Abstract
Purpose of Review
Secondary AML (s-AML) encompasses a distinct subgroup of AML with either therapy-related AML or AML arising from preexisting myeloid neoplasms. Despite recent advances in the treatment armamentarium of AML, outcomes remain poor in s-AML. The purpose of this review is to highlight distinct characteristics, prognostic factors, and treatment options for patients with s-AML. Further, we focus on a distinctly poor-risk subgroup of s-AML with previous exposure to hypomethylating agents (HMAs) and describe ongoing clinical trials in this patient population.
Recent Findings
CPX-351 (liposomal daunorubicin and cytarabine) is the first drug approved for s-AML and represents an advancement in the management of fit patients with this subtype of AML. Despite incremental improvement in remission rates and survival, long-term survival remains poor. Patients who have received prior HMAs for antecedent MDS rarely benefit from CPX-351 or other cytotoxic chemotherapy regimens. The approval of venetoclax in combination with azacitidine has led to a paradigm shift in the management of newly diagnosed older unfit AML patients; however, patients with s-AML and prior HMA therapy were excluded from the landmark randomized phase 3 study. Several early phase clinical trials with both low- and high-intensity therapies are ongoing for s-AML patients, though prior HMA exposure limits inclusion in many of these studies that include HMAs.
Summary
Patients with s-AML previously treated with an HMA have dismal outcomes with standard therapeutic options and are under-represented in clinical trials. Trials investigating novel therapeutic options in this population are critically needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a myeloid malignancy resulting from a clonal proliferation of myeloid progenitors leading to compromised hematopoiesis and bone marrow failure. Traditionally, AML has been classified by the morphology, immunophenotype, and cytogenetic abnormalities of the leukemic cells. Advances in molecular profiling over the last few decades has allowed for further classification of AML into prognostically distinct subgroups. The 2016 World Health Organization (WHO) Classification now includes a category for “AML with recurrent genetic abnormalities” that subclassifies AML by genetic abnormalities reflecting the known heterogeneity of this disease [1]. Updated risk stratification models by the European Leukemia Net (ELN) now utilize molecular abnormalities to determine risk [2]. The largest mutational analysis performed in AML to date with over 1500 patients illustrates how driver mutations can influence prognosis [3]. Papaemmanuil et al. therefore proposed a new classification system based on 14 individual genetic subgroups (Table 1). These changes demonstrate the ongoing progress in the classification of AML by unique genomic and molecular factors.
AML has historically been grouped by clinical ontogeny into 3 distinct categories: secondary-AML (s-AML) arising from an antecedent hematologic disorder (AHD) (often myelodysplastic syndrome [MDS] or myeloproliferative neoplasm [MPN]), therapy-related AML (t-AML) arising as a late complication of prior exposure to leukemogenic therapies, and de novo AML which is not preceded by an identified myeloid neoplasm or cytotoxic therapy. These historical classifications are only somewhat preserved in formalized classification schemes. The 2016 WHO Classification groups all myeloid neoplasms that are therapy-related into “therapy-related myeloid neoplasms” and does not include a specific classification for s-AML [1]. However, a subcategory of “AML with myelodysplasia-related changes (AML-MRC)” has replaced s-AML by WHO Classification, which includes one of the following: [1] a prior history of clinical MDS (but not MPN), [2] multilineage dysplasia (>50% dysplasia in 2 or more cell lines) without a mutation in NPM1 or biallelic CEBPA, or [3] the presence of an MDS-related cytogenetic abnormality (see Table 2). In addition to a set of well-defined cytogenetic abnormalities, s-AML is characterized by specific molecular features that highlight the unique leukemia driver mutations associated with preexisting MDS. Lindsley et al. reported a set of somatic mutations (SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, and STAG2) that were highly specific for a clinical history of s-AML preceded by MDS [5•, 6]. These advances may make it possible to discriminate patients with “biological s-AML” based on diagnostic molecular and cytogenetic characteristics, though this strategy has not been formally incorporated into standard AML classification.
It is critical to identify patients with s-AML at diagnosis as these patients have a particularly poor prognosis and commonly have adverse-risk cytogenetic features [7,8,9]. Overall outcomes are significantly worse in patients with s-AML compared with de novo AML and treatment options are more limited. Overall rates of complete remission (CR), including incomplete count recovery (CRi), are less than 40% in patients with s-AML treated with conventional chemotherapy, and median overall survival (OS) is often between 5 and 7 months [7,8,9,10]. Long-term (>5 year) OS may be possible with hematopoietic stem cell transplantation (HSCT), though few patients (<5%) receive this therapy [7,8,9]. Even among HSCT recipients, median OS is approximately 1 year after HSCT in patients with s-AML [10]. There remains an urgent, unmet need for novel therapeutic agents in this subgroup to improve clinical outcomes. The purpose of this review is to delineate the outcomes of patients with s-AML and AML with MRC, in particular highlighting patients with previous exposure to hypomethylating agents (HMAs)—an extremely poor-risk subgroup—and emphasizing the role of novel agents and clinical trials in this disease population.
Clinical Outcomes of Secondary AML with Intensive Induction Therapy
Prospective Clinical Trials
Comparatively few clinical trials have examined novel therapies specifically in s-AML patients (Table 3). s-AML is associated with poor clinical outcomes compared to de novo AML when treated with intensive induction therapy regimens involving anthracycline-based chemotherapy [16,17,18]. A randomized phase 3 trial evaluated 7+3 (cytarabine 200 mg/m2 continuous infusion IV days 1–7, daunorubicin 45 mg/m2 days 1–3) versus cytarabine plus amonafide (a novel topoisomerase II inhibitor) in newly diagnosed s-AML with antecedent MDS (n=215), t-AML with antecedent MDS (n=44), and t-AML without MDS (n=173) [11•]. The median age was 65 and 63 years old, respectively. There were no significant differences in median OS (7.0 months for both arms) or CR rates (45% vs. 46%, respectively) between the two induction strategies. Those with an antecedent MDS had a lower CR rate compared to those with t-AML without prior MDS (40% vs. 50%, respectively; p=0.04). These findings demonstrate the poor overall outcomes of patients with s-AML treated with intensive induction therapy and represent the only randomized phase 3 study performed specifically in this patient population.
