Abstract
Purpose of Review
The ability to detect minimal residual disease (MRD) in myeloma has improved due to advances in flow cytometry and sequencing methodologies. Here, we evaluate recent clinical trial data and explore the current and future roles of MRD assessment in the context of clinical trial design and clinical practice.
Recent Findings
A review of recent phase III studies reveals that achievement of MRD negativity is associated with improved progression-free survival (PFS) and/or overall survival (OS). Treatment arms that are more effective from a PFS or overall response rate perspective are also associated with superior MRD negativity rates. The current standard MRD methodologies are limited by requiring bone marrow samples and refinement of methodologies that can detect disease outside of the bone marrow is needed.
Summary
Currently, MRD is a prognostic biomarker and further efforts are required to determine whether it can serve as a surrogate endpoint. The use of MRD status to guide treatment decisions is currently not recommended outside the confines of a clinical trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to advances in therapeutics over the past several decades, more patients with multiple myeloma are achieving complete responses (CR), translating to improved survival [1]. Advances in technologies that enable detection of minimal residual disease (MRD) coupled with substantial data demonstrating achievement of MRD negativity is associated with improved survival outcomes [2••] has led to considerable interest in the routine incorporation of MRD assessment into clinical practice and clinical trial design. MRD status is now included in the International Myeloma Working Group (IMWG) response criteria [3••]. However, the ability of MRD to serve as a surrogate endpoint or to guide treatment decisions is not yet determined. We evaluate the current status of MRD assessment in myeloma, review the recent literature which has incorporated MRD testing into major clinical trials, and discuss what the future may hold for the role of MRD in myeloma.
Definition of MRD
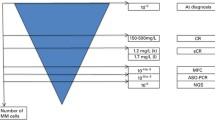
One of the first reports to discuss MRD in myeloma was published in 1993 and the authors used a PCR-based technique to evaluate immunoglobulin gene “fingerprinting” to try to determine whether patients in CR following allogeneic transplant were cured [4]. Since that time, numerous studies have been published utilizing different methodologies with varying sensitivities to assess MRD status. As these cross-trial differences in MRD assessment make it difficult to compare results, the IMWG focused on developing consensus criteria for both MRD assessment and for incorporating MRD status into the response criteria [3••]. With respect to the former, two methods utilizing bone marrow aspirate samples are currently considered standard: multiparametic flow cytometry (MFC) analysis (aka next-generation flow (NGF)) and next-generation sequencing (NGS). If MFC is to be utilized, then a validated method such as the eight-color, two-tube method should be used, as per the established EuroFlow procedure [5]. It is further specified that a minimum of five million cells are to be analyzed and the MFC method should have a sensitivity of at least 1 in 105 plasma cells. For NGS, the IMWG specified that a validated assay such as Lympho-SIGHT (Sequenta Inc., now Adaptive Biotechnologies) with a minimum sensitivity of 1 × 10−5 be utilized. Recently, the US Food and Drug Administration (FDA) granted De Novo designation for the clonoSEQ assay (Adaptive Biotechnologies) for the detection and monitoring of MRD in patients with myeloma or B cell acute lymphoblastic leukemia (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622004.htm). The clonoSEQ assay has a sensitivity of 1 × 10−6. One important limitation of evaluating MRD status by bone marrow aspirate analysis is that it is not uncommon for myeloma to be characterized by multifocal lesions and extramedullary disease [6]. Therefore, the IMWG also defined a category of “Imaging plus MRD-negative” in which patients are determined to be MRD negative in the bone marrow by either MFC or NGS, but also have achieved PET/CT-negativity. Finally, the concept of “sustained MRD negativity” was introduced as achieving imaging plus MRD negativity in assessments that are a minimum of 1 year apart.
