Abstract
Purpose of Review
Heart failure (HF) is a major public health problem worldwide, affecting more than 64 million people [1]. The complex and severe nature of HF presents challenges in providing cost-effective care as patients often require multiple hospitalizations and treatments. This review of relevant studies with focus on the last 10 years summarizes the health and economic implications of various HF treatment options in Europe and beyond. Although the main cost drivers in HF treatment are clinical (re)admission and decompensation of HF, an assessment of the economic impacts of various other device therapy options for HF care are included in this review. This includes: cardiovascular implantable electronic devices (CIEDs) such as cardiac-resynchronisation-therapy devices that include pacemaking (CRT-P), cardiac-resynchronisation-therapy devices that include defibrillation (CRT-D), implantable cardioverter/defibrillators (ICDs) and various types of pacemakers. The impact of (semi)automated (tele)monitoring as a relevant factor for increasing both the quality and economic impact of care is also taken into consideration. Quality of life adjusted life years (QALYs) are used in the overall context as a composite metric reflecting quantity and quality of life as a standardized measurement of incremental cost-effectiveness ratios (ICER) of different device-based HF interventions.
Recent Findings
In terms of the total cost of different devices, CRT-Ds were found in several studies to be more expensive than all other devices in regards to runtime and maintenance costs including (re)implantation. In the case of CRT combined with an implantable cardioverter-defibrillator (CRT-D) versus ICD alone, CRT-D was found to be the most cost-effective treatment in research work over the past 10 years. Further comparison between CRT-D vs. CRT-P does not show an economic advantage of CRT-D as a minority of patients require shock therapy. Furthermore, a positive health economic effect and higher survival rate is seen in CRT-P full ventricular stimulation vs. right heart only stimulation. Telemedical care has been found to provide a positive health economic impact for selected patient groups—even reducing patient mortality. For heart failure both in ICD and CRT-D subgroups the given telemonitoring benefit seems to be greater in higher-risk populations with a worse HF prognosis.
Summary
In patients with HF, all CIED therapies are in the range of commonly accepted cost-effectiveness. QALY and ICER calculations provide a more nuanced understanding of the economic impact these therapies create in the healthcare landscape. For severe cases of HF, CRT-D with telemedical care seems to be the better option from a health economic standpoint, as therapy is more expensive, but costs per QALY range below the commonly accepted threshold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction: Global burden and cost of heart failure (HF)
Heart failure (HF) is a major public health problem worldwide, affecting more than 64 million people [1]. The prevalence of HF is increasing due to aging populations, improved survival from cardiovascular diseases, and the growing burden of risk factors such as hypertension, diabetes, and obesity – each serving as a predictor for mortality [2].
The burden of HF is particularly high in Europe—it is the leading cause of hospitalization and readmission, and a major contributor to healthcare costs [3]. Even with continued advances in HF treatment and management, HF remains a major cause of morbidity and mortality, with a high risk of hospitalization, disability, and death [4].
According to a recent systematic review and meta-analysis of HF in Europe, the prevalence of HF ranges from 1.3% to 2.0% in the general population, and increases with age, reaching to 10% in people over the age of 70 [4]. The incidence of HF is high, with an estimated 1–2% of the population developing HF each year [5]. The burden of HF is especially high in Eastern Europe, where prevalence and incidence of HF are among the highest in the world [6].
HF is the main cause of hospitalization and readmission in Europe, accounting for up to 2.5 million admissions per year [7]. The economic burden of HF is also significant, with estimated costs ranging from €3,000 to €10,000 per patient per year. Here the length of stay and number of hospital admissions is a main driver of cost [8]. Therefore avoiding hospitalization is one of the main goals of every good therapy—including device therapy.
Device-based therapies must be considered especially in more severe cases of HF where the economic effect is higher compared to a healthier population (Fig. 1)
Different distribution of HF and local burden in the USA and different EU countries, modified and taken from Cowie MR et al. Improving care for patients with acute heart failure: before, during and after hospitalization in ESC Heart Fail. 2014. Total means HF patients over all, HF (I) & (II) means HF patients Degree I and II, HF total % means percentage of the population with HF. Credit: Distributable by creative commons CC BY-NC-ND 4.0 DEED (https://creativecommons.org/licenses/by-nc-nd/4.0/), Figure used from Cowie, Martin R., et al. "Improving care for patients with acute heart failure: before, during and after hospitalization." ESC heart failure 1.2 (2014): 110–145
Despite advances in treatment and management, the prognosis of HF remains still very bad compared to other treatment areas, with a 5-year mortality rate of up to 50% [9].