A randomized phase 2 study comparing CPX-351 (a liposomal formulation of cytarabine and daunorubicin) to conventional 7+3 was performed in newly diagnosed adults with AML between the ages of 60–75 years [12]. Although this study included both de novo and s-AML patients, the patient population was highly enriched for s-AML (percentage of s-AML patients: CPX-351 38.8%; 7+3 46.3%). Additionally, 39% and 37% of patients on CPX-351 and 7+3, respectively, had prior exposure to HMAs. Among all patients enrolled on this study, overall CR/CRi rates were 66.7% vs. 51.2% in patients treated with CPX-351 vs. 7+3, respectively (p=0.07). However, patients with s-AML appeared to have the most benefit with CPX-351 when compared with 7+3 (CR/CRi rates = 57.6% vs. 31.6%, p=0.06; median OS = 12.1 months vs. 6.1 months, p=0.01, respectively). These findings provided the impetus for the design of a randomized phase 3 study of CPX-351 vs. 7+3 in patients 60–75 years old with newly diagnosed AML-MRC (see the “Intensive Induction Chemotherapy in AML-MRC” section) [13•].
A randomized phase 2 trial of timed sequential alvocidib (cyclin-dependent kinase-9 inhibitor), cytarabine, and mitoxantrone versus 7+3 (with high dose daunorubicin, 90 mg/m2) induction in newly diagnosed AML patients < 70 years with non-favorable cytogenetics revealed overall CR rates of 60% vs. 42%, respectively in s-AML subsets compared with 79% vs. 70%, respectively, in de novo AML [4].
A phase 2 study investigated the clinical activity of G-CLAC (clofarabine 30 mg/m2 IV days 1–5 plus high-dose cytarabine 2 gm/m2 IV days 1–5 with G-CSF priming) in 50 newly diagnosed AML patients 18–64 years old. Of the 50 patients included in this study, 23 (46%) had s-AML or abnormal blood counts for >1 month prior to diagnosis of AML, and 2 (4%) had t-AML [14]. The overall CR rate for the whole cohort of newly diagnosed AML patients was 82%. There was no significant difference seen in overall CR rates between de novo AML and AML with AHD (85% vs. 78%, respectively); however, median OS was significantly decreased in patients with AHD (24 vs. 13 months, respectively; p=0.045).
Retrospective Analyses
Retrospective analyses have also reported poor outcomes with intensive induction chemotherapy for this population (Table 3). A single institution retrospective study by Rizzieri et al. of patients with s-AML who underwent intensive induction (mostly with 7+3) from 1995 to 2008 found that 58% (56/96) achieved CR with a median OS and event-free survival (EFS) of 13.6 months and 8 months, respectively [15]. The median age of patients included in the analysis was 55 years, with 53% having t-AML and 47% harboring a preexisting MDS or MPN. There was no difference in CR rates between those who received 7+3 and those who received other intensive induction regimens. Notably, patients with t-AML had a higher CR rate compared with s-AML arising from either MDS or a MPN (82% vs 62%; p=0.027), although this increase in CR did not translate to improved median EFS or OS.
A retrospective study of 998 AML patients treated with intensive chemotherapy at The University of Texas MD Anderson Cancer Center found that a longer duration of AHD was an independent poor prognostic factor for CR, 8-week mortality, and OS [18]. A more recent analysis of the experience at MD Anderson reported the results of 931 s-AML patients > 60 years old treated from 1990 through 2015 [10]. The median age was 68 years, with 58% having an adverse-risk cytogenetic profile. The CR/CRp rate for all patients regardless of intensity of therapy was 39.5%, with higher rates of CR in those who received intensive induction compared to HMAs or investigational therapies (46% vs. 36% vs. 27%, respectively). The median OS for the entire cohort was only 6 months. Clinical outcomes of patients who received a HSCT (7.1%) were better than those who did not proceed to HSCT (median OS: 16.2 months v. 5.5 months, p<0.001).
Large population-based studies corroborate the dismal outcomes reported by other studies in s-AML. A large Swedish registry (n=3,363) assessed outcomes of 630 patients with s-AML arising from an AHD and 259 patients with t-AML [16]. Compared to de novo disease, patients with s-AML were older (median 73 vs 70 years, p<0.001), had worse performance status, were more likely to have an adverse-risk cytogenetic profile (40% vs 26%, p<0.001), and were less likely to receive intensive treatment (39% vs 64%, p<0.001). Similar trends were seen when comparing t-AML to de novo AML patients. None of the patients with s-AML had favorable-risk cytogenetics. Based on this analysis, CR rates with intensive chemotherapy (n=1967) for those with de novo AML, t-AML, and s-AML were 72%, 54%, and 39%, respectively (p<0.001). On multivariable analysis, s-AML with AHD and t-AML were both independent risk factors for poor OS in patients treated with intensive induction (HR 1.51 and 1.72, respectively), and median OS was 6–7 months regardless of age. Similarly, an analysis of a large Danish registry of AML patients found that s-AML treated with intensive therapy (n=320) was associated with inferior OS compared to de novo AML as well as decreased CR rates (de novo AML: 75%, s-AML with prior MDS: 59%, other s-AML: 54%, t-AML: 61%) [17]. Patients with s-AML were older, less likely to receive intensive induction, and more likely to have intermediate or adverse-risk cytogenetics. Importantly, patients with s-AML were also less likely to enroll in clinical trials (s-AML with prior MDS: 19.6% vs. other AHD: 24.7% vs. de novo: 35.6%; p=<0.001). This is a common theme in trials assessing novel agents in newly diagnosed AML as s-AML patients are more likely excluded due to a high degree of comorbidities, frailty, poor performance status, poor prognosis and low likelihood of achieving a response, and overall treatment nihilism for this poor-risk subgroup [19•].
