor subsequent therapies [1••, 23]. This could be used in the future real time to guide management decisions. For example, detection of molecular disease with ctDNA after salvage chemotherapy in patients with r/r DLBCL who have achieved radiographic remission could be used to determine if patients should get consolidative autologous stem cell transplant or CAR T after salvage chemotherapy. Another example of the clinical application of ctDNA post treatment includes serial monitoring of ctDNA after completion of CAR T therapy to identify patients high-risk for relapse so treatment improving T cell function and minimizing T cell exhaustion such as checkpoint blockade or immunomodulatory agents can be initiated in these patients at the time of detection of molecular disease.
Follicular Lymphoma
ctDNA has also been shown to have an important role in prognostication as well as prediction of relapse and transformation in follicular lymphoma (FL). A prospective study evaluating ctDNA in FL patients demonstrated that plasma ctDNA correlated to TMTV and serial monitoring of ctDNA in patients without therapy demonstrated various patterns of fluctuation and undetectable ctDNA correlated with clinical regression [24•]. Plasma samples at diagnosis from 34 patients in the PRIMA trial was studied and in multivariate analysis, a high baseline level of ctDNA measured by VDJ immunoglobulin rearrangement sequencing was the only independent factor associated with shorter PFS in patients [25]. Other studies have also demonstrated the prognostic value of baseline ctDNA in FL, with higher ctDNA levels corresponding to higher tumor burden and shorter PFS [26]. ctDNA analyzed from 415 patients enrolled in the FOLLO5 trial was also evaluated both pre-treatment with conventional chemoimmunotherapy and at 12 and 24 months of follow-up after completion of treatment. Patients without a molecular marker or with a low molecular tumor burden showed higher CR rate and longer PFS rate. MRD negativity at 12 and 24 months post treatment resulted in an improved PFS both in patients who achieved complete remission and in patients who achieved partial remission thus suggesting that ctDNA may be a more sensitive prognostic marker for survival compared to radiographic response in FL [27•].
Clinical progression in FL patients may also lead to DLBCL transformation in around 20% of patients [28]. A study evaluating tumor and plasma samples of transformed FL patients showed that new mutations were detected at the time of transformation (not present a the time of diagnosis) and sometimes detected weeks to months prior to clinical evidence of transformation [9]. Thus, ctDNA may serve as a minimally invasive method of predicting transformation in FL patients.
Mantle Cell Lymphoma
MRD has been used for the past 20 years in the context of mantle cell lymphoma (MCL), initially based on evidence from PCR-based methods and more recently with NGS-based methods [5•]. A pooled analysis of transplant-eligible MCL patients treated with frontline chemoimmunotherapy found high rates of MRD negativity that could persist for years after ASCT [29]. MRD has been demonstrated to be correlated with PFS in another study evaluating MCL patients treated with bendamustine-rituximab induction followed by rituximab ± lenalidomide consolidation [30]. ctDNA dynamics was evaluated in a phase 2 study of 53 treatment-naïve MCL patients who received induction therapy with bortezomib and DA-R-EPOCH for 6 cycles followed by randomization to observation or bortezomib maintenance in responding patients [31]. Patients without detectable ctDNA after 2 cycles of induction had longer PFS and OS compared to those with detectable ctDNA. Monitoring ctDNA after induction demonstrated that molecular relapse can precede clinical relapse [31]. This study demonstrates that interim ctDNA negativity after induction therapy in MCL can correlate with improved response and thus further supports response-adapted strategies [31]. A phase II study of sequential chemoradioimmunotherapy followed by autologous stem cell rescue in MCL incorporated NGS-based ctDNA monitoring post treatment with samples collected from 16 patients. Five out of the 7 patients whose disease remained in remission had undetectable MRD. Of the 9 patients whose disease relapsed, 6 patients had MRD positivity at least 3 months before relapse and 1 patient had MRD positivity at the time of relapse. All patients who had at least 2 positive MRD tests clinically relapsed. This study demonstrated that NGS-based ctDNA could identify early molecular relapse [32]. The ongoing ECOG ACRIN 4151 trial evaluates the role of ASCT in MCL patients who achieve MRD negativity, as measured by ClonoSeq, in addition to radiographic complete remission after induction therapy. MRD data continues to evolve in MCL and increasing data is allowing ctDNA to be incorporated into landmark MCL clinical trials [5•].
