Abstract
Purpose of Review
Chimeric antigen receptor T-cell (CAR-T) therapy is a form of adoptive cellular therapy that has revolutionized the treatment landscape in hematologic malignancies, especially B-cell lymphomas. In this review, we will discuss some of the landmark data behind these therapies and then lay out our approach to utilizing this new therapy.
Recent Findings
CD19-directed CAR-Ts are the most common type currently used in treatment of relapsed B-cell lymphoid neoplasms. There are currently three FDA-approved products: axicabtagene ciluecel and tisagenlecleucel for the treatment of relapsed/refractory large B-cell lymphoma and pediatric B-cell acute lymphocytic leukemia (tisagenlecleucel only) and brexucabtagene autoleucel for the treatment of relapsed/refractory mantle cell lymphoma. These therapies are associated with distinctive acute toxicities such as cytokine release syndrome and neurotoxicity and chronic toxicities such as cytopenias and hypogammaglobulinemia.
Summary
CAR-T therapy provides significant potential in the treatment of relapsed B-cell lymphomas despite current limitations. Several novel CAR cell designs are currently being studied in clinical trials which include tandem CAR-Ts, allogeneic CAR-Ts, and CAR-NK cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-Hodgkin lymphomas affect about 70,000 patients a year in the USA and have an estimated 5-year survival of about 70% [1]. Diffuse large B-cell lymphoma (DLBCL) followed by follicular lymphoma (FL) and then mantle cell lymphoma (MCL) constitute the most common types of B-cell non-Hodgkin’s lymphoma (B-NHL). While chemoimmunotherapy approaches such as R-CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone with rituximab) in aggressive B-cell lymphomas and BR (bendamustine with rituximab) in indolent B-cell lymphomas have significantly improved outcomes for patients [2, 3], those with relapsed and refractory disease can have very poor prognosis. A good example of that is what was shown in the SCHOLAR-1 study wherein the median overall survival (OS) was about 6 months for patients with refractory DLBCL [4].
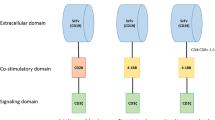
Chimeric antigen receptor T-cell (CAR-T) therapy is a form of adoptive cellular therapies that have revolutionized the treatment of relapsed B-cell lymphoid malignancies [5, 6]. The Food and Drug Administration (FDA) has approved both axicabtagene ciluecel (axi-cel) and tisagenlecleucel (tisa-cel) for the treatment of large B-cell lymphoma (LBCL) that have progressed after two lines of systemic therapies and brexucabtagene autoleucel for the treatment of relapsed and refractory MCL. All these products are second generation CAR-Ts directed against CD19 [7]. The history of CAR-T therapy started in the 1980s with the production of first generation CAR-Ts that lacked clinical efficacy and then later the addition of costimulatory molecules (CD28 or 4-1BB) to make second generations constructs which were shown to persist and have efficacy [8,9,10,11,12].
Most CAR-Ts are produced from autologous T-cells obtained from patients via leukopheresis. This process typically involves T-cell selection, gene transfer usually via a retrovirus, T-cell expansion, and transfer of a cryopreserved product and then infusion of the cells. This process can be as short as 14–21 days or up to even several weeks [13]. Patients receive lymphodepletion chemotherapy that commonly consists of fludarabine and cyclophosphamide for 3 days prior to CAR-T infusion [14]. In this review, we will discuss the current and new promising CAR-T constructs in the treatment of B-NHL and will also discuss our approach to choose the “optimal” CAR for patients.