Current Techniques for MRD Detection
Multiparametric Flow Cytometry
MFC can be used to detect the presence of plasma cells in bone marrow aspirate samples and to discriminate between normal and aberrant plasma cells [7•]. CD38 expression has generally been considered to be the most reliable marker for detecting plasma cells via flow cytometry, although it is expressed at lower levels on other hematopoietic cells [7•, 8]. While CD138 has also proven to be an important plasma cell marker [9], CD138 expression on other bone marrow populations has been observed as has downregulation following exposure of samples to heparin [7•]. The combined use of CD38 and CD138 is recommended for identifying plasma cells [7•]. The normal plasma cell phenotype is one which is lacking in CD20, CD22, and surface membrane immunoglobulins (smIg) and is heterogeneous for CD19, CD45b, and CD56 [7•]. In contrast, the phenotype of aberrant plasma cells can be characterized by the aberrant expression pattern of CD19, CD56, CD45, CD38, CD27, CD20, CD28, CD33, CD117, and smIg [7•]. However, variation in antigenic expression is common and should be taken into consideration when interpreting such flow cytometric data.
The goal of the International Myeloma Foundation’s Black Swan Research Initiative was to develop a consensus methodology for the detection of aberrant plasma cells by MFC and this work has led to the EuroFlow panel [7•, 10•]. This panel consists of two 8-color tubes (tube 1: CD138, CD27, CD38, CD56, CD45, CD19, CD117, CD81; tube 2: CD138, CD27, CD38, CD56, CD45, CD19, cIgκ, cIgλ). While there is agreement within the field regarding the identity of the epitopes to be analyzed, other variables such as the number of tubes, the commercial source of the antibodies, and preparation of the sample continue to be explored. A single ten-color tube methodology was recently reported [11]. Another group has compared their MFC methodology (“BuffaFlow”) to the EuroFlow methodology [12]. While the same epitopes are analyzed, there are differences with respect to when red blood cells are lysed. It was determined that although the bulk pre-lysis method used in the EuroFlow procedure is slightly less expensive, it does require a dedicated technologist and it significantly decreases CD138 intensity. While CD45, CD56, CD19, CD81, CD27, and CD117 were found to be insensitive to pre-lysis, the intensity of CD138 was reduced by approximately 25-fold following the bulk lysis procedure. The BuffaFlow methodology utilizes antibody incubation prior to red blood cell lysis.
There are limitations to using MFC. The quality of output depends directly on the quality of the bone marrow aspirate. Hemodilution, sampling error, and fragility of the malignant population can lead to false-negative results. It should also be noted that CD38-negative relapse has been reported following daratumumab therapy [13] and this could also result in false-negative results.
Next-generation Sequencing
NGS utilizes locus-specific primers for IGH-VDJH, IGH-DJH, or IGK. In comparison to allele-specific oligonucleotide (ASO)-PCR, this technique does not require the use of patient-specific primers. However, baseline bone marrow samples are still required in order to identify the dominant clonotype. The sensitivity of this technique can reach 10−6 [14]. The reported applicability of this technique is more than 90% [14,15,16]. There is considerable interest in determining whether this technique could be applied to the peripheral blood, obviating the need for an invasive procedure. A recent study compared NGS MRD results from the bone marrow and from circulating tumor (ct) cells in the peripheral blood [17•]. There was only 49% consistency between paired plasma and bone marrow results and the negative predictive value of MRD assessment of ctDNA was only 36%; thus, currently, this technology cannot be applied to the peripheral blood [17•].
PET/CT and MRI for MRD
Multiple myeloma does not always infiltrate the bone marrow in a homogeneous fashion. In fact, about 60% of patients show focal lesions representing local accumulations of plasma cells [18]. Therefore, the IMWG has recognized whole body imaging as a valuable addition to the MRD assessments described above [3••]. Imaging can not only provide information on the bone marrow but also of the soft tissue.
While magnetic resonance imaging (MRI) has high sensitivity, particularly in early stages of the disease and for myeloma characterized by diffuse infiltration, PET-CT provides information about the vitality of the focal lesions and is therefore currently considered the standard of care to assess residual infiltration after therapy [19]. Multiple groups have independently shown the prognostic and predictive benefit of fluorodeoxyglucose (FDG) PET-CT [20,21,22]. The recent study by Moreau et al. also compared PET-CT and MRI and found that there was no difference between the two techniques in detecting bone lesions at diagnosis, but that normalization of PET-CT, but not MRI, after therapy was predictive of PFS and OS [20]. A functional MRI technique called diffusion-weighted imaging might however challenge the superiority of PET-CT in the future, since two independent studies in small numbers of patients have shown a higher sensitivity [23, 24]. The sensitivity of PET-CT in myeloma can be limited by low expression of hexokinase-2 [25] and better PET tracers than the standard FDG are in development [26]. In addition, an antibody-based tracer directed against CD38 appears particularly promising at this time [27].