Hodgkin’s Lymphoma
Given the scarcity of Hodgkin’s Reed-Sternberg cells, the tumor volume in Hodgkin’s lymphoma (cHL) is significantly smaller than that of other aggressive lymphomas. However, despite that, the correlation between ctDNA levels and tumor volume on radiographic imaging is very similar in cHL to that in DLBCL [1••]. ctDNA is a rich and easily accessible source of tumor DNA for cHL mutation profiling, hence serving as a source for evaluating molecular bases of response and resistance to immunomodulatory therapy in clinical trials [1••]. In a retrospective study, the XPO1 E571K mutation was monitored in ctDNA via dPCR and NGS techniques from patients with cHL harboring the reporter and found to be present in 24% of patients. The presence of this mutation at the EOT correlated with shorter PFS [33]. Detection of XPO1 E571K mutation in plasma ctDNA may serve as a novel biomarker in HL and should be further evaluated in a prospective study. However, XPO1 E571K is the only recurrent single mutation but is limited to 10–20% of cases [1••] and most Hodgkin’s lacks a universal biomarker for monitoring given the absence of highly prevalent mutations. Thus, the quantification of ctDNA based on the detection of tumor-specific mutations in cHL can be challenging [1••]. A report recently assessing levels of plasma ctDNA using real-time PCR in pediatric HL cases found that ctDNA level in patients with HL correlated with B-symptoms and increase in ctDNA levels after the first cycle of chemotherapy was liked to predict worse prognosis [34]. Another ctDNA NGS study identified genomic imbalances in HRS cells at diagnosis with rapid normalization upon therapy initiation, thus suggesting a possible role of ctDNA in early response monitoring [35•]. A study using deep NGS-based ctDNA combined with PET imaging provided the proof of concept that ctDNA can track residual disease and may serve as a novel precision medicine biomarker in cHL and a method of early identification of chemorefractory patients with cHL [36]. Genotyping of longitudinal ctDNA samples collected before salvage treatment, at the time of relapse, and during salvage therapy with the use of novel agents and following transplantation has identified clonal evolution patterns in cHL [36]. Further studies are underway monitoring ctDNA in cHL pivotal trials to better understand the predictive and prognostic utility of MRD measurement.
Peripheral T Cell Lymphoma
There has been some recent emerging data regarding the use of ctDNA in T cell lymphomas. NGS-based high-throughput T cell receptor (TCR) sequencing of TCRβ and TCRγ genes provides a comprehensive analysis of distinct T cell clones which can be tracked in peripheral blood. Significant clonotypic heterogeneity of the TCR which may cause treatment resistance is present in peripheral T cell lymphoma (PTCL) patients. A prospective study assessing of tumor and blood samples of T cell lymphoma patients found that baseline ctDNA correlated with LDH and high level of ctDNA predicted treatment failure and worse PFS in a statistically significant manner. This study also demonstrated that change in ctDNA correlated with clinical outcomes in a more sensitive manner than PET/CT and ctDNA was predictive of relapse in T cell lymphoma patients [37]. Another study evaluating NGS-based ctDNA in 45 PTCL patients undergoing frontline treatment also demonstrated that patients with detectable ctDNA after therapy had worse survival [38•]. MRD negativity, however, defined as clearance of ctDNA after 2 cycles or at EOT did not significantly predict clinical outcomes, though these results are limited by small study sample size [38•]. For patients with MRD positivity, median lead time from clonotype detection to clinical progression was 12.5 months [38•]. Further studies are needed to optimize use of ctDNA in PTCL to guide therapeutic decisions.
Primary Central Nervous System Lymphoma
Primary CNS lymphoma (PCNSL) is a relatively rare type of disease that predominantly affects elderly patients and has a poor prognosis. This is a diagnostically challenging disease given anatomically hard-to-biopsy tumor with histological material that is often present in insufficient quantities. Hence, minimally invasive methods of diagnosing PCNSL through ctDNA and radiographic MRI imaging in lieu of tissue biopsy would be clinically extremely useful [4]. A retrospective study of 25 PCNSL patients with 32% displaying measurable somatic variants in ctDNA demonstrated the utility of ctDNA NGS in detecting gene alterations in PCNSL patients [39]. Several retrospective studies have demonstrated that in patients with MYD88 L265P PCNSL, ctDNA using droplet dPCR successfully identified this somatic variant in cerebrospinal fluid (CSF) samples in 71–77% cases, thus suggesting that detection of MYD88 L265P mutation in CSF or plasma could be an additional important diagnostic tool [40, 41]. Studies have also shown that CSF ctDNA can better detect CNS lesions than plasma ctDNA and flow cytometry [42]. A retrospective study of 19 patients found ctDNA in the CSF of all patients with restricted CNS lymphoma but only was detected in the plasma of 2/6 patients. Furthermore, CSF ctDNA was also found to be better at detecting residual disease than flow cytometry in patients after receiving treatment, thus demonstrating its utility in predicting relapse in patients with CNS lymphoma [42]. Another recent study applied CAPP-Seq methods to explore the utility of ctDNA in noninvasively distinguishing CNS lymphoma from other CNS tumors based on their mutational landscapes in plasma and CSF. This study found a high specificity and positive predictive value of ctDNA for non-invasive diagnosis of PCNSL. This study also observed a significant correlation of ctDNA concentrations with total radiographic tumor volumes measured by MRI. Baseline pre-treatment ctDNA levels and ctDNA positivity during curative-intent induction therapy also predicted survival [43•].