Intensive Induction Chemotherapy in AML-MRC
Based on subset analyses demonstrating improved outcomes with CPX-351 compared with 7+3 in s-AML from a randomized phase 2 trial of CPX-351 vs. 7+3 in newly diagnosed AML 60–75 years, a randomized phase 3 study of CPX-351 vs. 7+3 was performed in t-AML and AML-MRC [12, 13•]. Eligibility for this study included patients with newly diagnosed AML aged 60–75 years with t-AML, AML from preexisting MDS or CMML (with or without prior HMAs), or AML with MRC based on WHO-defined MDS-related cytogenetic abnormalities. Of note, patients were not eligible if they had a preexisting MPN. Additionally, those with multilineage dysplasia (satisfying criteria for WHO AML-MRC) were not included unless they had a prior history of MDS/CMML or MDS-related cytogenetics. A total of 309 patients were enrolled (CPX-351: n=153, 7+3: n=156). The primary endpoint was OS. Among all patients enrolled, there was a significantly higher overall CR/CRi rate with CPX-351 compared with 7+3 (47.7% vs. 33.3%, p=0.016) and median OS was 9.6 months with CPX-351 versus 6.0 months with 7+3 (p=0.003). One-year and 2-year OS estimates between CPX-351 and 7+3 were 41.5% and 27.6% and 31.1% and 12.3%, respectively. Subset analyses of median OS in patients treated with CPX-351 vs. 7+3 demonstrated differences between groups of enrolled patients (t-AML: 12.1 vs. 6.0 months, AML with antecedent MDS or CMML: 7.4 vs. 6.0 months, and de novo AML with MDS-related cytogenetic abnormalities: 10.1 vs. 7.4 months). The t-AML subgroup appeared to have the greatest overall benefit from CPX-351 compared with 7+3. Notably, 105/309 (34.0%) of patients enrolled on this study had MDS with previous exposure to HMAs prior to enrollment (CPX-351: n=50, 7+3: n=55). In this subgroup of patients, CR rates were 36% vs. 33% and median OS was 5.7 vs. 7.4 months with CPX-351 and 7+3, respectively. In comparison, patients with MDS without previous exposure to HMAs had CR rates of 67% vs. 37% and median OS of 15.7 vs. 5.1 months with CPX-351 vs. 7+3, respectively.
In conclusion, patients with s-AML from antecedent MDS/CMML with previous HMA therapy have dismal outcomes with intensive induction chemotherapy. These data reinforce the overall poor outcomes of patients with AML-MRC treated with intensive chemotherapy.
Clinical Outcomes of Secondary AML with Low-intensity Therapy
Patients who are unfit for intensive induction treatment strategies commonly receive low-intensity strategies such as HMAs, targeted oral therapies, and low-dose cytarabine (LDAC). Clinical trials reporting outcomes of these agents have variably enrolled and reported outcomes of patients with s-AML and those who have received prior HMAs (Table 4).
LDAC
Ara-C (cytarabine), a nucleoside analog that has cytotoxic effects, has been used for decades as a continuous infusion in combination with anthracyclines for induction therapy and with high doses in consolidation but has substantial toxicity in older patients. Lower doses are better tolerated and can be delivered in the outpatient setting. A randomized phase 3 trial compared LDAC versus hydroxyurea/best supportive care in 217 newly diagnosed AML or high-risk MDS patients deemed unfit to receive intensive chemotherapy [20]. Only 13% of all patients survived 1 year. However, median OS was significantly better with LDAC compared with hydroxyurea/best supportive care (OR 0.60, p<0.001, median OS not reported), related primary to the achievement of CR (18% v. 1%, p<0.001). Median OS of patients achieving CR was 575 days compared to only 66 days in patients who did not achieve CR. Of the 217 patients included in this trial, 58 (27%) had s-AML (LDAC: 28 patients). OS favored LDAC over hydroxyurea in this subgroup, though differences were not statistically significant (OR 0.69, 95% CI 0.38–1.24, no median OS provided). OS did not significantly differ between de novo and s-AML patients. Given the overall poor outcomes of patients treated with LDAC, this is uncommonly used in clinical practice for s-AML.
HMAs
DNA methylation of oncogenes and tumor suppressor genes is thought to contribute to the leukemogenesis and survival of leukemia cells. HMAs such as decitabine and azacitidine have been shown to reverse this process and induce normal cellular proliferation. Though HMAs were initially approved for the treatment of higher risk MDS, a post hoc analysis of an MDS trial initially demonstrated the utility of azacitidine for AML patients [32]. This randomized trial comparing azacitidine with conventional care regimens (physician’s choice of best supportive care, LDAC, or induction chemotherapy) was initiated when widespread use of the FAB criteria for MDS included RAEB-T (refractory anemia with excess blasts in transformation, between 20 and 30% blasts). Subsequently, the WHO redefined AML as ≥20% myeloblasts in the marrow or blood. As a result, this trial included a subset of patients with WHO-defined AML with 20–30% blasts. Though this analysis specified patients with oligoblastic AML (20–30% blasts) rather than s-AML, it is likely that many of these patients in fact had s-AML by modern definitions. The analysis demonstrated a significantly prolonged median OS of azacitidine (24.5 months) over conventional care regimens (16 months) with hazard ratio 0.47 (95% CI, 0.28–0.79; p = 0.005).
Subsequently, a randomized multi-center phase 3 study including newly diagnosed AML patients >65 years who were not candidates for HSCT investigated decitabine 20 mg/m2 IV days 1–5 every 28 days versus physician’s choice (LDAC or best supportive care) [21]. Eligibility for this study included de novo (n=312) and s-AML patients (n=171). Patients with favorable-risk cytogenetics or who received prior therapy (including HMAs) were excluded. Median OS of s-AML patients receiving decitabine (n=87) was 7.1 months vs. 4.9 months for physician’s choice (n=84). Median OS was slightly worse among s-AML patients (7.1 months) than for de novo patients (8.2 months, not statistically compared in the study).
A randomized multi-center phase 3 trial evaluated azacitidine versus physician’s choice (LDAC, best supportive care or intensive chemotherapy) for newly diagnosed older (> 65 years) AML patients (> 30% blasts) who were not candidates for HSCT [22]. The study design was similar to the randomized phase III study of decitabine versus physician choice though this study also included an option for intensive chemotherapy in the physician choice arm. Of the 488 patients enrolled, 87 patients (18%) had s-AML due to prior MDS. Median OS was 10.4 months vs. 6.5 months (p=.10) with azacitidine vs. physician choice, respectively, though outcomes were not reported separately for s-AML.
