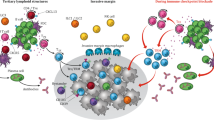
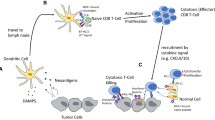
Abstract
Purpose of Review
Checkpoint inhibitors block co-inhibitory signals which serves to promote T cell activation/reinvigoration in the periphery and tumor microenvironment. A brief historical background as well as a summary of key observations related to the composition and prognostic value of tumor-infiltrating lymphocytes (TILs) is discussed.
Recent Findings
Solid tumor patients that respond to checkpoint inhibitors have greater CD8+ T cell densities (at the tumor margin) associated with a gene inflammation signature and high tumor mutational burden. The precise specificity of effector (CD8+ T cell) TIL remains poorly defined and this deficiency represents a major challenge for the field of cancer immunology.
Summary
High mutational burden cancers such as melanoma provides compelling evidence that missense mutations create neoantigens which can serve as target antigens for the immune system. Emerging evidence suggests that neoantigen-specific TILs are the major effector cells that mediate tumor regression due to checkpoint inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cellular infiltrate and composition of human cancers is complex and includes various hematopoietic cells, stromal cells, and tumor vasculature. The immune infiltrate of most cancers is dominated by macrophages (M0/M1/M2 subtypes) and conventional αβ T cells, including CD4+ memory cells, CD4+ regulatory cells, CD4+ follicular helper cells, and CD8+ effector cells [1, 2]. Smaller populations of hematopoietic cells within the tumor infiltrate include B cells, monocytes, dendritic cells, NK cells (activated and resting), γδ T cells, and mast cells; neutrophils and eosinophils are variable. In conventional terms, tumor-infiltrating lymphocytes (TILs) represent the heterogeneous population of αβ T cells, both CD4+ and CD8+ subsets, present within the tumor microenvironment (TME) [3]. TILs are often isolated ex vivo as a single cell suspension for analysis; however, with newer molecular technologies, the cellular infiltrate can be directly interrogated in situ by excision of the tumor mass without a need to dissociate the tissue. It is now apparent that the composition of the immune infiltrate can be highly variable even within a group of patients with the same malignant histology. For example, microsatellite instability (MSI)-high CRC have greater infiltrates of CD8+ T cells compared with sporadic MSS CRC which is dominated by M2 macrophages and have fewer infiltrating CD8+ T cells [4, 5].
Investigators often characterize tumors as inflamed (“hot”) or non-inflamed (“cold”) using a series of biomarkers to assess the degree of T cell infiltration or T cell activation within the TME [6]. The use of gene expression profiles, high CD8+ T cell density, and PD-L1 expression within the TME are now widely accepted biomarkers to assist investigators in classification of solid tumors and subsequent response to novel immunotherapy agents [7].
Based on the initial studies in melanoma, TILs are now recognized as a rich source of effector T cells that exhibit tumor recognition [8]. For many years, the significance of TILs within the broader context of cancer immunology was underappreciated for many reasons. Clinicians often argued that adoptive cell therapy with TIL seemed uniquely beneficial to melanoma patients and was less applicable to more common solid tumors such as lung or colon adenocarcinoma. Second, the culture methods to generate TIL for adoptive transfer were considered arcane, too costly, and required specialized manufacturing facilities and, as a result, few academic centers attempted to reproduce the results obtained at NIH until recently. Finally, the field of cancer immunotherapy was largely discredited until 2010–2012 when publication of clinical trial success using the immunomodulatory antibodies, anti-CTLA-4 and anti-PD-1, as well as chimeric antigen receptor T cells for treatment of hematological malignancies transformed the cancer treatment landscape. These therapeutic advances stimulated investigators to re-interrogate the TME in search of novel biomarkers—including TIL—that predict response to treatment, especially patients that received checkpoint inhibitors such as anti-PD-1 (or anti-PD-L1) alone or in combination with anti-CTLA-4 [9].
