Abstract
Purpose of Review
The incorporation of pegaspargase in chemotherapy regimens has significantly improved the prognosis of ALL in adults. However, pegaspargase use poses many challenges due to its unique toxicity profile. Here, we review pegaspargase’s most clinically significant toxicities, and provide guidance for their prevention and management in order to avoid unnecessary drug discontinuation and achieve maximum clinical benefit.
Recent Findings
Clinically significant toxicities of pegaspargase include thrombosis, hypersensitivity and inactivation, hepatotoxicity, pancreatitis, and hypertriglyceridemia. The majority of these toxicities are temporary, nonfatal, and can be managed supportively without permanent pegaspargase discontinuation. Special attention should be paid to inactivation, which can lead to treatment failure, as well as pancreatitis, which necessitates complete cessation of asparaginase therapy. The question of how to best proceed in patients who cannot tolerate pegaspargase remains unanswered, and is an important area of future investigation.
Summary
Pegaspargase is an essential component of the pediatric-inspired regimens that have improved survival in adult ALL. Although pegaspargase’s toxicity profile is unique, it is also highly manageable and should not be a barrier to achieving maximum clinical benefit using this drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukemia (ALL) in adults portends a worse prognosis when compared to the cure rates seen in the pediatric population [1]. Historically, adult patients have been able to achieve relatively high rates of complete remission (CR) (80–90%), but a high incidence of relapse has led to lower cure rates and poor long-term overall survival (OS) (5-year OS of 30–45% in adults, including approximately 20% in adults > 60 years old) [2,3,4,5,6]. These inferior outcomes in adults have been partially attributed to an increased frequency of high-risk ALL genetics contributing to relapse and chemotherapy resistance. Furthermore, adults tend to be treated with inferior chemotherapy regimens compared to their pediatric counterparts, due in part to concerns over increased treatment-related toxicities [3, 7, 8].
In recent years, the prognosis of ALL in adolescents and young adults has improved significantly with the incorporation of more intensive, pediatric-inspired regimens, which have been shown to be safe and feasible in this population [9–10, 11•]. An important component of these regimens is the use of asparaginase, a bacterial enzyme that hydrolyzes asparagine to aspartic acid and ammonia. Unlike normal cells, ALL cells are unable to synthesize adequate asparagine on their own. Deprivation of this amino acid leads to inhibited DNA, RNA, and protein synthesis, and ultimately results in apoptosis of the leukemic cells [12, 13]. In ALL, asparaginase is now most commonly administered as pegaspargase, in which the enzyme is covalently linked to polyethylene glycol (PEG) to prolong half-life and decrease immunogenicity [14].
Despite the improved outcomes with the integration of asparaginase into adult ALL regimens, its use remains challenging, as it can be associated with side effects requiring experienced management. The toxicity profile of asparaginase, most notably a risk of hypersensitivity and inactivation, thrombosis, hepatotoxicity, and pancreatitis, is unique when compared to other chemotherapies. Additionally, its use is primarily confined to ALL, a relatively rare condition in the adult population, leading to less experience and familiarity with the drug and its toxicities. Data from pediatric studies demonstrate that asparaginase’s benefit is dependent on dose intensity and that discontinuation due to toxicity could compromise survival rates, further emphasizing the importance of appropriate and effective management of treatment-related side effects [15, 16].
With the increased use of pegaspargase in adult ALL, it is imperative to be familiar with this treatment’s side effect profile to avoid unnecessary dose reductions or treatment discontinuations, and allow for maximum clinical benefit. In this article, we will review pegaspargase’s most clinically significant toxicities, and provide guidance for their prevention and management.
Asparaginase Formulation
Various preparations of the asparaginase enzyme have been utilized since its anti-tumor properties were originally noted in the 1960s. The Escherichia coli–derived asparaginase formulation has been used in the treatment of ALL for over 50 years [17]. As a bacterially derived enzyme, this preparation can be highly immunogenic and lead to antibody formation and hypersensitivity reactions [18]. E. coli asparaginase also has a short half-life and requires frequent administration (often at least three times per week), as does the alternative Erwinia chrysanthemi–derived asparaginase preparation [19, 20].
In an effort to reduce immunogenicity and prolong half-life, E. coli–derived asparaginase has been covalently linked to PEG. With its longer half-life, decreased immunogenicity, and comparable efficacy, pegylated asparaginase is now the preferred formulation over native E. coli asparaginase. Currently, there are two FDA-approved pegylated formulations of asparaginase. Pegaspargase was approved in 1994 for those with hypersensitivity to the native form of the enzyme, and received an additional approval in 2006 for children and adults with newly diagnosed ALL [21]. More recently, calaspargase pegol was approved in 2018 for ALL patients age 1 month to 21 years [22]. Pegaspargase remains the most widely used of these two pegylated formulations, and is the focus of this review.