Evolving MRD Assays
As noted above, there has been interest in the detection of ctDNA in the blood as a means to measure residual disease. Due to generally low levels of circulating myeloma cells and the clonal heterogeneity of this disease, it has thus far proven difficult to utilize genomic approaches to measure MRD in the blood. There has been significant variability in the reported yield of cell-free DNA (cfDNA) extracted from the blood of myeloma patients, which has limited the applicability of this technique [28].
Mass spectrometry-based methodologies for measuring residual monoclonal proteins are being developed [29]. The clonotypic peptide method involves the identification of unique peptides from digested immunoglobulins that can be followed over time. This method has a reported detection limit of 0.0001 g/dL which translates to a 2000-fold improvement in sensitivity compared to the standard serum protein electrophoresis [19]. Limitations of this method include complexity of the workflow leading to prolonged turn-around time, expense, and potential issues with identifying the patient-specific peptide, termed the M-protein complementarity determining region (CDR) [29]. The monoclonal immunoglobulin rapid accurate molecular mass (miRAMM) method uses the accurate molecular mass of the intact light chain as the marker of the disease [30]. This method can differentiate between endogenous monoclonal protein and exogenous monoclonal antibody drugs [29].
Review of MRD Analysis in Recent Phase III Trials
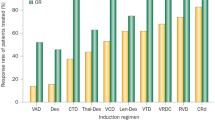
Several review articles have summarized the results from older studies which reported MRD status [3••, 31] and the consistent observation has been that achievement of MRD negativity is associated with improved PFS and/or OS outcomes. An analysis of 609 newly diagnosed patients enrolled in three PETHEMA/GEM clinical trials revealed that MRD negativity was associated with prolonged PFS and OS and that MRD negativity was more prognostic for PFS/OS than traditional CR [32]. Landgren et al. conducted a meta-analysis of studies published between 1990 and 2016 and concluded that achievement of MRD negativity is associated with superior PFS and OS [33]. Similar findings were reported in a second meta-analysis which gathered “real-world” data by adding patients who received older combination of therapies along with older methods (and likely less sensitive) of performing flow cytometry-based MRD assays [2]. In Table 1, we summarize the key phase III studies published/presented within the last 3 years that reported MRD analysis. Despite the established guidelines for MRD assessment [3••], there continues to be heterogeneity with respect to how MRD is assessed and at what level of sensitivity. However, what is evident from these more recent studies is that the more effective treatments (as evaluated by traditional response rates or PFS) are associated with higher rates of MRD negativity and that regardless of how MRD negativity is achieved, it is associated with superior survival outcomes.
Thus far there are limited data to determine whether how quickly MRD negativity is achieved correlates with survival outcomes or whether it is simply achievement of MRD negativity, regardless of the duration of treatment needed to reach that state, which is important long-term. A recent analysis of the IFM 2009 study did report that the PFS and OS of patients who were MRD negative at both tested time points (prior to maintenance and after completion of 12 months of maintenance) were similar to those who became MRD negative at the second time point [36]. While it is tempting to interpret these data as providing evidence that it does not matter how long it takes to achieve MRD negativity, it should also be noted that those patients who were MRD negative at the first time point, but then became MRD positive at the second time point, had inferior survival [36]. Thus, it is evident that more data related to timing and duration of MRD negativity are needed in order to fully understand the prognostic significance.
Current State of MRD in Trial Design
Currently, MRD is considered a prognostic biomarker, defined as a biomarker that can help identify patients at higher risk of adverse disease-related outcomes [42]. This is in contrast to response biomarkers which are defined as being dynamic assessments that show a biological response has occurred in a patient receiving a therapeutic intervention [43]. There is considerable interest in establishing MRD as a surrogate endpoint for OS as it would allow for the use of an endpoint with a much earlier read-out. However, there are a number of issues which must first be resolved from a regulatory perspective. These issues include determining the optimal threshold and time points of MRD assessment associated with clinical benefit, the role of other disease-specific factors (e.g., cytogenetics, extramedullary disease) on survival outcomes, the degree of MRD improvement that is clinically meaningful, and the impact of missing data on outcomes of previously performed studies [44, 45•].