Lastly, guadecitabine, a second-generation HMA whose active metabolite is decitabine, was investigated in an open-label phase 2 study in newly diagnosed AML, relapsed/refractory AML (including prior-HMA therapy), and HMA-naïve MDS who were not candidates for intensive induction therapies [23]. In this study, patients were randomized to receive either 60 or 90 mg/m2 of guadecitabine on days 1–5 every cycle. Thirty-six percent of enrolled patients had s-AML. Patients with s-AML had a non-significant lower likelihood of achieving a response to therapy on regression modelling (univariate 0.68, p=0.36; multivariable 0.81, p=0.34). Survival outcomes were not reported separately for s-AML patients. A randomized phase III study (ASTRAL) of guadecitabine versus physician’s choice (decitabine, azacitidine, or LDAC) in 815 newly diagnosed AML patients (298 with s-AML) who were not eligible for intensive induction was recently reported [24]. A combined primary endpoint of CR and OS in the intention-to-treat population was not reached with guadecitabine versus physician choice. Median OS on guadecitabine was 7.1 months versus 8.5 months for physician’s choice group (p=0.73). Outcomes of s-AML patients were not reported separately, though this study is not yet published. Importantly, patients with s-AML with prior HMA therapy were excluded in these studies.
Targeted Agents
Gemtuzumab ozogamicin (GO), an antibody drug-conjugate targeting CD33, was investigated in a randomized phase 3 study (AML-19) versus best supportive care in newly diagnosed older adults with AML unsuitable for intensive chemotherapy [25]. This study enrolled a total of 237 patients, 73 (31%) of whom had s-AML, though excluded patients who had received prior chemotherapy. Median OS for all patients was 4.9 months with GO vs. 3.6 months in the best supportive care group (HR 0.69, p=0.005). Overall CR rate was 25% in patients with s-AML treated with GO (compared to 28% in de novo AML). There was no difference in OS between secondary and de novo AML patients (HR 0.85, CI 0.64–1.13, p=0.25). Based on this data, GO was FDA-approved for newly diagnosed older adults who are not candidates for intensive chemotherapy though not commonly used as a single agent in this subgroup.
Ivosidenib is an oral, targeted chemotherapy that inhibits isocitrate dehydrogenase 1 (IDH1) and leads to the suppression of the oncometabolite 2-hydroxyglutarate. Ninety-four patients with s-AML were included in the phase 1 dose-escalation trial of ivosidenib for IDH1-mutated patients with relapsed or refractory AML [33]. Outcomes of s-AML patients were not reported separately; overall CR rate was 21.6% and median OS was 8.8 months for all patients included. An expansion cohort from this phase 1 study included newly diagnosed AML with IDH1 mutation unfit for intensive chemotherapy (n=34). Twenty-three patients and 15 patients in this cohort had s-AML and s-AML with prior HMA exposure, respectively [26]. In those with s-AML with prior HMAs, CRh (< 5% bone marrow myeloblasts with both ANC > 500/μl and platelet count > 50 x 109/L CR) and CR was 26.7% and 20.0%, respectively. Median OS was 12.6 months for all patients though OS was not reported separately for s-AML who received prior HMAs.
Enasidenib is an oral inhibitor of mutant isocitrate dehydrogenase-2 (IDH2) which plays a central role in abnormal epigenetic regulation leading to hypermethylation and blocked cellular differentiation. An open-label single-arm study evaluating enasidenib for newly diagnosed AML patients unfit for intensive chemotherapy included 27 patients with an AHD including 17 with prior history of MDS [27]. Median age of patients on this study was 77 years (range: 58–87 years). Five of 23 evaluable patients (22%) achieved an overall response with 3 (13%) achieving a CR. Median OS for patients with an AHD was 8.8 months, slightly less than the 11.3 months for all patients. Outcomes of s-AML patients with a prior exposure to HMAs was not reported.
Glasdegib
Glasedegib is a hedgehog pathway inhibitor that has been investigated in combination with LDAC in newly diagnosed older adults with AML who are not candidates for intensive chemotherapy. In a randomized phase 2 trial comparing LDAC versus LDAC/glasdegib, 88 patients with either AML (n=78) or high-risk MDS (n=10) were treated with combination therapy [28]. Fifteen of 88 (17%) had received prior HMA therapy. Median OS was 8.8 months overall with LDAC/glasdegib compared to 4.1 months with LDAC alone (HR 0.51, p<0.01). Interestingly, median OS of patients with s-AML (n=37) receiving glasdegib plus LDAC was substantially improved over LDAC alone (9.1 months v. 4.1 months, p < 0.001) [34]. Based on these data, glasdegib was FDA-approved for the frontline management of older unfit AML patients in combination with LDAC. However, given the modest clinical activity and comparison to LDAC, this regimen is uncommonly used in clinical practice.
Venetoclax
Venetoclax is an oral inhibitor of BCL2, an anti-apoptotic regulator in AML. Early clinical trials demonstrated modest activity when used as monotherapy in relapsed/refractory AML [35]. However, when combined with LDAC or HMAs, clinical activity appears to be synergistic [36]. In the VIALE-C study, a randomized phase III trial of LDAC plus venetoclax versus LDAC plus placebo in previously untreated AML >75 years or who were ineligible for intensive chemotherapy, 211 patients were randomized; 81 patients (38%) with s-AML were enrolled which included 42 (52% of s-AML) patients who had received prior HMAs [29•]. Median OS for patients receiving venetoclax with LDAC was 7.2 months as compared to only 4.1 months for those treated with LDAC plus placebo (HR 0.75, p=0.11). Median OS for patients with s-AML receiving venetoclax (n=58) was 5.5 months (versus 3.2 months for those receiving LDAC); overall CR/CRi was 36%. For s-AML patients who received prior HMAs (n=28), median OS was also 5.5 months (versus 4.1 months for those receiving LDAC) and CR/CRi was 25%. De novo AML patients had better outcomes compared with s-AML (hazard ratio=0.59; p=0.004).