Asparaginase-Associated Thrombotic Events
Increased risk of thrombosis is a consequence of asparagine depletion. The driving mechanism is related to the reduction of asparagine-dependent hemostatic protein synthesis, resulting in lower levels of antithrombin (ATIII), protein C, protein S, plasminogen, fibrinogen, and various coagulation proteins, thereby promoting coagulation [23].
The majority of thrombotic events (TEs) are venous rather than arterial, and can manifest across the lower or upper extremities, the pulmonary system, the portal system, or the atria [23, 24]. Asparaginase has also been shown to increase the risk of cavernous sinus thrombosis, which has a particularly high morbidity. Fortunately, it is a relatively rare complication occurring in 1–3% of adult patients; however, it carries a fatality rate of 5% [23, 24].
TEs occur most often during induction, but may also occur later in the treatment course [25, 26]. Multiple factors drive the predisposition for TEs during induction: corticosteroid administration often coincides with asparaginase treatments and may contribute to TEs via increasing the synthesis of some procoagulants and glucocorticoid-induced vascular changes [10, 27, 28]; other possible contributory elements include higher disease activity and acute illness during induction [23].
Age is also a significant risk factor for thrombosis [26]. The prevalence of all TEs in pediatric ALL patients has been estimated to be as high as 36.7%, although only 5% of these were classified as symptomatic TEs [29]. Moreover, a large meta-analysis of pediatric patients estimated the rate of symptomatic TEs at approximately 5.2% [25]. The incidence of adult symptomatic TEs varies between 5 and 41% in patients treated with asparaginase, with increased risk of TEs with older age, immobilization, and the presence of a central venous catheter [26, 30,31,32,33,34].
Treating asparaginase-related TEs requires therapeutic anticoagulation and generally parallels treating clots attributable to other etiologies (Fig. 1). While there are several available options, low molecular weight heparin (LMWH) is most commonly used [35•]. Notable exceptions are patients with renal failure or at an increased risk of bleeding, where unfractionated heparin (UFH) would be preferred. Upon diagnosis of a TE, pegaspargase treatment should continue along with concurrent anticoagulation for at least 3 months [36]. However, resuming asparaginase following central nervous system or life-threatening TEs remains controversial; more commonly, pegaspargase therapy is discontinued following such events [35•, 37]. As previously described, pegaspargase reduces native anticoagulants, including ATIII, which contains the non-essential amino acid asparagine. Since heparin is dependent on ATIII for its therapeutic effect, practices have varied over monitoring and repleting ATIII in patients treated with therapeutic heparin, with no strong evidence supporting its use or lack thereof [36]. The approach to patients with thrombocytopenia (particularly with a platelet count < 30,000/mcL) depends primarily on the bleeding risk. However, we make an effort to treat with full-dose anticoagulation with platelet support to raise the platelet count to at least > 30,000/mcL [38]. Furthermore, in cases where the platelet count remains < 30,000/mcL with 30 days elapsed since the onset of the acute TE, we temporarily hold anticoagulation and re-evaluate once the platelet count recovers to at least > 30,000/mcL. Sometimes, in an effort to balance the risks and benefits of anticoagulation, we decrease the dose of anticoagulation by half. Most recently, several case reports have described the successful treatment of asparaginase-related TEs with direct oral anticoagulants (DOACs), but large studies evaluating this approach are lacking [39, 40].
Data on the efficacy of prophylactic LMWH is limited in the adult ALL population. However, when patients aged 1 to 18 years old were randomized to receive prophylaxis with either LMWH, low-dose UFH, or activity-adapted ATIII throughout induction during treatment of ALL, both LMWH and activity-adapted ATIII lead to lower rates of thromboembolism without an increased risk of major hemorrhage [41]. A prospective trial evaluated the use of prophylactic anticoagulation in adult patients, beginning between the day of diagnosis and at least 7 days after the start of induction and continued > 6 weeks after the final dose of asparaginase during consolidation II. It showed a lower risk of TEs without an increased risk of bleeding events [42•]. Nevertheless, in the NOPHO ALL2008 study, despite treatment with LMWH, 13% of the patients aged 17 years or older still developed first-time TEs [32].
The replacement of ATIII as a prophylactic measure against asparaginase-related TEs is controversial due to both its cost and lack of consistent evidence demonstrating benefit. A few prospective pediatric trials using ATIII repletion showed a trend towards reducing TE without increasing the adverse events [29]. However, data in adults, with both retrospective and prospective studies, showed contradictory findings [43,44,45,46]. Therefore, it remains difficult to derive solid conclusions from these studies, as they are small and nonrandomized. Additionally, different institutional protocols have different thresholds for repletion of ATIII, and some may concurrently use prophylactic anticoagulation [44, 45].
While the data is more robust in the pediatric population, when compared with low-dose UFH, activity-targeted ATIII replacement and LMWH lead to a significant risk reduction for TE during ALL induction therapy without increasing the risk of bleeding. Therefore, it is reasonable to treat adult ALL patients receiving asparaginase with prophylactic LMWH, along with close monitoring of the platelet count. Activity-targeted ATIII replacement is an equally reasonable approach, but the added expense, the additional required monitoring, and the inconsistency of a threshold ATIII level for replacement make this approach more cumbersome and less standardized.