Conclusion
ctDNA has in the recent years transformed the approach to lymphoma diagnosis, monitoring, and early detection of clonal evolution. Advances in techniques including NGS, dPCR, and CAPP-seq have enabled the examination of multiple mutations in ctDNA and correlation of these mutations with survival outcomes, treatment response, and prediction of resistance and clonal selection. The use of ctDNA has been applied to multiple different lymphoma types including DLBCL, FL, MCL, HL, PTCL, and PCSNL among others. A variety of independent studies in these subtypes of lymphoma have demonstrated the utility of ctDNA measurement throughout the disease course of patients from baseline, to mid-treatment, to post-treatment in serving as a predictive and prognostic biomarker. ctDNA is increasingly being incorporated into secondary endpoints and correlative studies for ongoing clinical trials.
While there has been significant progress in the area of ctDNA and its application to lymphoma in recent years, there are still logistic hurdles to overcome prior translation real time into the clinical setting. While there is largest data in this space with DLBCL, there is still a dearth of information in development and application of ctDNA techniques in more rare subtypes of lymphoma such as HL and PTCL. Prospective translation into the clinical setting also requires real-time sample processing and reporting with a rapid turnaround in order to enable clinical decisions based on the results of ctDNA. There are a variety of methods of measuring ctDNA and MRD and further efforts are needed to standardize ctDNA quantification with other biomarkers including PET/CT. We also need to better understand how ctDNA as a prognostic marker integrates into other existing risk-stratification tools such as IPI, COO, and interim radiographic PET/CT scans. Furthermore, although ctDNA levels pretreatment and post treatment as well as dynamic changes during treatment have demonstrated to be prognostic and predictive of response and relapse, there remains work to be done in better understanding how to change clinical practice based on ctDNA and if these changes will improve patients’ long-term survival outcomes. Trials in the future need to explore novel therapies for high-risk patients with residual molecular disease or de-escalation of therapies based on achieving a favorable molecular response, and demonstrate improved survival outcomes based on this ctDNA adapted approach similar to studies previously conducted using a PET/CT-based approach such as the RATHL study [44] in cHL or S1001 study [45] in DLBCL.
Future studies in the future are needed to validate ctDNA genotyping for detection of mutations that can tailor targeted therapies, establish feasibility of using ctDNA real time to understand response and detect treatment resistance in complement to radiographic imaging and other biomarkers, implement ctDNA as earlier surrogate end points in clinical trials, and further investigate how to change management based on ctDNA.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Roschewski M, Rossi D, Kurtz DM, et al. Circulating tumor DNA in lymphoma: principles and future directions. Blood Cancer Discov. 2022;3:5–15. These references are key papers that review the general principles of ctDNA, methods of ctDNA measurement, and key clinical applications of ctDNA with respect to prognosis and early detection in lymphoma.
Hindson CM, Chevillet JR, Briggs HA, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10:1003–5.
Huet S, Salles G. Potential of circulating tumor DNA for the management of patients with lymphoma. JCO Oncol Pract. 2020;16:561–568. These references are key papers that review the general principles of ctDNA, methods of ctDNA measurement, and key clinical applications of ctDNA with respect to prognosis and early detection in lymphoma.
Camus V, Jardin F. Cell-free DNA and the monitoring of lymphoma treatment. Pharmacogenomics. 2019;20:1271–82.
Jung D, Jain P, Yao Y, et al. Advances in the assessment of minimal residual disease in mantle cell lymphoma. J Hematol Oncol. 2020;13:127. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Hussaini MO, Srivastava J, Lee LW, et al. Moffitt cancer center 2-year single-institution experience with next-generation sequencing minimal residual disease detection: clinical utility, application, and correlation with outcomes in plasma cell and lymphoid malignancies. Blood. 2019;134(Supplement_1):4654-.