Aggressive Lymphomas
Aggressive LBCLs were the very first diseases to get FDA approval for CAR-T products. This success is at least in part related to high unmet need for patients with active relapsed or refractory disease as seen in the SCHOLAR-1 study and due to the unique characteristics of CD19 which these CAR-T products have been targeting. The SCHOLAR-1 was an international multicenter retrospective study that evaluated the outcomes of patients with refractory DLBCL and those relapsed within 12 months of autologous stem cell transplant. The study showed that remission was only achievable in less than 10% of patients and the median OS was only around 6 months [4]. What has made CD19 an optimal therapeutic target is the fact that CD19 is ubiquitously expressed on the surface B-lymphocytes and patients can survive with B-cell aplasia despite having variable degrees of hypogammaglobulinemia. [15]. The two FDA-approved CAR-T products in this area are axi-cel and tisa-cel. Lisocabtagene maraleucel (liso-cel) is another CD19 CAR-T that has shown similar efficacy and is expected to get FDA approval in the near future [16••].
The safety and efficacy of axi-cel was evaluated in the multicenter phase 2 ZUMA-1 study [17••]. One hundred and one patients with relapsed and refectory LBCL were treated on this study with a target dose of 2 × 106 CAR-T/kg after receiving a lymphodepletion regimen that consisted of low-dose cyclophosphamide and fludarabine. The primary endpoint for this study was overall response rate that was found to be 82% (54% were complete response). With extended follow-up, we know now that about half of these patients will have a durable remission, which suggest a cure rate of 30–40% for these extremely high risk patients. Adverse events (AEs) were noted in 100% of patients, including 95% that were grade 3 or above. The most common AEs were fever and hematologic toxicities which happened in more than 80% of patients. The most common grade 3 toxicities were neutropenia (78%), anemia (43%), and thrombocytopenia (38%). AEs of notable interest were those that are somewhat unique to CAR-T therapies such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). CRS happened in 93% of patients; however, only 13% were grade 3 or above, including 1% that were grade 5. Neurotoxicity occurred in 64% of patients including 28% that were grade 3 or higher.
Similar results were also seen with standard of care use of axi-cel in “real world” setting [18••]. Nastoupil and colleagues retrospectively analyzed the data from 17 institutions in the USA. They found out that 92% of the patients that underwent leukapheresis were able to receive the CAR-T infusion, and 43% of them would not have met eligibility for ZUMA-1 study due to their comorbidities. Median progression-free survival (PFS) was 8.3 months and median OS was not reached at a median follow-up of 12.9 months. Overall response rate was 82% including 64% with complete remission. Nonrelapse mortality was 4.4%. Grade 3 or above CRS and neurotoxicity were seen in 7 and 31%, respectively.
The safety and efficacy of tisa-cel was evaluated in the phase 2 JULIET study which had a similar design to ZUMA-1 (Table 1). The study involved 93 patients, and the overall response rate was found to be 52% (40% were complete responses). AEs happened in 100% of the patients with CRS, fever, and hematologic toxicities being the most common. CRS occurred in 58% of patients with 22% being grade 3 or above. Neurotoxicity occurred in 21% of patients, with 12% being grade 3 or higher.
It is important to note few key differences between these two landmark studies apart from their outcomes. The ZUMA-1 study did not allow bridging therapy unlike JULIET wherein 90% of patients had received such therapies to control disease during the time between collection and infusion of CAR-T cells. The percentage of patients enrolled in ZUMA-1 and JULIET that actually were able to receive the CAR-T infusion were 91 and 40%, respectively, usually due to manufacturing issues or lymphoma progression and death. The median time from enrollment to CAR-T infusion was 17 and 54 days, respectively [19••]. The CRS toxicity grading was also different as ZUMA-1 used the Lee criteria while JULIET used Penn criteria [20].