In an effort to address these issues and establish MRD as a surrogate endpoint, the i2TEAMM (International Independent Team for Endpoint Approval of Myeloma MRD) Initiative has been developed and is led by Drs. N Munshi, J San Miguel, B Durie, and Q Shi (reviewed in [44]). This initiative is composed of myeloma research groups from the USA and Europe and members of the pharmaceutical industry as well as an independent statistical/analytical group. The i2TEAMM is in the process of developing a meta-analytic surrogacy analysis based on patient-level data from 14 clinical trials. A trial-level assessment of surrogacy will be performed in order to determine how precisely the treatment effect on the true endpoint can be predicted based on the observed treatment effect on the surrogate endpoint. Further complicating matters is the fact that surrogacy will need to be determined separately for each of the different myeloma patient populations (e.g., newly diagnosed vs relapsed/refractory).
Current State of MRD in Clinical Practice
There are multiple issues that have made real-world utilization of MRD assessment problematic. Several surveys of academic centers have demonstrated that there is significant variability in the utilization of MRD analysis [31, 46]. Although as noted above, consensus guidelines exist for the use of MFC, in reality many institutions continue to use their own home-grown panels. Implementation of the EuroFlow panel with the requisite level of sensitivity can be difficult because of the instrument time required to process sufficient numbers of cells, particularly in high-volume centers. Furthermore, there can be reimbursement issues related to MRD analysis by MFC as this is not an FDA-approved assay.
Perhaps an even more important issue, however, is what to do with the MRD result once it is in hand. At this time, there are no guidelines for how to tailor therapy based on MRD status. Many studies evaluating MRD status have done so at the day 100 post-ASCT time point and all have demonstrated that achievement of MRD negativity at that time point is associated with improved outcomes. Therefore, it has been speculated that maintenance therapy may not be required for those patients who are MRD negative at day 100. However, in the IFM 2009 study where lenalidomide maintenance was discontinued after 1 year, it was noted that relapses in the MRD-negative population began to occur once maintenance was discontinued [35, 36]. Furthermore, the Myeloma XI study has provided evidence that lenalidomide maintenance improves PFS even for patients who are MRD negative [38]. Thus, currently, there are no data that support the practice of withholding lenalidomide maintenance therapy based on MRD status. Perhaps a more important question is whether, following an as yet undefined period of sustained MRD negativity, treatment can be de-escalated or even discontinued. Several studies are planned to investigate this strategy, including the SWOG S1803 study. While the primary question that this phase 3 study is addressing is whether the addition of daratumumab to lenalidomide maintenance post-ASCT improves OS, the study also has been designed to evaluate outcomes following randomization to continuation vs discontinuation of maintenance therapy in patients who marrow tests are MRD negative after 2 years of maintenance therapy.
The role of ASCT as consolidation following induction therapy is continuing to evolve. The IFM 2009 study demonstrated superior PFS and MRD negativity rates for patients randomized to consolidation with ASCT vs additional cycles of RVD (lenalidomide, bortezomib, dexamethasone) [35]. The FORTE study is a randomized phase II study in which transplant-eligible patients are randomized to three groups: arm A (KCD (carfilzomib, cyclophosphamide, dexamethasone)-ASCT-KCD), arm B (KRD (carfilzomib, lenalidomide, dexamethasone)-ASCT-KRD), or arm C (KRD × 12 cycles). Following completion of the induction/consolidation, there is a second randomization to lenalidomide vs carfilzomib/lenalidomide maintenance therapy. The preliminary results presented in abstract form have revealed fairly similar MRD negativity rates in Arms B and C prior to maintenance (58% vs 54%, respectively) [47]. Longer follow-up is required to determine whether survival outcomes differ between the two groups and thus at this time, it is premature to conclude that ASCT can be withheld in the upfront setting. In addition, it will be important to investigate whether MRD status following completion of induction could be used to determine whether consolidation with ASCT is needed.