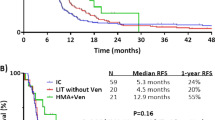
The VIALE-A study, in contrast, was a randomized phase 3 study of azacitidine plus venetoclax versus azacitidine plus placebo in previously untreated AML patients >75 years or who were ineligible for intensive induction therapy [30•]. The median OS was 14.7 months in the azacitidine–venetoclax group (n=286) and 9.6 months in the control group (n=145) (HR 0.66, p<0.001). 107 patients with either s-AML or t-AML (history of MDS or CMML (n=72), t-AML (n=35)) were included in the trial. Patients who had received prior chemotherapy for either MDS or CMML were excluded. Median OS was 16.4 months (95% CI, 9.7–24.4) for s-AML/t-AML patients receiving azacitidine plus venetoclax (n=72) vs. 10.6 months (95% CI, 4.9–13.2) with azacitidine plus placebo (n=35) (HR, 0.56; 95% CI 0.35–0.91). Interestingly, the reported median OS for this combined group of patients (s-AML and t-AML) receiving azacitidine plus venetoclax appeared longer than in patients with de novo AML (16.4 v. 14.1 months, no direct statistical comparison). CR/CRi among s-AML patients receiving azacitidine plus venetoclax was 66.7% (versus 22.9% for patients receiving azacitidine alone). Venetoclax has also been investigated in combination with intensified decitabine (20 mg/m2 IV days 1–10) in a cohort of patients with both newly diagnosed and relapsed/refractory AML in a single-center phase 2 trial [31]. This non-randomized study included 168 patients with AML: 70 patients with de novo disease, 43 patients with s-AML (15 who had not received prior therapy and 28 who had received prior chemotherapy including HMAs), and 55 patients with relapsed/refractory disease. CR/CRi for untreated and previously treated s-AML patients was 80% and 61%, respectively. Median OS was 7.8 months (CI: 2.9 to 10.7) in untreated s-AML patients and 6.0 (CI: 3.4 to 13.7) months in treated s-AML patients. Among the 50 patients included who had received prior HMA therapy (in either the secondary AML (n=25) or relapsed/refractory cohort (n=25)) ORR was 60% (combined CR 16%, CRi 24%, and MLFS of 20%); MRD-negativity was achieved in 48%; median OS was 6.0 months with 27% 1-year overall survival. On univariate analysis, Cox proportional hazards ratio (HR) for OS of prior HMA therapy was 2.00 (CI: 1.3–3.06, p < 0.01); multivariate analysis showed a HR of 2.4.
Clinical Outcomes of s-AML After Previous Exposure to HMAs
HMAs represent a standard-of-care first-line treatment strategy for high-risk MDS and are frequently used for CMML and other myeloid malignancies. Therefore, a high proportion of s-AML patients have had previous exposure to HMAs and have limited therapeutic options if they progress to AML given the widespread clinical use of HMAs with or without other agents in this setting. In fact, azacitidine plus venetoclax represents a paradigm shift and new standard-of-care for older patients (>75 years) with newly diagnosed AML who are not candidates for intensive chemotherapy. However, the poor outcomes seen with non-HMA-based strategies such as LDAC plus venetoclax in patients with s-AML after prior HMAs highlight the unmet clinical need in this patient population [37,38,39,40]. Furthermore, as discussed in the “AML with MRC” section, the randomized phase 3 study establishing CPX-351 as a standard-of-care option for patients with AML-MRC revealed dismal outcomes with CPX-351 in patients with s-AML with prior HMA therapy and no benefit compared with 7+3 in subset analyses [13•].
Retrospective analyses support the poor outcomes seen prospectively with conventional chemotherapy in patients with s-AML after prior HMA exposure (Table 5). A retrospective review at MD Anderson found that s-AML patients (n=254, 53 with t-AML) with a prior diagnosis of MDS, MPN, or aplastic anemia who had received at least one therapy for that diagnosis had significantly worse OS than those with an untreated AHD (n=215; OS 4.2 vs 9.2 months; p=0.001) [8]. This difference was found to be independent of age, cytogenetic risk, or treatment intensity. CR rates were also significantly lower in patients with s-AML previously treated with HMAs (32%) compared with t-AML (67.6%) and de novo AML (79.5%) (p<0.001). Most (73%) of these patients with previously treated AHD received HMAs. The previously treated s-AML group was also more frequently older with adverse-risk cytogenetics. A smaller retrospective study found that when patients with s-AML and a history of MDS or MDS/MPN were given an anthracycline-based induction therapy, those previously treated with a HMA or lenalidomide (n=25) had an inferior response rate and median OS compared to those who received supportive care alone for their AHD (n=36; CR/CRi: 32% vs 78%, OR 0.13, 95% CI, 0.04–0.42, p=0.001; OS: 3.7 vs 10.5 months, p<0.001) [41]. The majority of the patients in the previously treated group had received a HMA (24/25; 96%). A multi-center retrospective analysis examined 241 patients with s-AML who received prior HMA therapy for an AHD (MDS or CMML) and was treated with a variety of intensive induction chemotherapy regimens [42]. This analysis revealed that the CR/CRi rates for CLAG/M (cladribine, cytarabine, G-CSF, mitoxantrone; n=60), 7+3 (n=30), and CPX-351 (n=14) were 53%, 32%, and 41.2%, respectively, and that there was no significant difference in median OS among the three groups (approximately 7 months) highlighting the poor outcomes seen with any intensive induction strategy. Along these lines, a case-control study of patients with s-AML arising from MDS treated with a HMA compared outcomes of CLAG/M (n=28) to standard 7+3 induction (n=24) [43]. CLAG/M yielded a higher ORR (64% vs. 29%; p=0.014) and median OS (202 days vs. 86 days; p=0.025) than 7+3 and has encouraging clinical activity in this patient population.