Finally, although hypofibrinogenemia is a fairly common side effect of pegaspargase therapy, the bleeding risks are low even without fibrinogen repletion [23]. In fact, cryoprecipitate repletion may have a counterintuitive effect by increasing the risk of TEs during asparaginase therapy [23, 45, 46]. In the absence of active bleeding, cryoprecipitate repletion should be avoided or minimized.
Asparaginase-Induced Allergic Reactions: Hypersensitivity and Inactivation
As a bacterial-derived (and thus a foreign substance to the human body) enzyme, the introduction of pegaspargase could lead to antibody formation and allergic reactions [18]. Antibodies against pegaspargase are most commonly directed against the pegylated component of the enzyme [47•]. These antibodies could ultimately reduce the availability of the active asparaginase, leading to a risk of insufficient asparagine depletion. Given the inferior outcomes in patients who receive an inadequate course of asparaginase therapy, prompt identification and management of asparaginase inactivation is crucial to reduce the risk of treatment failure [18, 48].
Hypersensitivity reactions can mimic infusion reactions or non-antibody-mediated reactions in their presentation and, unfortunately, it is often difficult to make this distinction based on symptoms alone [47•, 48]. The latter include rash, pruritus, edema, cough, shortness of breath, chest pain, nausea, vomiting, and hypotension [18]. Less commonly, hypersensitivity may manifest as a “silent inactivation,” in which neutralizing antibodies are formed in the absence of clinical symptoms [18, 49, 50].
Hypersensitivity has been noted in 6–21% of adults receiving asparaginase therapy, with approximately 6% classified as grade 3–4 in severity [9, 23, 51•]. The prevalence is influenced by several factors, including patient characteristics (carrying HLA-DRB1*07:01 alleles could be associated with a higher rate of allergic reaction), the use of concurrent medications (notably corticosteroids), and route of asparaginase administration (intramuscular versus intravenous) [23, 49, 52]. The wider use of pre-medication prior to asparaginase therapy in adults has decreased the rate of asparaginase-associated hypersensitivity; however, this has contributed to an increased risk of silent inactivation [11•, 49, 50].
In the acute setting of a symptomatic hypersensitivity reaction, the infusion should be stopped immediately (Fig. 2). Treatment including antihistamines, corticosteroids, acetaminophen, and/or epinephrine should be utilized if indicated based on the scope and severity of each patient’s symptoms. Once the patient is stabilized, the clinician’s attention should then be directed towards appropriately classifying the reaction. Certain characteristics may favor a non-antibody-mediated reaction, which would not alter drug efficacy or clearance and allow for continuation of pegaspargase. For instance, as antibodies against pegaspargase are more likely to develop with prolonged exposure to the drug, a patient receiving their infusion for the first time with no prior exposure to other asparaginase formulations is less likely to be experiencing a true antibody-mediated reaction [47•, 49]. Moreover, obtaining both asparaginase activity and ammonia levels at the time of the reaction can provide additional and objective data that may aid in the classification of the reaction. An elevated ammonia level (which is often associated with headaches, nausea, and vomiting) in the setting of normal asparaginase activity argues against a true hypersensitivity reaction, and is more consistent with a non-antibody-mediated infusion reaction due to ammonia accumulation seen following pegaspargase administration [47•, 49].
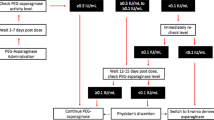
Approach to pegaspargase infusion adverse reaction. Asterisk indicates a redraw to confirm subtherapeutic level can be considered. However, this is a send-out lab for the majority of institutions, and the time delay required for a result may limit the utility of this approach in the clinical setting
Alternatively, when a patient has a reaction after receiving several doses of asparaginase or is found to have reduced asparaginase activity, the highest suspicion should be for a true hypersensitivity reaction [18, 49, 50]. In this setting, therapeutic drug monitoring (TDM) of asparaginase is essential (Fig. 2). Recent consensus recommendations suggest a target level of > 0.1 IU/mL must be achieved for adequate response, as asparaginase levels below this do not consistently result in complete depletion of asparagine [48, 49, 53]. When concerns for an antibody-mediated pegaspargase reaction lead to cessation of the infusion, asparaginase levels should initially be checked 4–7 days post-dose if the patient received > 50% of the dose, or 3–5 days post-dose if the patient received 10–50% of the dose. If < 10% of the initial dose was received, a level should be deferred and instead checked with the following dose (if it is deemed clinically safe to resume the drug) [48, 54]. The goal is to maintain therapeutic levels for at least 14 days after each dose of pegaspargase [48, 49, 53, 54].