Dubois S, Viailly PJ, Mareschal S, et al. Next-generation sequencing in diffuse large B-cell lymphoma highlights molecular divergence and therapeutic opportunities: a LYSA study. Clin Cancer Res. 2016;22:2919–28. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Bohers E, Viailly PJ, Becker S, et al. Non-invasive monitoring of diffuse large B-cell lymphoma by cell-free DNA high-throughput targeted sequencing: analysis of a prospective cohort. Blood Cancer J. 2018;8:74. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Scherer F, Kurtz DM, Newman AM, et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med. 2016;8:364ra155.
Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–86.
Rossi D, Spina V, Bruscaggin A, et al. Liquid biopsy in lymphoma. Haematologica. 2019;104:648-652. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Bratman SV, Newman AM, Alizadeh AA, et al. Potential clinical utility of ultrasensitive circulating tumor DNA detection with CAPP-Seq. Expert Rev Mol Diagn. 2015;15:715–9.
Roschewski M, Dunleavy K, Pittaluga S, et al. Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: a correlative biomarker study. Lancet Oncol. 2015;16:541–9. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Kurtz DM, Green MR, Bratman SV, et al. Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood. 2015;125:3679–87. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Alig S, Macaulay CW, Kurtz DM, et al: Short diagnosis-to-treatment interval is associated with higher circulating tumor DNA levels in diffuse large B-cell lymphoma. J Clin Oncol. 2021;39:2605–2616. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Kurtz DM, Scherer F, Jin MC, et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J Clin Oncol. 2018;36:2845–2853. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Herrera AF, Tracy S, Croft B, et al. Risk profiling of patients with relapsed/refractory diffuse large B-cell lymphoma by measuring circulating tumor DNA. Blood Adv. 2022;6(6):1651–60.
Frank MJ, Hossain NM, Bukhari A, et al. Monitoring of circulating tumor DNA improves early relapse detection after axicabtagene ciloleucel infusion in large B-cell lymphoma: results of a prospective multi-institutional trial. J Clin Oncol. 2021;39:3034–3043. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Cirillo M, Craig AFM, Borchmann S, et al. Liquid biopsy in lymphoma: molecular methods and clinical applications. Cancer Treat Rev. 2020;91:102106.
Rossi D, Diop F, Spaccarotella E, et al. Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood. 2017;129:1947–1957. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Camus V, Bohers E, Dubois S, et al. Circulating tumor DNA: an important tool in precision medicine for lymphoma. Expert Rev Precis Med Drug Dev. 2018;3:11–21. These references are key papers that review the general principles of ctDNA, methods of ctDNA measurement, and key clinical applications of ctDNA with respect to prognosis and early detection in lymphoma.
Kumar A, Westin J, Schuster SJ, et al. Interim analysis from a prospective multicenter study of next-generation sequencing minimal residual disease assessment and ct monitoring for surveillance after frontline treatment in diffuse large b-cell lymphoma. Blood. 2020;136(Supplement 1):46–7.
Kurtz DM, Soo J, Co Ting Keh L, et al. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat Biotechnol. 2021;39:1537–1547.
Distler A, Lakhotia R, Phelan JD, et al. A prospective study of clonal evolution in follicular lymphoma: circulating tumor DNA correlates with overall tumor burden and fluctuates over time without therapy. Blood. 2021;138:1328–1328. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Sarkozy C, Huet S, Carlton VE, et al. The prognostic value of clonal heterogeneity and quantitative assessment of plasma circulating clonal IG-VDJ sequences at diagnosis in patients with follicular lymphoma. Oncotarget. 2017;8:8765–74.
Delfau-Larue MH, van der Gucht A, Dupuis J, et al. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: distinct prognostic value in follicular lymphoma. Blood Adv. 2018;2:807–16.
Galimberti S, Luminari S, Ciabatti E, et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: the FIL FOLL05 trial. Clin Cancer Res. 2014;20:6398–405. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Sarkozy C, Trneny M, Xerri L, et al. Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial. J Clin Oncol. 2016;34:2575–82.
Merryman RW, Edwin N, Redd R, et al. Rituximab/bendamustine and rituximab/cytarabine induction therapy for transplant-eligible mantle cell lymphoma. Blood Adv. 2020;4:858–67.