Effective management of HF requires a multidisciplinary approach, including self-driven lifestyle modifications, pharmacological therapy, device therapy, and cardiac rehabilitation [10]. Evidence-based therapies such as angiotensin-converting enzyme inhibitors, beta-blockers, and mineralocorticoid receptor antagonists have been shown to improve survival and reduce hospitalization in patients with HF [11]. However, these therapies are underutilized in clinical practice, particularly in older patients and those with comorbidities [12].
In summary, HF is a major public health burden in Europe, with a high prevalence, incidence, and negative economic effect. Effective management of HF requires a comprehensive approach, including prevention, early diagnosis, and evidence-based therapy. Device-based therapies have emerged as significant contributors to the management and treatment of HF, having evolved especially to address the challenges associated with decompensated and chronic HF. Both, device-based therapy and telemedical approaches are cornerstones in HF treatment. These therapies must be considered especially in more severe cases of HF [13] where the economic effect is higher compared to a healthier population.
Introduction to heart failure (HF) management
Heart failure (HF) is known as progressive and severe chronic condition as it affects the heart's ability to pump blood efficiently to meet metabolic demands. HF thereby remains a pivotal concern in healthcare, demanding an integrative care approach. There are multiple components necessary for HF management to have a seamless continuum of care, including both ambulatory and inpatient care settings that may or may not be supported by telemedical measures.
The complex nature, high incidence and severity of the illness present intricate economic challenges in the treatment of HF. Care is often fragmented as patients often must travers multiple care settings and interact with different physicians and care staff [14].
Ambulatory care forms a cornerstone of HF management, providing a setting for early detection, monitoring, and management of the disease. Utilizing resources like echocardiography, BNP/NT-proBNP testing and wearable telemonitoring technology, healthcare providers can specially monitor HF progression in this setting. Additionally, providing education on lifestyle modifications, medication management and symptom self-recognition empowers patients to manage their condition and can potentially reduce hospital admissions [15].
Patients with acute decompensated HF often require hospitalization for intensive care management. Worsening of the heart condition, hospital (re)admission as well as the length of stay in the hospital is responsible for most of the economic impact of HF [16]. A major cost reduction strategy is therefore to manage the reduction of HF events and the length of hospital stays.
State of the art HF management requires a concerted effort across different healthcare settings. By fostering a seamless transition between ambulatory and inpatient care and leveraging emerging technologies, the healthcare community can significantly improve the quality of life for HF patients while optimizing resource utilization.
An integrative treatment approach underlines the importance of a patient centered model in fighting the multiple challenges of HF. This includes incorporating device-based therapies in differentiated settings. Precisely pairing patients with the adequate therapy and including more CRT-D and CRT-P devices in their overall treatment plans is on the rise [17] in the field of HF therapy and must therefore also be seen in a standardized health economic perspective [18], as these therapies are very cost-intensive [19]. This review intends to give a review and overview on that matter.
Health economic metrics and a standardized evaluation framework
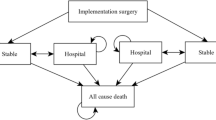
To measure how efficiently financial resources are used, the field of health economics has implemented the standard measurement of “Quality Adjusted Lifetime Years” (QALYs). This is a well-accepted approach used to assess the value derived from medical interventions.
To measure how efficiently financial resources are used, the field of health economics has implemented the standard measurement of “Quality Adjusted Lifetime Years” (QALYs)—this is a well-accepted approach used to assess the value derived from medical interventions (Fig. 2).
The application of QALYs in heart failure treatment can specifically support medical decision-making and enable the comparison of different treatment modalities with various device options for therapy. Thereby QALYs play a pivotal role in resource allocation and can help maximize overall HF health outcomes [20].
When using QALY calculations, health economic calculations often also use an Incremental Cost-Effectiveness Ratio (ICER) approach. An ICER is another fundamental concept in health economics, providing a systematic approach to evaluate the cost-effectiveness of different interventions. An ICER is computed by dividing the difference in costs between two possible interventions by the difference in their effectiveness measured in QALYs.
The combined usage of an ICER and QALYs computations allows for a clearer calculation in budget impact analysis as it shows the real effect of the used device, measurement alone or in differentiation to the use of another device. In an HF therapy scenario the use of ICER/QALYS analysis can be an especially useful approach, as many effects of the burdens of HF are spread over populations with comorbitities [21]. Using ICER in these populations, healthcare systems can have a profound impact, predicting the financial implications of adopting new device-based therapies for HF over a long-term period of time.