Future directions
Given the highly unfavorable prognosis of s-AML, it would be preferable to prevent the evolution of antecedent hematologic disorders to AML rather than treating advanced leukemia that has developed mechanisms of resistance. Advances in sequencing technologies and analyses of clonal evolution continue to refine our understanding of the biological continuum between antecedent hematologic diseases and s-AML. Accordingly, diagnostic criteria and prognostic scoring systems will certainly progress and influence the management of these patients. For example, knowledge of the details of genetic progression gained from single-cell sequencing techniques and studies involving paired samples of MDS and s-AML from the same patient raises the prospect of using molecular signatures to serially monitor clonal changes with the goal of earlier recognition, treatment, and perhaps prevention of progression to AML [44]. Such knowledge, coupled with expanding access to alternative stem cell sources for HSCT may augment our ability to prevent s-AML. Furthermore, as our ability to map the progression from AHDs to s-AML improves, our capability to recognize cases of AML that have arisen from a previously undiagnosed AHD also improves. This capacity has implications for the appropriateness of HSCT, therapies directed toward s-AML, and clinical trials seeking to enroll such patients. Mutations in spliceosome genes, BCOR, STAG2, and EZH2 are noted to be highly enriched in AML that has evolved from MDS without a known prior diagnosis of MDS, and it is likely that such molecular data will be incorporated into future diagnostic schemas [5, 45]. Greater understanding of the key sequences and driver mutations behind progression to s-AML may lead to the identification of critical pathways to target therapeutically.
Despite the recognized need, there remains a paucity of clinical trials focusing on the s-AML patient population (Table 6). Although CPX-351 is an FDA-approved option for patients with AML-MRC of all ages, the phase 3 trial only enrolled patients ages 60–75. There is an ongoing phase 2 trial investigating CPX-351 in patients ≤60 years (NCT04269213; Table 6) [13•]. CLAG-M has demonstrated encouraging clinical activity in retrospective studies of s-AML patients leading to the design of an ongoing phase 2 trial of CLAG-M in newly diagnosed s-AML with antecedent MDS (NCT03150004; Table 6) [42, 43]. There is also an ongoing trial of CD8+ depleted, non-engrafting, HLA-mismatched unrelated donor lymphocytes in combination with cytarabine-based induction chemotherapy in patients 60–79 years with newly diagnosed AML with AHD (NCT04620681; Table 6).
Another area in need of further research is s-AML arising from MPNs. The classification of AML-MRC (and by extension the studies of CPX-351) does not include these patients, and there is currently no standard-of-care for these patients. Even with HSCT, 5-year survival rates of 10% have been observed in this poor-risk subgroup [46]. Similar to s-AML arising from MDS, further elucidating the unique leukemogenesis of MPNs will hopefully lead to new targets as well as improved prognostic and diagnostics systems, which are already beginning to incorporate molecular information [47,48,49]. Given the known efficacy of JAK2 inhibitors for the treatment of MPNs, ongoing phase 1/2 trials are investigating ruxolitinib in combination with standard cytarabine-based and CPX-351 induction and consolidation regimens followed by ruxolitinib maintenance in s-AML evolving from a MPN (NCT03558607, NCT03878199, Table 6). A phase 2 trial of decitabine and ruxolitinib has demonstrated encouraging activity in accelerated and blast phase MPNs, and an ongoing phase 2 study is evaluating decitabine in combination with either ruxolitinib or fedratinib as a bridge to HSCT in MDS or AML arising from a MPN or MDS/MPN overlap syndrome (NCT04282187; Table 6) [50]. Furthermore, based on preclinical and retrospective data of combined JAK2 and IDH inhibition, a trial of ruxolitinib with enasidenib in IDH2 mutated s-AML from an antecedent MPN is planned (NCT04281498; Table 6) [51, 52]. The MDM2 inhibitor KRT-232 is also being studied in combination with low-dose cytarabine or decitabine in a phase 1B/2 trial of newly diagnosed or R/R AML secondary to MPN (NCT04113616; Table 6). Based on promising phase 1 data, an ongoing randomized phase 2 trial is investigating whether the addition of veliparib to topotecan and carboplatin induction therapy in newly diagnosed or R/R s-AML arising from an antecedent MPN improves clinical outcomes (NCT03289910; Table 6) [53].
Targeted therapies are notably under-represented in currently enrolling trials that specifically enroll s-AML. The mutations that frequently characterize s-AML have been notoriously difficult to target pharmacologically. For example, the spliceosome modulator H3B-8800 demonstrated the ability to induce lethality in preclinical models of myeloid neoplasms with mutations in genes encoding for the spliceosome machinery, but displayed a striking lack of activity in a phase I/II trial enrolling patients with myeloid malignancies harboring the same mutations [54, 55]. Furthermore, though EZH2 inhibitors have demonstrated efficacy in lymphomas with activating mutations in EZH2, myeloid malignancies tend to harbor loss-of-function mutations in EZH2, rendering such inhibitors less likely to benefit s-AML patients. Despite the disappointing results in targeting these mutations, several studies suggest encouraging developments in the treatment of TP53-mutated leukemias, a relatively common mutation in s-AML. For instance, an ongoing phase Ib trial of azacitidine with the anti-CD47 monoclonal antibody magrolimab, a macrophage checkpoint inhibitor, has demonstrated a 71% objective response rate with median duration of response of 9.9 months among a subset (n=21) of AML patients with mutated TP53 [56]. Furthermore APR-246, a small molecule that preclinically restores function of mutation-inactivated TP53 has demonstrated synergy with azacitidine and an overall response rate of 87% with significant prolongation of OS in responding patients (12.8 vs. 3.9 months for non-responding patients, p<0.001) [57]. Although promising, both these agents are chiefly being developed in combination with azacitidine, a strategy not likely to benefit s-AML patients who have previously been treated with HMAs. There remains a critical need to develop novel therapeutic combinations for s-AML patients previously treated with HMAs.
Finally, the reasons why s-AML arising after treatment with HMAs represents a prognostically distinct high-risk disease category are not known, but efforts to understand the biology of this clinical phenotype may lead to insights regarding treatment. One hypothesis is that the cytotoxic pressure exerted by HMAs may impact clonal evolution by selecting for resistant or aggressive subclones and have been shown to induce potentially pathogenic C>G transversions [58, 59]. Other hypotheses include metabolic changes resulting in decreased cytarabine incorporation and resistance, upregulation of multidrug resistance protein-1 (MDR1) via promoter hypomethylation, and increased expression of immune checkpoints [60,61,62]. There are currently no clinical trials focused on this subgroup of patients, and it represents one of the highest unmet needs in the field.