Additional important unanswered questions include how frequently MRD status should be measured, what to do if a patient converts from MRD negativity to MRD-positivity, whether treatment should be changed if a patient does not initially achieve MRD-negative status, and whether any MRD-based treatment decisions ultimately translate to superior survival outcomes. In an analysis of 50 patients who had achieved at least a VGPR on the RB-MM-EMN-441 study, it was determined that MRD progression predated clinical relapse by median of 9 months and biochemical relapse by a median of 4 months [48]. Thus, given the relative ease of monitoring for biochemical relapse in the blood as compared to MRD status in the bone marrow, it is not clear that repeated assessment of MRD status is currently justified outside of the confines of a clinical trial. While there are data from recent trials demonstrating that MRD negativity rates improve over time with increasing duration of therapy (e.g., [49, 50]), there are insufficient data to guide us with respect to changing therapy if MRD negativity is not achieved.
Conclusions and Future Directions
There is currently strong evidence that achievement of MRD negativity does matter with respect to serving as a prognostic marker for survival. However, currently, we would not recommend routine assessment of MRD outside the confines of a clinical trial as data are lacking to support MRD status-driven treatment decisions. It is anticipated that if MRD can be used as a surrogate endpoint, this will allow for more rapid completion of clinical trials. The results of trials that are incorporating MRD status-based treatment decisions are eagerly awaited. It is evident that a single result of MRD negativity from a random bone marrow aspirate specimen does not equate cure. However, it is anticipated that in the future, as developing methodologies improve our ability to measure residual disease in the blood, bone marrow, and via imaging techniques, we will be able to identify patients who have been functionally cured of their disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Costa LJ, Brill IK, Omel J, Godby K, Kumar SK, Brown EE. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017;1:282–7.
. Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3:28–35 Meta-analysis of the association between MRD status and survival.
•• Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46 Incorporation of MRD into the IMWG response criteria.
Bird JM, Russell NH, Samson D. Minimal residual disease after bone marrow transplantation for multiple myeloma: evidence for cure in long-term survivors. Bone Marrow Transplant. 1993;12:651–4.
Stetler-Stevenson M, Paiva B, Stoolman L, Lin P, Jorgensen JL, Orfao A, et al. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry B Clin Cytom. 2016;90:26–30.
Came N, Nguyen V, Westerman D, Harrison S. Aggressive and extramedullary plasma cell myeloma evade bone marrow flow cytometric minimal residual disease detection. Br J Haematol. 2016;173:947–9.
• Flores-Montero J, de Tute R, Paiva B, Perez JJ, Bottcher S, Wind H, et al. Immunophenotype of normal vs. myeloma plasma cells: toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry B Clin Cytom. 2016;90:61–72 Development of consensus panels for MRD assessment by flow cytometry.
Orfao A, Garcia-Sanz R, Lopez-Berges MC, Belen Vidriales M, Gonzalez M, Caballero MD, et al. A new method for the analysis of plasma cell DNA content in multiple myeloma samples using a CD38/propidium iodide double staining technique. Cytometry. 1994;17:332–9.
Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93:431–8.
• Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, Garcia-Sanchez O, Bottcher S, et al. Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:2094–103 Development of consensus panels for MRD assessment by flow cytometry.
Roshal M, Flores-Montero JA, Gao Q, Koeber M, Wardrope J, Durie BGM, et al. MRD detection in multiple myeloma: comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood Advances. 2017;1:728–32.
Soh KT, Tario JD Jr, Wallace PK. Diagnosis of plasma cell dyscrasias and monitoring of minimal residual disease by multiparametric flow cytometry. Clin Lab Med. 2017;37:821–53.
Minarik J, Novak M, Flodr P, Balcarkova J, Mlynarcikova M, Krhovska P, et al. CD38-negative relapse in multiple myeloma after daratumumab-based chemotherapy. Eur J Haematol. 2017;99:186–9.
Avet-Loiseau H, Corre J, Lauwers-Cances V, Chretien M-L, Robillard N, Leleu X, et al. Evaluation of minimal residual disease (MRD) by next generation sequencing (NGS) is highly predictive of progression free survival in the IFM/DFCI 2009 trial. Blood. 2015;126:191.
Martinez-Lopez J, Lahuerta JJ, Pepin F, Gonzalez M, Barrio S, Ayala R, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073–9.