However, there are conversations in the healthcare economics community about employing a more differentiated use of QALYs—specifically in older populations or in cases of severe illnesses such as extensive HF [22]. It is argued by critics that healthcare resources should not be allocated solely based on the concept of health maximization as this can lead to discrimination against certain groups e.g., older and/or chronically ill people. Additionally, some health economists note how QALYs evaluations can lead to potential treatment biases as it is easier to compare treatments for illnesses where alternative treatments are widely known and discussed versus those that are not [23].
Nevertheless, using QALYs and ICER is a widely accepted measurement in the scientific community to objectivize treatment options and their respective outcomes in terms of financial implications and quality of life impact. Therefore, they will both be included in this review as a base concept.
The use of QALY/ICER metrics underpins the following three criteria which when combined create a suggested framework to evaluate a standardized health economic impact of HF treatments.
Holistic assessment
Through the lens of QALYs and ICER, stakeholders can be involved in informed decision-making, steering towards a setting that is not only cost-effective but also patient-centric and outcome-driven. QALYs provide a holistic assessment by accounting for both the length and quality of life. This is crucial in HF where interventions may extend life but also significantly impact the quality of life due to side effects or required lifestyle changes.
Standardized comparisons – specifically of device-based therapies
Employing QALYs facilitates standardized comparisons across different interventions and disease areas, aiding in the prioritization of healthcare resources based on cost-effectiveness. Especially when evaluating device-based therapies for heart failure, ICER can help determine the additional cost associated with achieving a better health outcome (measured in QALYs) with one device over another.
Informing policy and practice
The ICER informs policy and practice by providing a clear and comparable metric to evaluate the value proposition of new or existing interventions for HF thus helping to direct limited healthcare resources to interventions that provide the best health economic outcomes. This is integral for providers and payers making evidence-based decisions regarding the adoption and reimbursement of new technologies.
Overall, using these criteria provides a robust toolkit to evaluate the impact of different treatment modalities in HF. Combined the three areas offer a harmonized approach to measure and compare the effectiveness of healthcare interventions in the treatment of HF.
HF therapy, device-based options and their evolution
HF treatment, including device therapy, aims to enhance functional capacity, increase survival, and prevent progression to end-stage heart failure. Devices for monitoring patient status can furthermore anticipate exacerbations and preemptively adjust therapy—thereby regulating the frequency and necessity of hospital admissions.
Clinical trials give hard evidence, that device therapy is beneficial for some heart failure patients, showing an effect even in those without a history of ventricular arrhythmia. Evolution of heart failure therapies, including devices, is expected to have a significant impact on patients with end-stage heart failure [24].
Device therapy for treatment of HF has played an increasing role in the past decades. Device-based therapies are employed across the complete spectrum of heart failure management, from prehospitalization decompensation detection and inpatient management of acute decompensated heart failure (ADHF) to stabilization of cardiogenic shock and durable mechanical circulatory support. The number, indication, and complexity of these therapies continue to grow, making the recognition and familiarity with available technologies increasingly crucial for both research and clinical practice [25].
As stated above—in the aspect of innovation and new approvals there has been a notable change in device-based therapies over the last decade. Several device-based therapies were recently approved for HF management and play an integral role in managing different phenotypes of HF. Part of these new devices target structural or neurohormonal abnormalities that are not directly amenable to pharmacologic interventions.
A recent classification scheme categorizes new device-based therapies based on their mechanisms of action, including dilators, removers, inotropes, interstitials, pushers, pullers, and selective devices. These new devices aim to alleviate symptoms and recover heart and renal function in acute HF decompensation. The development of device-based therapy targeting specific paths is further evolving. It addresses the complex physiopathology of acute decompensated heart failure (ADHF) and is broadly described in literature [26].
Telemedical care for heart failure therapy
Telemonitoring uses different means of communication technology to remotely monitor patients' health status. It is a crucial aspect of managing chronic diseases, especially heart failure.
Telemonitoring for heart failure has evolved over almost 25 years, from 1999 to 2022. As stated before, implant-based telemonitoring can provide early detection of worsening HF and enable pre-emptive interventions that improve patient outcomes.
One of the milestone trials to use telemonitoring, was the IN-TIME clinical trial [27]. This trial involved automatic daily implant-based multiparameter telemonitoring. The authors proved, that daily multiparameter telemonitoring is effective in reducing clinical endpoints in patients with chronic systolic heart failure both in ICD and CRT-D subgroups. But they also noted that selection of patient groups is highly success-relevant, and the absolute benefit seems to be higher in higher-risk populations with a worse prognosis.
Telemonitoring modalities in HF are often proposed as essential for the organization and transition of HF care. However, their efficacy and the best way to measure has always been a point of debate in different publications [28, 29].