Historical Background
Rosenberg and colleagues provided the initial demonstration that CD8+ TILs from tumor-bearing C57BL/6 mice could be isolated and expanded in vitro by short-term culture using IL-2 [10]. TILs were shown to lyse syngeneic murine carcinomas in an MHC-restricted and antigen-specific manner using standard 51Cr release assays. Re-administration of the in vitro expanded effector TIL populations by adoptive transfer could mediate tumor regression and in certain instances, cure animals bearing large established liver or lung metastases. In their initial report using MC-38 cells derived from C57BL/6 colon adenocarcinoma, Rosenberg et al. found that when compared with LAK cells, TIL cultured in IL-2 for 15 days were > 50-fold more potent at eliminating pulmonary micrometastasis. Eradication of large established (14d) liver metastases by cultured TIL required pre-conditioning with cyclophosphamide and administration of IL-2 for 5 days. A follow-up report providing confirmatory evidence using a series of murine tumor cell lines was published the following year [11]. Parenthetically, the same MC-38 colon adenocarcinoma cell line was recently used to define multiple unique MHC class I-restricted immunogenic neoantigens (mutated Adpgk, Reps1, Dppagt1) using next-generation sequencing (NGS) methods in conjunction with mass spectrometry analysis of MHC-eluted peptide ligands [12]. Neoantigen peptide vaccination together with anti-CD40 and poly(I:C) could elicit CD8+ tumor–specific T cells and resulted in rejection of established MC-38 adenocarcinoma, in essence, validating Rosenberg’s original observation.
Human Studies
Two independent reports were published in 1987 demonstrating the presence of TILs in patients with metastatic cancer. Rabinowich et al. examined TILs from surgically resected lung cancer specimens and demonstrated the lytic activity of CD8+ T cells specific for autologous tumor cells by 51Cr release assay [13]. Fresh TILs harvested from resected lung cancer specimens were dissociated from tumor and cultured in vitro for 3–5 weeks in IL-2. The effector T cells were then shown to exhibit some degree of specificity using autologous tumor cells as targets cells. The second report provided clear demonstration of TIL lytic activity specific for autologous melanoma but not allogeneic melanoma cell lines consistent with antigen-specific tumor recognition by CD8+ T cells expanded in vitro from TILs [14].
A multitude of clinical studies confirming the presence of TIL isolated from various surgically resected cancers followed and substantiated the initial observations that TILs did, in fact, contain a population of effector T cells that were distinct from NK cells based on specificity for autologous tumor.
TIL-Based Cell Therapy in Melanoma
The in vitro studies (vide supra) prompted the NIH team to translate their findings into a series of pilot/phase 1 clinical trials using TIL as a source of effector T cells for adoptive cell transfer into patients with metastatic disease. A report of 20 melanoma patients pre-conditioned with cyclophosphamide (25 mg/kg i.v.) followed by 3–75 × 1010 TILs followed by high-dose IL-2 (100,000 U/kg i.v. every 8 h) for 5 days demonstrated a 55% ORR; however, most responses were not durable [15]. The next significant step described the young TIL culture method to generate higher quality CD8+ T cells for adoptive cell therapy in patients that received more intensive conditioning chemotherapy with cyclophosphamide (60 mg/kg) × 2 days plus fludarabine (25 mg/m2) × 5 days. Patients continued to receive high-dose IL-2 every 8 h for up to 5 days after cell transfer. The final study results for the 93 melanoma patients treated at NIH with autologous selected TIL adoptive cell transfer confirmed a 22% CR rate (56% ORR) and a 5-year overall rate of 29% for all patients [16]. Data from other centers using autologous TIL cell transfer in similar protocols provides confirmatory evidence for clinical activity in patients with metastatic melanoma [17, 18].
TIL Therapy and Tumor Antigens
One of the major questions related to autologous TIL cell therapy was the antigen specificity of the cell product and the recognition of shared versus unique tumor antigens. For many years, it was assumed that recognition of non-mutated melanoma differentiation antigens and cancer-testis antigens were the relevant targets of TILs [19]. Studies from several laboratories provided initial evidence that TIL products contained both CD8+ and CD4+ T cells that recognized non-mutated shared antigens which included gp100, MART-1/Melan-A, tyrosinase, and several MAGE-family members [20,21,22]. However, in instances when T cell reactivity to non-mutated antigens was found in TIL or peripheral blood, the precursor frequencies were often less than 1%. Despite the presence of tumor-reactive T cells as defined by recognition of autologous tumor using standard in vitro assays, most TIL products remained incompletely characterized with regard to tumor antigen specificity.
In 1995, two case reports provided initial evidence that melanoma patient CD8+ T cells could recognize amino acid–substituted (AAS, mutated) peptides restricted to HLA class I molecules [23, 24]. Using conventional cDNA expression cloning methods and screening with T cell lines (derived from TIL or PBMC) to assess recognition of autologous tumor, it became evident that cancer patients could, in fact, develop spontaneous immunity to tumor-encoded missense mutations. A series of single-patient case reports emerged from various groups using a similar strategy of cDNA expression cloning to define a single neoantigen per patient primarily in melanoma as well as several lung carcinomas, renal cell carcinomas, and a head and neck cancer [25].