Conclusions
Overall, these findings suggest that s-AML with previous HMA therapy represents a distinct biologic subgroup of s-AML. Despite the expansion of therapeutic options across AML, patients with s-AML with prior HMA exposure have not yet achieved substantial benefit from novel therapies and continue to have particularly dismal clinical outcomes with both intensive and non-intensive strategies. These patients are vastly under-represented in recent and current clinical trials. There remains an urgent, unmet need for novel therapeutic agents in this subgroup.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–21.
Zeidner JF, Foster MC, Blackford AL, Litzow MR, Morris LE, Strickland SA, et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100(9):1172–9.
Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125(9):1367–76 This analysis is the first to specifically define secondary AML by the presence of specific molecular mutations.
Higgins A, Shah MV. Genetic and genomic landscape of secondary and therapy-related acute myeloid leukemia. Genes (Basel). 2020;11(7):749.
Borthakur G, Lin E, Jain N, Estey EE, Cortes JE, O’Brien S, et al. Survival is poorer in patients with secondary core-binding factor acute myelogenous leukemia compared with de novo core-binding factor leukemia. Cancer. 2009;115(14):3217–21.
Boddu P, Kantarjian HM, Garcia-Manero G, Ravandi F, Verstovsek S, Jabbour E, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv. 2017;1(17):1312–23.
Kennedy JA, Atenafu EG, Messner HA, Craddock KJ, Brandwein JM, Lipton JH, et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood. 2013;121(14):2725–33.
Boddu PC, Kantarjian HM, Ravandi F, Garcia-Manero G, Verstovsek S, Jabbour EJ, et al. Characteristics and outcomes of older patients with secondary acute myeloid leukemia according to treatment approach. Cancer. 2017;123(16):3050–60.
Stone RM, Mazzola E, Neuberg D, Allen SL, Pigneux A, Stuart RK, et al. Phase III open-label randomized study of cytarabine in combination with amonafide L-malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia. J Clin Oncol. 2015;33(11):1252–7 This is the first randomized phase 3 study specifically done in a secondary AML patient population. Ultimately, there was no significant difference in outcomes between cytarabine + amonafide versus cytarabine + daunorubicin in newly diagnosed secondary AML.
Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–46.
Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–92 This landmark randomized phase 3 study revealed that CPX-351 led to significantly improved overall survival and clinical outcomes compared with 7+3 in newly diagnosed older adults with secondary AML or AML with MDS-Related Changes rednering CPX-351 a new standard-of-care for this patient population.
Becker PS, Medeiros BC, Stein AS, Othus M, Appelbaum FR, Forman SJ, et al. G-CSF priming, clofarabine, and high dose cytarabine (GCLAC) for upfront treatment of acute myeloid leukemia, advanced myelodysplastic syndrome or advanced myeloproliferative neoplasm. Am J Hematol. 2015;90(4):295–300.
Rizzieri DA, O'Brien JA, Broadwater G, Decastro CM, Dev P, Diehl L, et al. Outcomes of patients who undergo aggressive induction therapy for secondary acute myeloid leukemia. Cancer. 2009;115(13):2922–9.
Hulegårdh E, Nilsson C, Lazarevic V, Garelius H, Antunovic P, Rangert Derolf Å, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90(3):208–14.
Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641–9.
Kantarjian H, O'Brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090–8.
Borate U, Norris BA, Statler A, Fu R, Bucy T, Sekeres MA. Representation of therapy-related myelodysplastic syndrome in clinical trials over the past 20 years. Blood Adv. 2019;3(18):2738–47 This important analysis revealed the underrepresentation of therapy-related MDS on prospective clinical trials reinforcing the importance of inclusion of this patient population and the lack of generalizability of our treatments for this patient population.
Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114–24.
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–7.
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–9.
Kantarjian HM, Roboz GJ, Kropf PL, Yee KWL, O’Connell CL, Tibes R, et al. Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase 1/2 trial. Lancet Oncol. 2017;18(10):1317–26.
Fenaux P, Gobbi M, Kropf PL, Mayer J, Roboz GJ, Döhner H, et al. S879 Results of ASTRAL-1 study, a phase 3 randomized trial of guadecitabine (G) vs treatment choice (TC) in treatment naïve acute myeloid leukemia (TN-AML) not eligible for intensive chemotherapy (IC). HemaSphere. 2019;3(S1):394–5.
Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972–9.
Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463–71.
Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33(11):2575–84.
Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–89.
Wei AH, Montesinos P, Ivanov V, CD DN, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–45 This randomized phase 3 study compared LDAC + venetoclax versus LDAC + placebo in newly diagnosed older or unfit AML patients who are not candidates for intensive chemotherapy. Although response rates were higher with LDAC + venetoclax vs. LDAC + placebo, overall survival improvements were more modest and not statistically significant. Further, those with prior HMAs had dismal outcomes with LDAC + venetoclax.
CD DN, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–29 This landmark randomized phase 3 study established azacitidine + venetoclax as a new standard-of-care in newly diagnosed older or unfit AML patients who are not candidates for intensive chemotherapy. Azacitidine + venetoclax led to significant improvement in overall survival and clinical outcomes compared with azacitidine + placebo. However, patients previously treated with HMAs for MDS or other myeloid malignancies were excluded on this study; thus, these results are not generalizable to secondary AML with prior HMA treatment.
DiNardo CD, Maiti A, Rausch CR, Pemmaraju N, Naqvi K, Daver NG, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7(10):e724–e36.
Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–9.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–98.
Heuser M, Robak T, Montesinos P, Leber B, Fiedler WM, Pollyea DA, et al. Glasdegib (GLAS) plus low-dose cytarabine (LDAC) in AML or MDS: BRIGHT AML 1003 final report and four-year overall survival (OS) follow-up. J Clin Oncol. 2020;38(15_suppl):7509.
Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and biological correlates of response in a phase ii study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–17.
Konopleva M, Letai A. BCL-2 inhibition in AML: an unexpected bonus? Blood. 2018;132(10):1007–12.
Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–40.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32.
Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–803.
Lübbert M, Suciu S, Baila L, Rüter BH, Platzbecker U, Giagounidis A, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29(15):1987–96.
Bello C, Yu D, Komrokji RS, Zhu W, Wetzstein GA, List AF, et al. Outcomes after induction chemotherapy in patients with acute myeloid leukemia arising from myelodysplastic syndrome. Cancer. 2011;117(7):1463–9.