With respect to increasing the likelihood of detecting silent inactivation, it may be beneficial to monitor asparaginase levels in all patients receiving pegaspargase (Fig. 3). Current guidelines suggest obtaining asparaginase levels at days 7 and 14 following each dose [18, 55]. Suspicion for silent inactivation should arise if asparaginase levels are < 0.1 IU/mL on day 7 or day 14 [55]. A redraw to confirm subtherapeutic levels can be considered. However, we note that this activity level is a send-out lab for the majority of institutions, and the time delay required for a result may limit the utility of this approach in the clinical setting.
Approach to therapeutic drug monitoring (TDM) with pegaspargase. Allo-HCT, allogeneic hematopoietic stem cell transplant; TDM, therapeutic drug monitoring. Asterisk indicates a redraw to confirm subtherapeutic level can be considered. However, this is a send-out lab for the majority of institutions, and the time delay required for a result may limit the utility of this approach in the clinical setting
If therapeutic levels of asparaginase activity cannot be achieved, a switch from the more common E. coli–derived formulation to an Erwinia-based asparaginase would be indicated. Erwinia use is complicated by more frequent administrations as well as increased cost. Furthermore, at the time of this publication, we recognize that the ongoing shortage of Erwinia-derived asparaginase may complicate this approach, and recommend that institutions use their associated protocols to determine whether or not to continue asparaginase in this setting.
Asparaginase-Induced Hepatotoxicity
In contrast to pediatric patients, asparaginase-related hepatotoxicity is the most commonly encountered adverse effect in adults [37]. The mechanism of toxicity is unknown, but may be a result of impaired hepatocyte protein synthesis, and manifests as hyperbilirubinemia, transaminitis, or both [56•]. Hepatotoxicity of any grade may arise in up to 90% of adults treated with pediatric-inspired regimens, and grade 3–4 toxicities are frequent, occurring at rates of 25–40% for hyperbilirubinemia and > 50% for transaminitis [23]. Studies suggest a link between hepatic steatosis and pegaspargase-related hepatotoxicity, but the true clinical significance remains unclear [57, 58].
High-grade hyperbilirubinemia usually arises after the first induction dose of asparaginase, with a median onset of 2 weeks [56•]. High-grade transaminitis also occurs most often following the initial induction dose. Recovery to low-grade hepatotoxicity is usually prolonged and may take up to 4 weeks; however, it is often reversible and rarely leads to liver failure [56•, 59]. Obesity, older age, hypoalbuminemia, and higher doses of asparaginase all appear to increase the risk of high-grade hyperbilirubinemia in adult patients [10, 23, 59, 60].
Fortunately, asparaginase-related hepatotoxicity has low rates of morbidity and mortality, with studies reporting hepatic failure and associated deaths in less than 1% of adult patients [10, 11•]. Subsequent doses of pegaspargase in patients with prior hepatotoxicity do not confer an increased risk of additional liver injury [56•]. Most importantly, discontinuing pegaspargase following hepatotoxicity results in worse patient outcomes [61, 62]. While the incidence of high-grade hepatotoxicity appears low with Erwinia-derived asparaginase, no substantial data exists to support switching from E. coli pegaspargase after an episode of hepatotoxicity [63, 64]. Therefore, it is recommended to resume the treatment with pegaspargase upon resolution of high-grade hepatotoxicity (Fig. 4), recognizing that the impact of this treatment delay is uncertain [59].
Asparaginase-Associated Pancreatitis
Due to its significant morbidity and mortality, asparaginase-associated pancreatitis (AAP) is the most common reason for the discontinuation of asparaginase therapy in adult ALL [53]. While the mechanism of AAP remains unclear, it is estimated to occur in 5–14% of individuals receiving pegaspargase [9, 11•, 23, 51•]. Pancreatitis is generally grade 3–4 in severity, and most commonly occurs during the first few cycles of therapy. Older age, high-risk ALL, higher pegaspargase dose, and longer duration of therapy are all associated with an increased risk of developing AAP [65]. APP mortality is estimated at 2%, most often from necrotizing pancreatitis [53, 66]. Recurrent abdominal pain in the setting of chronic pancreatitis and hyperglycemia with insulin dependence are potential long-term APP complications [66]. Given this degree of morbidity and mortality, it is crucial to educate patients on the symptoms of pancreatitis and to report them as soon as they develop so that an intervention is not delayed.
Supportive care, with a goal-directed intravenous fluids, analgesia, and correction of metabolic and electrolyte abnormalities, are the mainstay of the treatment for APP. After an APP episode, complete cessation of any asparaginase therapy is required [53]. The risk of recurrent pancreatitis with re-exposure to pegaspargase is significant, with rates greater than 40% reported following re-challenge [66]. Switching to another asparaginase-based formulation (such as Erwinia) should be avoided as well. Finally, it is important to distinguish clinical pancreatitis from chemical pancreatitis [53], as the latter is only associated with an increase in amylase and/or lipase levels without corresponding pancreatitis symptoms or imaging findings. Chemical pancreatitis is not a contraindication to continuation of asparaginase therapy.