With the advent of NGS technologies and improved bioinformatics pipelines, it became possible to identify tumor-encoded genomic alterations (missense mutations and indels) in a clinically relevant timeframe and define all putative neoantigens encoded by high mutational burden malignancies such as cutaneous melanoma. The initial reports in 2013 successfully identified neoantigen-specific CD8+ T cells from resected TIL that demonstrated reactivity for AAS (mutated) peptides that were restricted by HLA class I molecules in several patients [26]. The NIH group performed NGS of melanoma specimens from three patients and used synthetic AAS peptide–pulsed HLA-matched antigen-presenting cells to screen autologous TIL for reactivity in cytokine release assays; in each patient, 2–3 neoantigens were identified. In a separate report from Van Rooij et al., NGS of a resected melanoma lesion yielded 448 candidate AAS peptides that were formulated into p-MHC multimers that were used to screen patient TIL by flow cytometry. Their analysis yielded 2 neoantigen-specific CD8+ T cell populations, including a dominant response (3.3% TIL stained by p-HLA multimer) directed against mutated ATR [27].
Evidence supporting the role for neoantigen-specific T cells as key effector cells involved the elimination of metastatic cancer has accumulated primarily from studies performed at the NIH. Neoantigen-reactive T cells, both CD8+ and CD4+, can be identified in TIL products from most patients when coupled with NGS technologies and bioinformatics algorithms. An exemplary patient with stage 4 melanoma was reported to harbor CD8+ T cell reactivity to 10 unique neoantigens obtained from TIL that mediated complete regression of all metastatic deposits [28]. This reference patient had a high mutational burden (> 4000 non-synonymous mutations) melanoma and many of the tumor-reactive TCR clonotypes could be detected in peripheral blood 1 year after cell transfer therapy. Patients with common epithelial cancers that are often regarded as low mutational burden malignancies, in fact, harbor neoantigen-specific T cells that recognize AAS (mutated) peptides. In a seminal report of 10 patients with metastatic gastrointestinal malignancies, neoantigen-reactive T cells could be identified in 9 of the individuals (range, 10–155 mutations) directed against 1–3 unique neoantigens per patient. Several neoantigens were presented by HLA class II molecules [29]. A recent review summarizes the published studies in melanoma and GI cancers performed by the NIH investigators [30]. A recent publication further extends their observations using neoantigen-reactive TIL therapy successfully administered in a metastatic breast cancer patient [31] resulting in complete remission. Work from other investigators supports the finding that neoantigen-specific T cells, both CD4+ and CD8+ subsets, are present at significant precursor frequencies in TIL products that promote regression of metastatic melanoma [32, 33].
Prognostic Value of TIL
It was recognized as far back as 1989 that the density (brisk, non-brisk, or absent) of TILs within primary melanoma skin lesions had prognostic significance for overall survival [34]. More recent confirmatory studies in melanoma [35], breast cancer [36], lung cancer [37], HCC [38], CRC [39••], and other malignancies provide strong support for the underlying thesis and relative importance of TIL density as a prognostic feature of most primary malignancies. The work by Galon and colleagues on colorectal cancer provides perhaps the most comprehensive picture for any single solid tumor type emphasizing the extent of CD3+ and CD8+ effector T cells within the tumor and invasive margin to determine the Immunoscore as an objective measure of TIL density [40]. This body of work spanning more than a decade in collaboration with numerous international investigators in 17 countries validates the power of the Immunoscore using the IHC platform as a prognostic test for disease-free survival, disease-specific survival, and overall survival [41].
The TCGA-sponsored Immune Landscape of Cancer project is especially noteworthy since this effort included > 10,000 tumor samples (from 30 solid tumor types) that were subjected to extensive genomic and histological analysis using modern molecular technologies and bioinformatics algorithms in order to define how immune response (and TIL density) impacts patient prognosis [42]. Six immune subtypes were defined that span most major solid tumors and molecular subtypes (see Figure 1B in ref [42] for key characteristics of each immune subtype). The C2 (IFN-g dominant) and C3 (Inflammatory) subtypes were associated with the best overall survival which is consistent with known correlations with type-1 (Th1/Th17) immunity and high TIL density scores. In contrast, C4 (lymphocyte depleted) and C6 (TGF-β dominant) subtypes were associated with the poorest prognosis which is consistent with high macrophage content, low TIL density, and an immunosuppressive TME. The Immune Landscape of Cancer project provides a rich resource for investigators to critically assess the genomic and histological features of cancer patients enrolled on novel immunotherapy trials in order to identify mechanisms of response and resistance.