Talati C, Goldberg AD, Przespolewski A, Chan O, Ali NA, Kim J, et al. Comparison of induction strategies and responses for acute myeloid leukemia patients after resistance to hypomethylating agents for antecedent myeloid malignancy. Leuk Res. 2020;93:106367.
Jaglal MV, Duong VH, Bello CM, Al Ali NH, Padron E, Fernandez HF, et al. Cladribine, cytarabine, filgrastim, and mitoxantrone (CLAG-M) compared to standard induction in acute myeloid leukemia from myelodysplastic syndrome after azanucleoside failure. Leuk Res. 2014;38(4):443–6.
Menssen AJ, Walter MJ. Genetics of progression from MDS to secondary leukemia. Blood. 2020;136(1):50–60.
Yokoyama K, Shimizu E, Yokoyama N, Nakamura S, Kasajima R, Ogawa M, et al. Cell-lineage level-targeted sequencing to identify acute myeloid leukemia with myelodysplasia-related changes. Blood Adv. 2018;2(19):2513–21.
Tefferi A, Mudireddy M, Mannelli F, Begna KH, Patnaik MM, Hanson CA, et al. Blast phase myeloproliferative neoplasm: Mayo-AGIMM study of 410 patients from two separate cohorts. Leukemia. 2018;32(5):1200–10.
Grinfeld J, Nangalia J, Baxter EJ, Wedge DC, Angelopoulos N, Cantrill R, et al. Classification and personalized prognosis in myeloproliferative neoplasms. N Engl J Med. 2018;379(15):1416–30.
Tefferi A, Guglielmelli P, Lasho TL, Gangat N, Ketterling RP, Pardanani A, et al. MIPSS70+ Version 2.0: mutation and karyotype-enhanced international prognostic scoring system for primary myelofibrosis. J Clin Oncol. 2018;36(17):1769–70.
Tefferi A, Guglielmelli P, Nicolosi M, Mannelli F, Mudireddy M, Bartalucci N, et al. GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia. 2018;32(7):1631–42.
Mascarenhas JO, Rampal RK, Kosiorek HE, Bhave R, Hexner E, Wang ES, et al. Phase 2 study of ruxolitinib and decitabine in patients with myeloproliferative neoplasm in accelerated and blast phase. Blood Adv. 2020;4(20):5246–56.
McKenney AS, Lau AN, Somasundara AVH, Spitzer B, Intlekofer AM, Ahn J, et al. JAK2/IDH-mutant-driven myeloproliferative neoplasm is sensitive to combined targeted inhibition. J Clin Invest. 2018;128(2):789–804.
Cahill K, Patel AA, Liu H, Gurbuxani S, Thirman M, Kosuri S, et al. Outcomes of IDH-mutated advanced phase Ph-negative myeloproliferative neoplasms treated with idh inhibitors. Blood, 2019;134(Supplement_1):4176.
Pratz KW, Rudek MA, Gojo I, Litzow MR, McDevitt MA, Ji J, et al. A phase I study of topotecan, carboplatin and the PARP inhibitor veliparib in acute leukemias, aggressive myeloproliferative neoplasms, and chronic myelomonocytic leukemia. Clin Cancer Res. 2017;23(4):899–907.
Seiler M, Yoshimi A, Darman R, Chan B, Keaney G, Thomas M, et al. H3B-8800, an orally available small-molecule splicing modulator, induces lethality in spliceosome-mutant cancers. Nat Med. 2018;24(4):497–504.
Steensma DP, Wermke M, Klimek VM, Greenberg PL, Font P, Komrokji RS, et al. Results of a clinical trial of H3B-8800, a splicing modulator, in patients with myelodysplastic syndromes (MDS), acute myeloid leukemia (AML) or chronic myelomonocytic leukemia (CMML). Blood, 2019;134(Supplement_1):673.
Sallman D A A A, Kambhampati S, Al Malki MM, Zeidner JF, Donnellan W, et al. The first-in-class anti-CD47 antibody magrolimab combined with azacitidine is well-tolerated and effective in AML patients: phase 1b results. Blood. 2020.
Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Phase 2 results of APR-246 and azacitidine (AZA) in patients with TP53 mutant myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia (AML). Blood. 2019;134(Supplement_1):676.
Uy GL, Duncavage EJ, Chang GS, Jacoby MA, Miller CA, Shao J, et al. Dynamic changes in the clonal structure of MDS and AML in response to epigenetic therapy. Leukemia. 2017;31(4):872–81.
Jacoby MA, Duncavage EJ, Chang GS, Miller CA, Shao J, Elliott K, et al. Subclones dominate at MDS progression following allogeneic hematopoietic cell transplant. JCI Insight. 2018;3(5).
Qin T, Jelinek J, Si J, Shu J, Issa JP. Mechanisms of resistance to 5-aza-2'-deoxycytidine in human cancer cell lines. Blood. 2009;113(3):659–67.
Kantharidis P, El-Osta A. deSilva M, Wall DM, Hu XF, Slater A, et al. Altered methylation of the human MDR1 promoter is associated with acquired multidrug resistance. Clin Cancer Res. 1997;3(11):2025–32.
Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280–8.
Funding
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
DRR- None
SDG- None
MCF has served as a consultant for Daiichi-Sankyo and Macrogenics and has received institutional research funding from Bellicum Pharmaceuticals and Macrogenics.
JFZ has received honoraria from Agios, Bristol-Myers Squibb/Celgene, Daiichi-Sankyo, Genentech, Pfizer, and Takeda; has served as a consultant for AbbVie, AsystBio Laboratories, Celgene, and Takeda; and has received institutional research funding from AROG, Forty Seven, Merck, Sumitomo Dainippon Pharma, and Takeda.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Acute Myeloid Leukemias
Rights and permissions
About this article
Cite this article
Richardson, D.R., Green, S.D., Foster, M.C. et al. Secondary AML Emerging After Therapy with Hypomethylating Agents: Outcomes, Prognostic Factors, and Treatment Options. Curr Hematol Malig Rep 16, 97–111 (2021). https://doi.org/10.1007/s11899-021-00608-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00608-6