Asparaginase-Induced Hypertriglyceridemia
Hypertriglyceridemia is a relatively benign but common complication of pegaspargase therapy, with grade 3–4 elevations occurring in up to 50% of patients [53, 67]. Elevations in triglyceride levels typically occur during consolidation cycles as opposed to induction, and usually resolve spontaneously. The risk of developing hypertriglyceridemia is strongly associated with an increased body mass index and is inversely correlated with an older age [53]. Unlike in the general population, where it has long been recognized that hypertriglyceridemia increases the risk of clinical pancreatitis, asparaginase-induced hypertriglyceridemia does not confer an increased risk of AAP [23, 68, 69]. The development of asparaginase-related hypertriglyceridemia does not require any change to the treatment regimen. Some experts suggest the use of triglyceride-lowering agents (i.e., fibrates), diet changes, insulin infusions, or even plasmapheresis as means of primary or secondary prevention; however, there is a lack of evidence that these interventions decrease the risk of hypertriglyceridemia or AAP [51•].
Unanswered Questions and Future Directions
Despite the increasing integration of pegaspargase into adult ALL regimens over the past decade, many questions on its use and management remain unanswered:
-
1)
What is the upper age limit at which pegaspargase (and intensive pediatric regimens) can be safely administered to adult ALL patients? Thus far, the upper age limit across recent major trials incorporating asparaginase into adult ALL regimens has ranged from 39 to 60 years old, with variations in treatment regimens and patient characteristics making cross-trial comparison challenging [10, 11•, 70, 71]. Although the use of these pediatric-inspired regimens has improved outcomes in older adults, these studies are not without concerns regarding increased treatment-related toxicity, as both the GRALL-2003 and GRALL-2005 trials noted a poorer tolerance of asparaginase in the older population of their study cohort [70, 71].
-
2)
What is the optimal formulation of asparaginase? With its prolonged half-life, the PEGylated form of asparaginase is currently the preferred option for most regimens. As previously mentioned, the PEG component of pegaspargase is the most common cause of antibody formation (and subsequent hypersensitivity and inactivation). Asparaginase glycosylation is being investigated as an alternative to PEGylation, with evidence that this approach could yield an enzyme that is still active, but less prone to an immunogenic response and resultant inactivation [72, 73]. Efforts to reduce incidence of asparaginase inactivation will be especially important in light of the ongoing shortage of Erwinia-derived asparaginase, which limits treatment options in patients who are no longer able to receive pegaspargase due to hypersensitivity and inactivation.
-
3)
How else can we optimize prophylaxis and/or treatment of pegaspargase-related toxicities? Much of this management remains uncertain and would benefit from larger, prospective clinical trials specifically in the adult population. Areas of interest include the role of prophylactic anticoagulation and ATIII repletion [44, 45]; the utility of L-carnitine in treating asparaginase-induced hyperbilirubinemia [60, 74, 75]; and the use of prophylactic octreotide in APP, which has been used in case reports of patients who developed APP and required re-exposure to pegaspargase [76, 77].
-
4)
What are the options if a patient is unable to tolerate asparaginase? Even with appropriate toxicity management, some ALL patients will require permanent asparaginase discontinuation due to side effects. The question of how to best manage these patients remains unanswered, and future studies are required to determine the best course of action in these instances, including earlier incorporation of allo-HCT in the treatment paradigm.
Conclusion
Pegaspargase is an essential component of the pediatric-inspired regimens that have significantly improved the outcomes in adult ALL. Although a lack of familiarity with its unique toxicity profile can make pegaspargase a challenge to manage, the majority of these toxicities are reversible, do not lead to significant mortality, and can be treated supportively and without its permanent discontinuation. By properly identifying and managing pegaspargase side effects and avoiding premature or unnecessary discontinuation, we could ensure that adult ALL patients achieve maximum clinical benefit from this medication.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120(6):1165–74.
Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–801.
Sive JI, Buck G, Fielding A, Lazarus HM, Litzow MR, Luger S, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157(4):463–71.
Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121(15):2517–28.
Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2016;91(11):1645–66.
Aldoss I, Forman SJ, Pullarkat V. Acute lymphoblastic leukemia in the older adult. J Oncol Pract. 2019;15(2):67–75.
Moorman AV. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev. 2012;26(3):123–35.
O’Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113(8):2097–101.
Rytting ME, Thomas DA, O’Brien SM, Ravandi-Kashani F, Jabbour EJ, Franklin AR, et al. Augmented Berlin-Frankfurt-Münster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL). Cancer. 2014;120(23):3660–8.
DeAngelo DJ, Stevenson KE, Dahlberg SE, Silverman LB, Couban S, Supko JG, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29(3):526–34.
• Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–59 This prospective trial incorporated more extensive use of pegaspargase, glucocorticoids, vincristine, and CNS prophylaxis in ALL patients up to 40 years old, resulting in significant improvement in EFS and OS compared to historical data.
Bussolati O, Belletti S, Uggeri J, Gatti R, Orlandini G, Dall’Asta V, et al. Characterization of apoptotic phenomena induced by treatment with L-asparaginase in NIH3T3 cells. Exp Cell Res. 1995;220(2):283–91.