Liso-cel was evaluated in the multicenter TRANSCEND NHL 001 study. The study design was similar to the aforementioned trials but with few key differences that included the sequential infusions of two T-cell components, CD4 and CD8 CAR-T cells, and also the inclusion of patients with secondary CNS involvement. Two hundred sixty-nine patients out of a total of 344 patients who had undergone leukapheresis were able to receive the CAR-T infusion indicating a success rate of 78% in achieving cell infusion. Failure to receive CAR-T therapy was primarily due to death from disease progression which occurred in 33 patients. The median time from pheresis to infusion was 37 days. The objective response rate was 73% including 53% with complete remission. Ninety-nine percent of the patients experienced AEs with the most common being neutropenia (63%), anemia (48%), and fatigue (44%). CRS occurred in 42% of patients, but grade 3 or above was only 2%. Neurotoxicity occurred in 30% of patients including 10% that were grade 3 and above. No treatment-related deaths were reported. Bridging therapy was allowed in this study similar to JULIET and was utilized in 59% of the patients. Bridging therapy was associated with poorer efficacy and higher incidence of CRS and ICANS [16••]. Liso-cel is currently undergoing FDA review for clinical approval [21]. Table 1 shows a comparison of the results of these three main studies.
The CD19 CAR-T brexucabtagene autoleucel (formerly known as KTE-X19) was evaluated in the treatment of relapsed MCL in the ZUMA-2 trial. Seventy-four patients were enrolled, but only 68 were able to receive the infusion which indicated about 92% success rate in delivering the treatment to patients. The objective response rate was 85% in the treated patients including 59% with complete remission. The PFS was 61% at 1 year, and the authors also reported durable remissions in some of the early treated patients. AEs occurred in 100% of the patients, and most commonly were fevers (94%) and hematologic toxicities (>70%). CRS occurred in 91% of patients including 15% that were grade 3 or higher. ICANS occurred in 63% of patients including 31% with grade 3 or higher. A total of two patients had grade 5 AE, one was from organizing pneumonia and the other from staphylococcus bacteremia, both were thought to be related to conditioning chemotherapyapy [22]. Brexucabtagene autoleucel was approved by the FDA in July 2020 for the treatment of relapsed or refractory MCL [23].
It is also important that long-term toxicities can also happen with these therapies more commonly including cytopenias and hypogammaglobulinemia potentially requiring prolonged replacement therapy [24,25,26,27]. Jain and others retrospectively analyzed the time to hematologic recovery in patients that have received CAR-T therapy. They found that normalization of blood counts only happened in about two thirds of the patients at 1-year post infusion and was inversely associated with higher grade of CRS or ICANS [24]
Indolent Lymphomas
FL is the most common indolent lymphoma. While the prognosis of most patients with FL is favorable and often they can be observed for long periods of time without requiring treatment, however, the prognosis for those relapsing within 2 years of initial chemoimmunotherapy (POD24) can be quite poor. A study by Casulo and others showed that 5-year OS is about 50% in these patients, compared to 90% in those with late relapses [28].
Axi-cel was evaluated for the treatment of indolent B-cell lymphomas in the ZUMA-5 study. ZUMA-5 was a multicenter phase 2 study that included patients with relapsed and refractory FL (grades 1–3a) or marginal zone lymphoma (MZL) who had received two lines of therapy including anti-CD20 monoclonal antibody and alkylating agent. The study design was otherwise very similar to ZUMA-1. Ninety-four patients including 84 with FL had been treated at the time of interim results reporting. The majority (73%) of patients had advanced and refractory disease, and 66% met criteria for POD24. Despite that, all the patients had good performance status (ECOG PS 0-1). The objective response rate was found to be 94% with 79% achieving complete remission. Patients with FL had ORR of 95% with 80% achieving CR. Sixty-eight percent of patients had ongoing response at the time of data cut-off. In terms of safety, 83% experienced grade 3 or above AEs, most commonly hematologic toxicities. CRS grade 3 or above occurred in 11% of patients and neurotoxicity occurred in 19% of patients. There were two grade 5 toxicities including one related to axi-cel [29].