Ladetto M, Bruggemann M, Monitillo L, Ferrero S, Pepin F, Drandi D, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia. 2014;28:1299–307.
• Mazzotti C, Buisson L, Maheo S, Perrot A, Chretien ML, Leleu X, et al. Myeloma MRD by deep sequencing from circulating tumor DNA does not correlate with results obtained in the bone marrow. Blood Adv. 2018;2:2811–3 Study demonstrating lack of concordance between marrow and blood MRD results using next-generation sequencing.
Hillengass J, Landgren O. Challenges and opportunities of novel imaging techniques in monoclonal plasma cell disorders: imaging “early myeloma”. Leuk Lymphoma. 2013;54:1355–63.
Bergen HR 3rd, Dasari S, Dispenzieri A, Mills JR, Ramirez-Alvarado M, Tschumper RC, et al. Clonotypic light chain peptides identified for monitoring minimal residual disease in multiple myeloma without bone marrow aspiration. Clin Chem. 2016;62:243–51.
Moreau P, Attal M, Caillot D, Macro M, Karlin L, Garderet L, et al. Prospective evaluation of magnetic resonance imaging and [(18)F]fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial: results of the IMAJEM study. J Clin Oncol. 2017;35:2911–8.
Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989–95.
Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–76.
Pawlyn C, Fowkes L, Otero S, Jones JR, Boyd KD, Davies FE, et al. Whole-body diffusion-weighted MRI: a new gold standard for assessing disease burden in patients with multiple myeloma? Leukemia. 2016;30:1446–8.
Sachpekidis C, Mosebach J, Freitag MT, Wilhelm T, Mai EK, Goldschmidt H, et al. Application of (18)F-FDG PET and diffusion weighted imaging (DWI) in multiple myeloma: comparison of functional imaging modalities. Am J Nucl Med Mol Imaging. 2015;5:479–92.
Rasche L, Angtuaco E, McDonald JE, Buros A, Stein C, Pawlyn C, et al. Low expression of hexokinase-2 is associated with false-negative FDG-positron emission tomography in multiple myeloma. Blood. 2017;130:30–4.
Pandit-Taskar N. Functional imaging methods for assessment of minimal residual disease in multiple myeloma: current status and novel ImmunoPET based methods. Semin Hematol. 2018;55:22–32.
Ghai A, Maji D, Cho N, Chanswangphuwana C, Rettig M, Shen D, et al. Preclinical development of CD38-targeted [(89)Zr]Zr-DFO-daratumumab for imaging multiple myeloma. J Nucl Med. 2018;59:216–22.
Pugh TJ. Circulating tumour DNA for detecting minimal residual disease in multiple myeloma. Semin Hematol. 2018;55:38–40.
Thoren KL. Mass spectrometry methods for detecting monoclonal immunoglobulins in multiple myeloma minimal residual disease. Semin Hematol. 2018;55:41–3.
Barnidge DR, Dasari S, Botz CM, Murray DH, Snyder MR, Katzmann JA, et al. Using mass spectrometry to monitor monoclonal immunoglobulins in patients with a monoclonal gammopathy. J Proteome Res. 2014;13:1419–27.
Holstein SA, Avet-Loiseau H, Hahn T, Ho CM, Lohr JG, Munshi NC, et al. BMT CTN Myeloma Intergroup Workshop on Minimal Residual Disease and Immune Profiling: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant. 2018;24:641–8.
Lahuerta JJ, Paiva B, Vidriales MB, Cordon L, Cedena MT, Puig N, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–10.
Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone Marrow Transplant. 2016;51:1565–8.
Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378:518–28.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132:2456–64.
Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Phase 3 randomized study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant (MAIA). Blood. 2018;132:LBA-2.
de Tute RM, Cairns D, Rawstron A, Pawlyn C, Davies FE, Jones JR, et al. Minimal residual disease in the maintenance setting in myeloma: prognostic significance and impact of lenalidomide. Blood. 2017;130:904.
Gambella M, Omede P, Spada S, Muccio VE, Gilestro M, Saraci E, et al. Minimal residual disease by flow cytometry and allelic-specific oligonucleotide real-time quantitative polymerase chain reaction in patients with myeloma receiving lenalidomide maintenance: a pooled analysis. Cancer 2018. https://doi.org/10.1002/cncr.31854.
Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103:2079–87.
Dimopoulos MA, San-Miguel J, Belch A, White D, Benboubker L, Cook G, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018;103:2088–96.
Anderson KC, Auclair D, Kelloff GJ, Sigman CC, Avet-Loiseau H, Farrell AT, et al. The role of minimal residual disease testing in myeloma treatment selection and drug development: current value and future applications. Clin Cancer Res. 2017;23:3980–93.
Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98:34–46.
Holstein SA, Ye JC, Howard A, Bhutani M, Gormley N, Hahn T, et al. Summary of the second annual BMT CTN Myeloma Intergroup Workshop on Minimal Residual Disease and Immune Profiling. Biol Blood Marrow Transplant 2018. https://doi.org/10.1016/j.bbmt.2018.11.001.
• Gormley NJ, Turley DM, Dickey JS, Farrell AT, Reaman GH, Stafford E, et al. Regulatory perspective on minimal residual disease flow cytometry testing in multiple myeloma. Cytometry B Clin Cytom. 2016;90:73–80. FDA perspective on MRD testing in myeloma.
Flanders A, Stetler-Stevenson M, Landgren O. Minimal residual disease testing in multiple myeloma by flow cytometry: major heterogeneity. Blood. 2013;122:1088–9.
Gay F, Cerrato C, Rota Scalabrini D, Galli M, Belotti A, Zamagni E, et al. Carfilzomib-lenalidomide-dexamethasone (KRd) induction-autologous transplant (ASCT)-Krd consolidation vs KRd 12 cycles vs carfilzomib-cyclophosphamide-dexamethasone (KCd) induction-ASCT-KCd consolidation: analysis of the randomized FORTE trial in newly diagnosed multiple myeloma (NDMM). Blood. 2018;132:121.
Oliva S, Gambella M, Gilestro M, Muccio VE, Gay F, Drandi D, et al. Minimal residual disease after transplantation or lenalidomide-based consolidation in myeloma patients: a prospective analysis. Oncotarget. 2017;8:5924–35.
Voorhees PM, Rodriguez C, Reeves B, Nathwani N, Costa LJ, Lutska Y, et al. Efficacy and updated safety analysis of a safety run-in cohort from Griffin, a phase 2 randomized study of daratumumab (Dara), bortezomib (V), lenalidomide (R), and dexamethasone (D; Dara-Vrd) vs. Vrd in patients (Pts) with newly diagnosed (ND) multiple myeloma (MM) eligible for high-dose therapy (HDT) and autologous stem cell transplantation (ASCT). Blood. 2018;132:151.
Zimmerman T, Raje NS, Vij R, Reece D, Berdeja JG, Stephens LA, et al. Final results of a phase 2 trial of extended treatment (tx) with carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (KRd) plus autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (NDMM). Blood. 2016;128:675.
Acknowledgments
SK, JH, PLM, and SAH have no acknowledgments for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shalin Kothari declares no potential conflicts of interest. Jens Hillengass reports received honoraria and travel support from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Takeda and research funding from Celgene and Sanofi, outside the submitted work. Philip McCarthy reports receiving honoraria from Bristol-Myers Squibb, Celgene, Sanofi-Aventis, Takeda and Binding Site, research funding from Celgene, and has served on advisory committees/review panels/board membership for Bristol-Myers Squibb, Celgene, Sanofi-Aventis, Takeda, Binding Site and Karyopharm, outside the submitted work. Sarah Holstein reports receiving honoraria from Adaptive Biotechnologies, Celgene, Takeda and has served on advisory committees/review panels for Celgene, Takeda, Adaptive Biotechnologies, Sorrento, GlaxoSmithKline, outside the submitted work.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This Article is part of the Topical Collection on Stem Cell Transplantation
Rights and permissions
About this article
Cite this article
Kothari, S., Hillengass, J., McCarthy, P.L. et al. Determination of Minimal Residual Disease in Multiple Myeloma: Does It Matter?. Curr Hematol Malig Rep 14, 39–46 (2019). https://doi.org/10.1007/s11899-019-0497-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-019-0497-7