Covini D, Tardito S, Bussolati O, Chiarelli LR, Pasquetto MV, Digilio R, et al. Expanding targets for a metabolic therapy of cancer: L-asparaginase. Recent Pat Anticancer Drug Discov. 2012;7(1):4–13.
Fu CH, Sakamoto KM. PEG-asparaginase. Expert Opin Pharmacother. 2007;8(12):1977–84.
Abshire TC, Pollock BH, Billett AL, Bradley P, Buchanan GR. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood. 2000;96(5):1709–15.
Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–8.
Kurtzberg J, Asselin B, Bernstein M, Buchanan GR, Pollock BH, Camitta BM. Polyethylene glycol-conjugated L-asparaginase versus native L-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a Children’s Oncology Group Study (POG 8866). J Pediatr Hematol Oncol. 2011;33(8):610–6.
van der Sluis IM, Vrooman LM, Pieters R, Baruchel A, Escherich G, Goulden N, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101(3):279–85.
Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol. 1999;457:621–9.
Heo YA, Syed YY, Keam SJ. Pegaspargase: a review in acute lymphoblastic leukaemia. Drugs. 2019;79(7):767–77.
Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist. 2007;12(8):991–8.
Li RJ, Jin R, Liu C, Cao X, Manning ML, Di XM, et al. FDA approval summary: calaspargase pegol-mknl for treatment of acute lymphoblastic leukemia in children and young adults. Clin Cancer Res. 2020;26(2):328–31.
Aldoss I, Douer D, Behrendt CE, Chaudhary P, Mohrbacher A, Vrona J, et al. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur J Haematol. 2016;96(4):375–80.
Couturier MA, Huguet F, Chevallier P, Suarez F, Thomas X, Escoffre-Barbe M, et al. Cerebral venous thrombosis in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma during induction chemotherapy with l-asparaginase: The GRAALL experience. Am J Hematol. 2015;90(11):986–91.
Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, de Gaetano G, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–22.
Underwood B, Zhao Q, Walker AR, Mims AS, Vasu S, Long M, et al. Incidence of venous thrombosis after peg-asparaginase in adolescent and young adults with acute lymphoblastic leukemia. Int J Hematol Oncol. 2020;9(3):IJH28.
van Zaane B, Nur E, Squizzato A, Gerdes VE, Büller HR, Dekkers OM, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost. 2010;8(11):2483–93.
Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia. 2013;27(3):553–9.
Mitchell LG, Andrew M, Hanna K, Abshire T, Halton J, Anderson R, et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer. 2003;97(2):508–16.
Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Donati MB. Venous thrombotic complications in adults undergoing induction treatment for acute lymphoblastic leukemia: results from a meta-analysis. J Thromb Haemost. 2007;5(3):621–3.
Grace RF, Dahlberg SE, Neuberg D, Sallan SE, Connors JM, Neufeld EJ, et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol. 2011;152(4):452–9.
Rank CU, Toft N, Tuckuviene R, Grell K, Nielsen OJ, Frandsen TL, et al. Thromboembolism in acute lymphoblastic leukemia: results of NOPHO ALL2008 protocol treatment in patients aged 1 to 45 years. Blood. 2018;131(22):2475–84.
Athale UH, Siciliano SA, Crowther M, Barr RD, Chan AK. Thromboembolism in children with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute protocols: effect of age and risk stratification of disease. Br J Haematol. 2005;129(6):803–10.
Tuckuviene R, Ranta S, Albertsen BK, Andersson NG, Bendtsen MD, Frisk T, et al. Prospective study of thromboembolism in 1038 children with acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology (NOPHO) study. J Thromb Haemost. 2016;14(3):485–94.
• Zwicker JI, Wang TF, DeAngelo DJ, Lauw MN, Connors JM, Falanga A, et al. The prevention and management of asparaginase-related venous thromboembolism in adults: guidance from the SSC on Hemostasis and Malignancy of the ISTH. J Thromb Haemost. 2020;18(2):278–84 This review summarizes the most recent recommendations from the International Society of Thrombosis and Hemostasis (ISTH) on the management of asparaginase-related thromboembolism.
Aldoss I, Douer D. How I treat the toxicities of pegasparaginase in adults with acute lymphoblastic leukemia. Blood. 2020;135(13):987–95.
Stock W, Douer D, DeAngelo DJ, Arellano M, Advani A, Damon L, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymphoma. 2011;52(12):2237–53.
Tufano A, Guida A, Di Minno MN, Prisco D, Cerbone AM, Di Minno G. Prevention of venous thromboembolism in medical patients with thrombocytopenia or with platelet dysfunction: a review of the literature. Semin Thromb Hemost. 2011;37(3):267–74.
Plander M, Szendrei T, Bodó I, Iványi JL. Successful treatment with rivaroxaban of an extended superficial vein thrombosis in a patient with acquired antithrombin deficiency due to peg-asparaginase treatment. Ann Hematol. 2015;94(7):1257–8.