An early phase study by Hirayama showed similar results using CD19 CAR-T cells with 4-1BB costimulation in FL. The study included 8 patients with R/R FL and 13 patients with transformed FL. Complete remission was reported to be 88% in patients with FL along with all those patients remaining in remission at median follow-up of 24 months. CRS occurred in 50% and neurotoxicity in 39% of FL patients; however, none of them were grade 3 or above [30].
Geyer et al. reported on a phase 1 study investigating CD19 CAR-T cells with CD28 costimulation primarily in relapsed/refractory chronic lymphocytic leukemia (CLL). The study included 16 patients with CLL and 4 patients with indolent B-NHL (2 with MZL, 1 with MCL, and 1 with FL). Three of the twelve evaluable patients with CLL had complete remission including two with negative minimal residual disease. All patients that achieved CR remained in remission at median follow-up of 53 months. CRS occurred in all patients, but grade 3 or above was only in 10%. Neurotoxicity grade 3 or above was seen in 10% of patients as well [31].
Finally, liso-cel is being currently investigated in in the international phase 2 (TRANSCEND FL) study in adult patients with R/R FL and MZL (NCT04245839).
Novel CAR-T Designs
CAR-T cell therapy has fundamentally changed the field of immunoncology and adoptive cellular therapies; however, further progress is still greatly needed. Autologous CD19 CAR-T cell therapy faces challenges such as barriers to successful cell manufacturing and delivery, low treatment efficacy, immune exhaustion, and immune escape. As a result, investigators continue to work on novel and improved CAR-T designs to improve efficacy (Table 2).
Shah and colleagues developed on-site noncryopreserved bispecific anti-CD-20 and anti-CD19 CAR-T cells. They enrolled 26 patients with B-NHL and CLL in their phase 1 study, and 22 patients received the target CAR-T dose. Grade 3 or higher CRS and neurotoxicity were only seen in 5% and 14% of patients, respectively. Objective treatment response was seen in 82% of patients including 64% with CR. The investigators also showed that CD19 loss was not seen in patients who relapsed [32]. A similar CD19/CD20 tandem CAR-T study was conducted by investigators in China. Tong et al. enrolled 33 patients with R/R B-NHL however only 28 were able to receive the cell infusion. Grade 3 or above CRS occurred in 14% of patients. No grade 3 or higher neurotoxicity were seen. The overall response rate was seen in 79% of patients including 71% with complete response. The 1-year PFS was 64% [33].
Off-the-shelf allogeneic CAR-Ts that utilize T-cells from healthy donors are another promising cell therapy platform. This approach could offer many advantages that include faster access to treatment, healthier T-cell products, less risk of contamination with lymphoma cells, ability for redosing when needed, and potentially lower costs [36]. Potential disadvantages include risks of graft versus host disease (GVHD) and CAR-T failure/elimination by patient’s own immune system [37, 38]. Neelapu and others presented first in human data of ALLO-051 and ALLO-647 in R/R LBCL and R/R FL. ALLO-051 is an allogeneic anti-CD19 CAR-T wherein the TCR alpha gene is disrupted to prevent GVHD, in addition to disrupting the CD52 gene to allow for voluntary host lymphodepletion via ALLO-647 which is an anti-CD52 monoclonal antibody. The investigators successfully treated 5 patients with LBCL and 4 with FL. None of the patients experienced GVHD. Most common grade 3 or higher AEs were hematologic toxicities, which occurred in more than half of the patients. Two patients experienced CRS, but both were less than grade 3. Neurotoxicity occurred in 1 patient and was grade 1. Three patients had complete remission, and four had partial response [34]. Other investigators reported success in using donor-derived allogeneic CAR-Ts in stem cell transplant patients who relapsed after allogeneic stem cell transplantation. These small studies showed clinical efficacy with low incidence of GVHD using this approach [39,40,41,42].