TIL Density and Checkpoint Inhibition
Clinical development of immunomodulatory antibodies that block PD-1/PD-L1 interaction as well as the CTLA-4/B7 interaction provided a unique opportunity to investigate biomarkers of response and resistance in various solid tumors [43]. The landmark study from Tumeh and colleagues developed a predictive model based on CD8+ T cell density at the invasive tumor margin in melanoma patients receiving anti-PD-1 monotherapy [35]. By performing serial biopsies, the authors provided strong evidence that responding patients have a higher density of CD8+ T cells prior to treatment and moreover, demonstrate higher T cell clonality based on TCR sequencing suggestive of a specific anti-tumor response. This observation has been independently confirmed in several other studies [44]. In melanoma patients receiving ipilimumab (anti-CTLA-4) monotherapy [45], investigators demonstrated a correlation of clinical response with high tumor mutational burden. Multiple reports confirmed a similar association of clinical response with high mutational burden in melanoma or non-small cell lung cancer patients receiving anti-PD-1 monotherapy [46]. Confirmatory evidence linking clinical response to anti-PD-1 therapy with tumor mutational burden appeared for other tumor types, including bladder, MSI-high GI malignancies, and head and neck cancers [47].
A recent report investigating tumor mutational burden and a T cell–inflamed gene signature profile evaluated > 300 tumor specimens from cancer patients treated with anti-PD-1 monotherapy in an effort to assess biomarkers of response [48]. Both the tumor mutational burden and the T cell–inflamed gene signature independently predicted clinical response to anti-PD-1 monotherapy. When the two biomarkers were used jointly, the rates of clinical response were highest for the subgroup with a high tumor mutational burden and a high T cell inflammation score. For example, in the pan-tumor group, the TMBhi/T cell inflamedhi patients exhibited a 37% clinical response rate (CI 19–57%), while the no responses were seen in the TMBlo/T cell–inflamedlo patients. As expected, patients defined by one positive biomarker showed a low response rate to anti-PD-1 compared with the double positive (TMB/inflamed) biomarker subgroup. Collectively, these studies (along with many others) provide strong evidence that TIL density in pre-treatment tumor (in particular, CD8+ T cells) is an important biomarker of clinical response and long-term benefit in patients with melanoma and many other solid tumors. In many studies, TIL density appears to correlate with high mutational burden and T cell inflammation suggesting that patients can develop spontaneous T cell immunity directed against neoantigens encoded by their malignancy [49].
Since high mutational burden tumors (encoding hundreds-thousands of missense mutations) have the potential to create an abundance of cancer-specific neoantigens, it is logical to envision how the host immune system can recognize AAS (mutant) peptides presented by the cancer [50]. A recent neo-adjuvant study in patients with resectable non-small cell lung cancer provided evidence for T cell clonal expansion and trafficking into tumor after 2 doses of anti-PD-1 monotherapy. The authors identified 3-T cell clonotypes specific for a single neoantigen encoded by the patient’s tumor and detected increased frequencies of all 3 clonotypes in peripheral blood after anti-PD-1 administration [51••]. Importantly, all 3 clonotypes were detected at relatively high frequency in tumor prior to treatment and could still be detected in surgically resected post-treatment tumor specimens and regional nodes. In a separate study of patients with MSI-high cancers, the authors using a similar strategy by TCR Vβ CDR3 sequencing in order to show similar clonal expansions of neoantigen-reactive T cells in peripheral blood after administration of anti-PD-1 [52]. Despite the limited sample sizes, these two studies provide tantalizing evidence to suggest that neoantigen-specific TIL can mediate tumor regression after checkpoint inhibitor therapy.
Conclusions
TILs are a rich source of effector T cells that mediate recognition and elimination of solid tumors as demonstrated by adoptive cell therapy approaches for high mutational burden malignancies such as melanoma. CD8+ T cell density assays (i.e., Immunoscore) are now regarded as a reliable biomarker for inflamed (“hot”) TME for most solid tumors, including patients that receive checkpoint inhibitors. Although the precise specificity of the effector CD8+ T cells in most instances is unknown, there is now emerging evidence to suggest that tumor-encoded genomic alterations that create neoantigens are the primary targets of the effector T cells. A major challenge for investigators going forward will be to develop new therapeutic strategies that effectively recruit neoantigen-specific T cells from the periphery to the TME and improve the efficacy of checkpoint inhibitors in solid tumor patients.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80.
Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18(1):248–62.
Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169(4):750–65 e17.
Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–22.
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51.
Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69(6):2685–93.
Keenan TE, Burke KP, Van Allen EM. Genomic correlates of response to immune checkpoint blockade. Nat Med. 2019;25(3):389–402.
Feldman SA, Assadipour Y, Kriley I, Goff SL, Rosenberg SA. Adoptive cell therapy--tumor-infiltrating lymphocytes, T-cell receptors, and chimeric antigen receptors. Semin Oncol. 2015;42(4):626–39.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e51.
Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233(4770):1318–21.
Spiess PJ, Yang JC, Rosenberg SA. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. J Natl Cancer Inst. 1987;79(5):1067–75.
Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–6.
Rabinowich H, Cohen R, Bruderman I, Steiner Z, Klajman A. Functional analysis of mononuclear cells infiltrating into tumors: lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. 1987;47(1):173–7.
Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987;138(3):989–95.
Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–80.
Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7.
Rohaan MW, van den Berg JH, Kvistborg P, Haanen J. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. 2018;6(1):102.
Met O, Jensen KM, Chamberlain CA, Donia M, Svane IM. Principles of adoptive T cell therapy in cancer. Semin Immunopathol. 2019;41(1):49–58.
Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4(127):127ps8.
Andersen RS, Thrue CA, Junker N, Lyngaa R, Donia M, Ellebaek E, et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 2012;72(7):1642–50.
Kvistborg P, Shu CJ, Heemskerk B, Fankhauser M, Thrue CA, Toebes M, et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology. 2012;1(4):409–18.
Frosig TM, Lyngaa R, Met O, Larsen SK, Donia M, Svane IM, et al. Broadening the repertoire of melanoma-associated T-cell epitopes. Cancer Immunol Immunother. 2015;64(5):609–20.
Coulie PG, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, et al. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci U S A. 1995;92(17):7976–80.
Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269(5228):1281–4.
Lu YC, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28(1):22–7.
Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19(6):747–52.
van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31(32):e439–42.
Prickett TD, Crystal JS, Cohen CJ, Pasetto A, Parkhurst MR, Gartner JJ, et al. Durable complete response from metastatic melanoma after transfer of autologous T cells recognizing 10 mutated tumor antigens. Cancer Immunol Res. 2016;4(8):669–78.
Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350(6266):1387–90.
Tran E, Robbins PF, Rosenberg SA. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol. 2017;18(3):255–62.
Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24(6):724–30.
Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21(1):81–5.
Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536(7614):91–5.
Clark WH Jr, Elder DE, Guerry D, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893–904.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50.
Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107(3):dju435.
Ding W, Xu X, Qian Y, Xue W, Wang Y, Du J, et al. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2018;97(50):e13301.
•• Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–39. Findings from this study validate the power of the Immunoscore classifier as a reliable estimate of the risk of recurrence in stages I–III resected colon cancer. Tissue samples from 2681 patients collected at 14 centers in 13 countries were included in this analysis.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4.
Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199–209.
Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. 2018;48(4):812–30 e14.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Wong PF, Wei W, Smithy JW, Acs B, Toki MI, Blenman KRM, et al. Multiplex quantitative analysis of tumor-infiltrating lymphocytes and immunotherapy outcome in metastatic melanoma. Clin Cancer Res. 2019;25(8):2442–9.
Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–11.
Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44.
Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56.
Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593.
Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4.
Linette GP, Carreno BM. Neoantigen vaccines pass the immunogenicity test. Trends Mol Med. 2017;23(10):869–71.
•• Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–86 Two doses of neo-adjuvant nivolumab in early-stage resectable NSCLC resulted in major pathological responses in 45% patients ( n =20). In most patients studied, tumor-specific T cell clones could be identified in tumor and peripheral blood which increased in frequency after anti-PD1 administration. Interestingly, TMB was predictive of pathological response to anti-PD1.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Funding
GPL and BMC are financially supported by CA204261, CA205794, CA217805, and the Abramson Cancer Center Translational Research Pilot Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on CART and Immunotherapy
Rights and permissions
About this article
Cite this article
Linette, G.P., Carreno, B.M. Tumor-Infiltrating Lymphocytes in the Checkpoint Inhibitor Era. Curr Hematol Malig Rep 14, 286–291 (2019). https://doi.org/10.1007/s11899-019-00523-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-019-00523-x