Talamo L, Douvas M, Macik BG, Ornan D. Successful treatment with apixaban of sinus venous thrombosis due to pegylated asparaginase in a young adult with T cell acute lymphoblastic leukemia: case report and review of management. Ann Hematol. 2017;96(4):691–3.
Greiner J, Schrappe M, Claviez A, Zimmermann M, Niemeyer C, Kolb R, et al. THROMBOTECT - a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica. 2019;104(4):756–65.
• Grace RF, DeAngelo DJ, Stevenson KE, Neuberg D, Sallan SE, Mourad YRA, et al. The use of prophylactic anticoagulation during induction and consolidation chemotherapy in adults with acute lymphoblastic leukemia. J Thromb Thrombolysis. 2018;45(2):306–14 This multicenter, prospective study of adult ALL patients receiving asparaginase showed prophylactic anticoagulation decreased risk of thromboembolism without increasing risk of bleeding events.
Hunault-Berger M, Chevallier P, Delain M, Bulabois CE, Bologna S, Bernard M, et al. Changes in antithrombin and fibrinogen levels during induction chemotherapy with L-asparaginase in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of supportive coagulation therapy and clinical outcome: the CAPELAL study. Haematologica. 2008;93(10):1488–94.
Farrell K, Fyfe A, Allan J, Tait RC, Leach M. An antithrombin replacement strategy during asparaginase therapy for acute lymphoblastic leukemia is associated with a reduction in thrombotic events. Leuk Lymphoma. 2016;57(11):2568–74.
Chen J, Ngo D, Aldoss I, Shayani S, Tsai NC, Pullarkat V. Antithrombin supplementation did not impact the incidence of pegylated asparaginase-induced venous thromboembolism in adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60(5):1187–92.
Orvain C, Balsat M, Tavernier E, Marolleau JP, Pabst T, Chevallier P, et al. Thromboembolism prophylaxis in adult patients with acute lymphoblastic leukemia treated in the GRAALL-2005 study. Blood. 2020;136(3):328–38.
• Liu Y, Smith CA, Panetta JC, Yang W, Thompson LE, Counts JP, et al. Antibodies predict pegaspargase allergic reactions and failure of rechallenge. J Clin Oncol. 2019;37(23):2051–61 This study evaluated serum samples from ALL patients who received pegaspargase versus L-asparaginase. Presence of anti-pegaspargase antibodies was associated with increased drug clearance and drug failure upon re-challenge. The PEG component of pegaspargase was found to be the most common cause of antibody formation.
Burke MJ, Rheingold SR. Differentiating hypersensitivity versus infusion-related reactions in pediatric patients receiving intravenous asparaginase therapy for acute lymphoblastic leukemia. Leuk Lymphoma. 2017;58(3):540–51.
Cooper SL, Young DJ, Bowen CJ, Arwood NM, Poggi SG, Brown PA. Universal premedication and therapeutic drug monitoring for asparaginase-based therapy prevents infusion-associated acute adverse events and drug substitutions. Pediatr Blood Cancer. 2019;66(8):e27797.
Tong WH, Pieters R, Kaspers GJ, te Loo DM, Bierings MB, van den Bos C, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123(13):2026–33.
• Douer D, Aldoss I, Lunning MA, Burke PW, Ramezani L, Mark L, et al. Pharmacokinetics-based integration of multiple doses of intravenous pegaspargase in a pediatric regimen for adults with newly diagnosed acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9):905–11.
Fernandez CA, Smith C, Yang W, Daté M, Bashford D, Larsen E, et al. HLA-DRB1*07:01 is associated with a higher risk of asparaginase allergies. Blood. 2014;124(8):1266–76.
Burke PW, Hoelzer D, Park JH, Schmiegelow K, Douer D. Managing toxicities with asparaginase-based therapies in adult ALL: summary of an ESMO Open-Cancer Horizons roundtable discussion. ESMO Open. 2020;5(5).
Bleyer A, Asselin BL, Koontz SE, Hunger SP. Clinical application of asparaginase activity levels following treatment with pegaspargase. Pediatr Blood Cancer. 2015;62(6):1102–5.
Salzer W, Bostrom B, Messinger Y, Perissinotti AJ, Marini B. Asparaginase activity levels and monitoring in patients with acute lymphoblastic leukemia. Leuk Lymphoma. 2018;59(8):1797–806.
• Burke PW, Aldoss I, Lunning MA, Devlin SM, Tallman MS, Pullarkat V, et al. Pegaspargase-related high-grade hepatotoxicity in a pediatric-inspired adult acute lymphoblastic leukemia regimen does not predict recurrent hepatotoxicity with subsequent doses. Leuk Res. 2018;66:49–56 This retrospective analysis of 51 adult ALL patients reported that, despite high rates of high-grade pegaspargase-associated hepatotoxicity, patients could be safely rechallenged with pegaspargase.