Another promising approach is the use of CAR-NK cells. Natural killer (NK) cells can recognize tumor antigens independent of patient’s human leukocyte antigen (HLA) phenotype and potentially can lead to development of cell banks that make treating large number of patients feasible with this type of a product. Several studies have now shown the viability and efficacy of CAR-NK production and their tumor immunogenicity [43,44,45]. Liu et al. reported using CAR-NK cells directed towards CD19 in CD19-positive lymphoid malignancies. They treated 11 patients using HLA-mismatched anti-CD19 CAR-NK cells derived from cord blood. Interestingly, they noted mild toxicity including no CRS, and the maximum tolerated dose was not reached in the study. Eight out of the 11 patients had responses including 7 with complete response [35••]. CAR-NK cells are being investigated in clinical trials for other diseases too such as BCMA CAR-NK in multiple myeloma (NCT03940833) and ROBO1 CAR-NK in solid tumors (NCT03940820) [46].
Lastly, armored CAR-T cell designs are another area of research to optimize these therapies. These cells undergo additional genetic modification that allow them to secrete cytokines or express proteins that enable them to interact with other immune cells and potentially overcome the immunosuppressive tumor microenvironment [47]. It is important to point out that most of these novel CAR-T constructs have only been evaluated in small studies with relatively short follow-up. While these results can serve as a proof of principle, it is still very early to predict their clinical efficacy and future success.
How to Optimize CAR-T Therapy
CAR-T cell therapy is a very promising treatment option for DLBCL; however, more than 50% of patients treated in the relapsed refractory setting do not attain a durable response. Several mechanisms of resistance to autologous CAR-T cell therapy have been identified, and this continues to be an active area of research [48]. Broadly speaking, resistance mechanisms can be due to tumor evasion or CAR-T cell failure [49]. In terms of tumor-based mechanisms of resistance, tumor cells can undergo antigen loss such as loss of CD19 in the case of CD19 directed CAR-T [50,51,52]; tumor cells can also express antigen masking [53] or take advantage of their suppressive microenvironment [49]. There are various mechanisms of CAR-T failure, these include destruction of the CAR-T cells by patient’s immune system [54], poor T-cell fitness [55, 56], or insufficient costimulation [57]. Targeting these known factors may increase efficacy of this technology [58,59,60]. This would entail that future research efforts should not only focus on improving and designing novel CAR-T constructs but also better understand the disease and patient’s characteristic that lead to treatment failure. Possibly off-the-shelf healthy donor-derived allogeneic CAR-T cell products may bypass the need for autologous T-cells and decrease time to first infusion as well as overcome poor T-cell fitness [61].
Selection of Optimal CAR-T Cell Therapy and Future Directions
CAR-T therapy is a major milestone in the treatment of relapsed/refractory B-cell lymphomas and has lead to a change in treatment paradigm. Due to the favorable outcome data reported for CAR-T cells, they are now preferred in comparison to allogeneic hematopoietic stem cell transplant, which should be restricted to situations where CAR-T cell therapy is deemed not feasible [62]. The results of clinical trials studies evaluating autologous CD-19 CAR-T for the treatment of DLBCL in the second line setting compared with high dose chemotherapy and autologous stem cell transplant are anxiously awaited. In our current treatment model, our initial choice for patients in first relapse for DLBCL, we prefer to enroll them on a trial with randomization between autologous transplant and CAR-T cell therapy. In the absence of clinical trial options, for patients who are beyond first relapse, we tend to use axi-cel for fit patients with aggressive disease given the rapid turnaround for cell manufacture with the caveat that axi-cel tends to have a higher incidence of CRS and neurotoxicity. Most commonly, these patients require an inpatient stay after infusion of cells for observation and treatment of toxicity. For older, less fit patients and more indolent disease, we tend to choose tisa-cel with the added benefit that a considerable number of patients can be treated in the outpatient setting.
The next frontier in this field will be expanding the efficacy of CAR-T cell therapy while making it safer, more accessible, and perhaps even cheaper.