Smith MR, Jegede O, Martin P, et al. ECOG-ACRIN E1411 randomized phase 2 trial of bendamustine-rituximab (BR)-based induction followed by rituximab (R) ± lenalidomide (L) consolidation for mantle cell lymphoma: effect of adding bortezomib to front-line BR induction on PFS. J Clin Oncol. 2021;39:7503–7503.
Lakhotia R, Melani C, Dunleavy K, Pittaluga S, Saba N, Lindenberg L, Mena E, Bergvall E, Lucas AN, Jacob A, Yusko E, Steinberg SM, Jaffe ES, Wiestner A, Wilson WH, Roschewski M. Circulating tumor DNA predicts therapeutic outcome in mantle cell lymphoma. Blood Adv. 2022;6(8):2667–2680. https://doi.org/10.1182/bloodadvances.2021006397
Kumar A, Bantilan KS, Jacob AP, et al. Noninvasive monitoring of mantle cell lymphoma by immunoglobulin gene next-generation sequencing in a phase 2 study of sequential chemoradioimmunotherapy followed by autologous stem-cell rescue. Clin Lymphoma Myeloma Leuk. 2021;21:230–237 e12.
Camus V, Stamatoullas A, Mareschal S, et al. Detection and prognostic value of recurrent exportin 1 mutations in tumor and cell-free circulating DNA of patients with classical Hodgkin lymphoma. Haematologica. 2016;101:1094–101.
Primerano S, Burnelli R, Carraro E, et al. Kinetics of circulating plasma cell-free DNA in paediatric classical Hodgkin lymphoma. J Cancer. 2016;7:364–6.
Vandenberghe P, Wlodarska I, Tousseyn T, et al. Non-invasive detection of genomic imbalances in Hodgkin/Reed-Sternberg cells in early and advanced stage Hodgkin's lymphoma by sequencing of circulating cell-free DNA: a technical proof-of-principle study. Lancet Haematol. 2015;2:e55–65. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Spina V, Bruscaggin A, Cuccaro A, et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood. 2018;131:2413–25.
Zhang W, Wang W, Han X, et al. Circulating tumor DNA by high-throughput sequencing of T cell receptor monitored treatment response and predicted treatment failure in T cell lymphomas. Int J Lab Hematol. 2021;43:1041–9.
Miljkovic MD, Melani C, Pittaluga S, et al. Next-generation sequencing-based monitoring of circulating tumor DNA reveals clonotypic heterogeneity in untreated PTCL. Blood Adv. 2021;5:4198–4210. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Fontanilles M, Marguet F, Bohers E, et al. Non-invasive detection of somatic mutations using next-generation sequencing in primary central nervous system lymphoma. Oncotarget. 2017;8:48157–68.
Rimelen V, Ahle G, Pencreach E, et al. Tumor cell-free DNA detection in CSF for primary CNS lymphoma diagnosis. Acta Neuropathol Commun. 2019;7:43.
Watanabe, Jun et al. High detection rate of myd88 mutations in cerebrospinal fluid from patients with cns lymphomas. JCO Precs Oncol. 2019;3:1–13. https://doi.org/10.1200/PO.18.00308
Bobillo S, Crespo M, Escudero L, et al. Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas. Haematologica. 2021;106:513–21.
Mutter JA, et al. Profiling of circulating tumor DNA for noninvasive disease detection, risk stratification, and MRD monitoring in patients with CNS lymphoma. Blood. 2021;138(Supplement 1):6–6. These references are key papers pertaining to the application of ctDNA to specific lymphoma subtypes namely DLBCL, FL, MCL, HL and PTCL.
Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by Interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–29.
Persky DO, Li H, Stephens DM, et al. Positron emission tomography-directed therapy for patients with limited-stage diffuse large B-cell lymphoma: results of Intergroup National Clinical Trials Network Study S1001. J Clin Oncol. 2020;38:3003–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
S.K.: none; J.Z.: Secura Bio, DaichiSankyo, Abbvie: research funding; Kiyoaw Kirin, Secura Bio, Seattle Genetics: honoraria; Secura Bio, Ono, Legend, Kiyowa Kirin, Myeloid Therapeutics Verastem Daichi Sankyo: consultancy.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on T-Cell and Other Lymphoproliferative Malignancies
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kambhampati, S., Zain, J. Circulating Tumor DNA in Lymphoma. Curr Hematol Malig Rep 17, 298–305 (2022). https://doi.org/10.1007/s11899-022-00677-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-022-00677-1