Sahoo S, Hart J. Histopathological features of L-asparaginase-induced liver disease. Semin Liver Dis. 2003;23(3):295–9.
Kamal N, Koh C, Samala N, Fontana RJ, Stolz A, Durazo F, et al. Asparaginase-induced hepatotoxicity: rapid development of cholestasis and hepatic steatosis. Hepatol Int. 2019;13(5):641–8.
Goekbuget N, Baumann A, Beck J, Brueggemann M, Diedrich H, Huettmann A, et al. PEG-asparaginase intensification in adult acute lymphoblastic leukemia (ALL): significant improvement of outcome with moderate increase of liver toxicity in the German Multicenter Study Group for Adult ALL (GMALL) study 07/2003. Blood. 2010;116(21):494.
Rausch CR, Marini BL, Benitez LL, Elias A, Burke PW, Bixby D, et al. PEGging down risk factors for peg-asparaginase hepatotoxicity in patients with acute lymphoblastic leukemia. Leuk Lymphoma. 2018;59(3):617–24.
Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48(3):254–61.
Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646–54.
Plourde PV, Jeha S, Hijiya N, Keller FG, Silverman LB, Rheingold SR, et al. Safety profile of asparaginase Erwinia chrysanthemi in a large compassionate-use trial. Pediatr Blood Cancer. 2014;61(7):1232–8.
Horvat TZ, Pecoraro JJ, Daley RJ, Buie LW, King AC, Rampal RK, et al. The use of Erwinia asparaginase for adult patients with acute lymphoblastic leukemia after pegaspargase intolerance. Leuk Res. 2016;50:17–20.
Oparaji JA, Rose F, Okafor D, Howard A, Turner RL, Orabi AI, et al. Risk Factors for asparaginase-associated pancreatitis: a systematic review. J Clin Gastroenterol. 2017;51(10):907–13.
Wolthers BO, Frandsen TL, Baruchel A, Attarbaschi A, Barzilai S, Colombini A, et al. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte di Legno Toxicity Working Group study. Lancet Oncol. 2017;18(9):1238–48.
Daley RJ, Rajeeve S, Kabel CC, Pappacena JJ, Stump SE, Lavery JA, et al. Tolerability and toxicity of pegaspargase in adults 40 years and older with acute lymphoblastic leukemia. Leuk Lymphoma. 2021;62(1):176–84.
Raja RA, Schmiegelow K, Sørensen DN, Frandsen TL. Asparaginase-associated pancreatitis is not predicted by hypertriglyceridemia or pancreatic enzyme levels in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2017;64(1):32–8.
Persson L, Harila-Saari A, Hed Myrberg I, Heyman M, Nilsson A, Ranta S. Hypertriglyceridemia during asparaginase treatment in children with acute lymphoblastic leukemia correlates with antithrombin activity in adolescents. Pediatr Blood Cancer. 2017;64(10).
Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–8.
Huguet F, Chevret S, Leguay T, Thomas X, Boissel N, Escoffre-Barbe M, et al. Intensified therapy of acute lymphoblastic leukemia in adults: report of the randomized GRAALL-2005 clinical trial. J Clin Oncol. 2018;36(24):2514–23.
Effer B, Lima GM, Cabarca S, Pessoa A, Farías JG, Monteiro G. L-Asparaginase from. Prep Biochem Biotechnol. 2019;49(7):679–85.
Effer B, Kleingesinds EK, Lima GM, Costa IM, Sánchez-Moguel I, Pessoa A, et al. Glycosylation of Erwinase results in active protein less recognized by antibodies. Biochem Eng J. 2020;163:107750.
Roesmann A, Afify M, Panse J, Eisert A, Steitz J, Tolba RH. L-carnitine ameliorates L-asparaginase-induced acute liver toxicity in steatotic rat livers. Chemotherapy. 2013;59(3):167–75.
Schulte RR, Madiwale MV, Flower A, Hochberg J, Burke MJ, McNeer JL, et al. Levocarnitine for asparaginase-induced hepatic injury: a multi-institutional case series and review of the literature. Leuk Lymphoma. 2018;59(10):2360–8.
Buie LW, Moore J, van Deventer H. Successful use of octreotide as a chemoprotectant for prevention of PEG-asparaginase-induced pancreatitis. Pharmacotherapy. 2014;34(8):e149–51.
Sakaguchi S, Higa T, Suzuki M, Fujimura J, Shimizu T. Prophylactic use of octreotide for asparaginase-induced acute pancreatitis. Int J Hematol. 2017;106(2):266–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
D. O. R., J. M. S., H. C. V. V. V, A. L. M., M. K. K., and F. E. C. declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Acute Lymphocytic Leukemias
Rights and permissions
About this article
Cite this article
Riley, D.O., Schlefman, J.M., Vitzthum Von Eckstaedt V, H.C. et al. Pegaspargase in Practice: Minimizing Toxicity, Maximizing Benefit. Curr Hematol Malig Rep 16, 314–324 (2021). https://doi.org/10.1007/s11899-021-00638-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00638-0