References
Papers of particular interest, published recently, have been highlighted as:•• Of major importance
Cancer Stat Facts: Non-Hodgkin lymphoma [Internet]. Available from: https://seer.cancer.gov/statfacts/html/nhl.html. Accessed Dec 2020.
Kesavan M, Eyre TA, Collins GP. Front-line treatment of high grade B cell non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2019;14:207–18.
Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95:316–27.
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8.
Bachanova V, Perales MA, Abramson JS. Modern management of relapsed and refractory aggressive B-cell lymphoma: a perspective on the current treatment landscape and patient selection for CAR T-cell therapy. Blood Rev. 2020;40:100640.
Kallam A, Vose JM. Recent advances in CAR-T cell therapy for non-Hodgkin lymphoma. Clin Lymphoma, Myeloma Leuk. 2019;19:751–7.
Vitale C, Strati P. CAR T-cell therapy for B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia: clinical trials and real-world experiences. Front Oncol. 2020;10:849.
Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–8.
Van Der Stegen SJC, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14:499–509.
Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35.
Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunol. 2019;8(5):e1049.
Freyer CW, Porter DL. Advances in CAR T therapy for hematologic malignancies. Pharmacotherapy. 2020;40:741–55.
Abramson JS, Lunning M, Palomba ML. Chimeric antigen receptor T-cell therapies for aggressive B-cell lymphomas: current and future state of the art. Am Soc Clin Oncol Educ B. 2019:446–53.
Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005. p. 111–7.
Watkins MP, Bartlett NL. CD19-targeted immunotherapies for treatment of patients with non-Hodgkin B-cell lymphomas. Expert Opin. Investig. Drugs. 2018. p. 601–11.
•• Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52 Largest clinical trial investigating efficacy and safety of liso-cell in patients with R/R LBCL.
•• Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44 Landmark phase 2 study that led to the approval of Axi-cel in R/R LBCL.
•• Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38:3119–28 Real world data for standard of care Axi-Cell outside of clinical trials. Comparable effiacy and safety despite less strict patient eligbility.
•• Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell Lymphoma. N Engl J Med. 2019;380:45–56 Landmark phase 2 study that led to the approval of tisa-cel in R/R LBCL.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019. p. 625–38.
Squibb BM. Bristol Myers Squibb provides regulatory update on lisocabtagene maraleucel (liso-cel) [Internet]. Available from: https://news.bms.com/news/details/2020/Bristol-Myers-Squibb-Provides-Regulatory-Update-on-Lisocabtagene-Maraleucel-liso-cel/default.aspx. Accessed Dec 2020.
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42.
U. S. Food and Drug Administration (FDA). FDA approves brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma [Internet]. 07/27/2020. 2020. p. 1–2. Available from: https://www.fda.gov/drugs/fda-approves-brexucabtagene-autoleucel-relapsed-or-refractory-mantle-cell-lymphoma. Accessed Dec 2020.
Jain T, Knezevic A, Pennisi M, Chen Y, Ruiz JD, Purdon TJ, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4:3776–87.
Nahas GR, Komanduri KV, Pereira D, Goodman M, Jimenez AM, Beitinjaneh A, et al. Incidence and risk factors associated with a syndrome of persistent cytopenias after CAR-T cell therapy (PCTT). Leuk Lymphoma. 2020;61:940–3.
Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54:1643–50.
Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26:26–33.
Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33:2516–22.
Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles GA, et al. Interim analysis of ZUMA-5: A phase II study of axicabtagene ciloleucel (axi-cel) in patients (pts) with relapsed/refractory indolent non-Hodgkin lymphoma (R/R iNHL). J Clin Oncol [Internet]. 2020;38:8008–8 Available from: https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.8008. Accessed Dec 2020.
Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Pender BS, et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood. 2019;134:636–40.
Geyer MB, Rivière I, Sénéchal B, Wang X, Wang Y, Purdon TJ, et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight. 2019;4.
Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26:1569–75.
Tong C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. 2020;136:1632–44.
Neelapu SS, Munoz J, Locke FL, Miklos DB, Brown R, McDevitt JT, et al. First-in-human data of ALLO-501 and ALLO-647 in relapsed/refractory large B-cell or follicular lymphoma (R/R LBCL/FL): ALPHA study. J Clin Oncol [Internet]. 2020;38:8002–2 Available from: https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.8002.
•• Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–53 Phase 1 clinical trial evaluating the safety and efficacy of CD19 CAR-NK in patients with relpased CD19 lymphomas.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020. p. 185–99.
Aftab BT, Sasu B, Krishnamurthy J, Gschweng E, Alcazer V, Depil S. Toward “off-the-shelf” allogeneic CAR T cells. Adv Cell Gene Ther. 2020;3.
Kim DW, Cho JY. Recent advances in allogeneic CAR-T cells. Biomolecules. 2020.
Hua J, Zhang J, Wu X, Zhou L, Bao X, Han Y, et al. Allogeneic donor-derived anti-CD19 CAR T Cell is a promising therapy for relapsed/refractory B-ALL after allogeneic hematopoietic stem-cell transplantation. Clin Lymphoma, Myeloma Leuk. 2020;20:610–6.
Brudno JN, Somerville RPT, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21.
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39.
Chen Y, Cheng Y, Suo P, Yan C, Wang Y, Chen Y, et al. Donor-derived CD19-targeted T cell infusion induces minimal residual disease-negative remission in relapsed B-cell acute lymphoblastic leukaemia with no response to donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation. Br J Haematol. 2017;179:598–605.
Sivori S, Meazza R, Quintarelli C, Carlomagno S, Della Chiesa M, Falco M, et al. NK Cell-based immunotherapy for hematological malignancies. J Clin Med. 2019;8:1702.
Ingegnere T, Mariotti FR, Pelosi A, Quintarelli C, De Angelis B, Tumino N, et al. Human CAR NK cells: A new non-viral method allowing high efficient transfection and strong tumor cell killing. Front Immunol. 2019;10.
Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: clinical transformation and future prospects. Cancer Lett. 2020. p. 175–80.
Clinicaltrials.gov. Website [Internet]. Available from: https://clinicaltrials.gov/ct2/home. Accessed Jan 2021.
Yeku OO, Brentjens RJ. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans. 2016;44:412–8.
Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019. p. 372–85.
Singh N, Orlando E, Xu J, Xu J, Binder Z, Collins MA, et al. Mechanisms of resistance to CAR T cell therapies. Semin Cancer Biol. 2020. p. 91–8.
Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24:1504–6.
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–95.
Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–10.
Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018
Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–56.
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–71.
Das RK, Vernau L, Grupp SA, Barrett DM. Naïve T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov. 2019;
Curran KJ, Seinstra BA, Nikhamin Y, Yeh R, Usachenko Y, Van Leeuwen DG, et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther. 2015;23:769–78.
Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020.
Wudhikarn K, Park JH. Dissecting factors influencing response to CAR T cell therapy in B lymphoid hematologic malignancies: from basic to practice. Leuk Lymphoma. 2020;61:2324–34.
Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020
Zhang E, Xu H. A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J. Hematol. Oncol. 2017.
Dreger P, Fenske TS, Montoto S, Pasquini MC, Sureda A, Hamadani M. Cellular immunotherapy for refractory diffuse large B cell lymphoma in the chimeric antigen receptor-engineered T cell era: still a role for allogeneic transplantation? Biol Blood Marrow Transplant. 2020;26:e77–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on T-Cell and Other Lymphoproliferative Malignancies
Rights and permissions
About this article
Cite this article
Al-Juhaishi, T., Ahmed, S. Selecting the Optimal CAR-T for the Treatment of B-Cell Malignancies. Curr Hematol Malig Rep 16, 32–39 (2021). https://doi.org/10.1007/s11899-021-00615-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00615-7