To further elaborate on the real effectiveness of the telemonitoring approach, a comprehensive meta-analysis was conducted in 2023 to study home telemonitoring systems (hTMS) in HF and its effect on clinical outcomes [30]. The meta-analysis showed that telemedical care resulted in a reduction of all-cause mortality and a downturn in HF-related hospitalizations.
Still, the methods of telemedical care and population selection remain diverse in the researched studies. For this reason, the authors suggest a more standardized approach to better compare health economic effects. Future research should strive to standardize these modes of effective telemedical care for HF patients.
Economic effects of device-based therapies for HF
The health economic impact of Cardiac Resynchronization Therapy (CRT) Devices, Implantable Cardioverter Defibrillators (ICD) Therapy, and Pacemakers in heart failure therapy has been a subject of several studies.
Besides the approach of telemedical care in HF there are different fields of discussion targeting the health economic effects of device-based HF therapy. It can be summarized in the following fields with actual results in different health economic studies.
Studies evaluating CRT-Ds and ICDs demonstrate the cost-effectiveness of CRT-D devices
A systematic review investigated the cost-effectiveness of CRT combined with an implantable cardioverter-defibrillator (CRT-D) versus ICD alone in patients with heart failure (HF). The authors concluded that CRT-D compared to ICD alone was the most cost-effective treatment in patients with HF. The highest and the lowest Incremental cost-effectiveness ratio (ICER) were reported in the US ($138,649 per quality-adjusted life years (QALY)) and the UK ($41,787 per QALY) respectively [31].
This is an interesting result, as the device-related primary costs of implanting the CRT are higher, but generate an effect, that is feasible from a health economic perspective—a fact that should not mislead one to interpret lower overall cost. The treatment is more cost intensive but generates QALYs at an accepted rate of costs.
CRT-D is from that interpretation the better strategy in most liberal budgets. In fact, if the cost of the CRT-D devices is further lowered or their battery longevity increased, it would become an even more attractive option. Even with an ICER considered at the World Health Organization WTP threshold of 35.000 USD per QALY [32] it is already today (in selected studies) the preferable health economic strategy.
Looking at the drivers for this cost effectiveness, it is a mixture of mortality reduction and a reduction of (re)hospitalisation rates. For example, Mealing et al. showed that CRT-D reduced monthly hospitalization rate by 30% while ICDs decreased monthly hospitalization rates by 20% [33]. Vice versa it is clear, based on the results of the current stated systematic review above, that more expensive CRT-D devices or a shorter battery life made the cost-effectiveness of CRT-D less advantageous.
One relevant specific health economic question is targeting on ICER of CRT-D vs. CRT-P with eye on the necessity of shocks in the relevant population
A review discussed economic evaluation models for cardiac resynchronization therapy with a biventricular pacemaker (CRT-P) and implantable cardioverter defibrillator (CRT-D). It highlighted that adding an ICD may further reduce the risk of sudden cardiac death but questioned the cost-effectiveness ratio of CRT-D compared to CRT-P if most patients do not require shock therapy [34]. For many patients it is not health economically feasible to add the “D” option to the CRT device, as it lowers battery life (at higher costs for the device) and it has no primary necessity in these populations.
Further one study on different left and right ventricular stimulation showed in a cost-effectiveness analysis that patient survival was 6.78 years with right ventricular only stimulation and 7.52 years with CRT-P, indicating an increase in survival of 10.9% [35].
This aspect once more shows, that there is no “one size fits all” approach in device-based therapy. Selecting the right device always must incorporate the state of the patient: severity of the illness, life expectancy, necessity of shocks etc. for the best selection of the device. A CRT-D device should consider critical versus a standard CRT-P in most of the patients believing this systematic review.
A very interesting aspect focuses on long-term runtime costs of CRT devices in the sense of a “total cost of ownership” view on the devices
A study investigated the runtime and costs of biventricular defibrillators (CRT-D) and biventricular pacemakers (CRT-P), providing insights into the costs associated with cardiac resynchronization therapy (CRT) device runtime across different manufacturers [36]. The study used health claims data from a large German health insurance provider to analyze the runtime of CRT devices, defined as the time between implantation and the date of generator change or removal. The median costs for implantation, change, and removal of a CRT device were also calculated in this work of Hadwiger et al.
The study included 17,826 patients with 4,296 complete runtimes for CRT-D devices and 429 for CRT-P devices observed. Median device runtime was 6.04 years for CRT-D devices and 8.16 years for CRT-P devices, with CRT-D devices having a significantly shorter runtime (p < 0.0001). The median cost of implantation for a CRT-D device was 14,270 EUR, and for a CRT-P device, it was 9,349 EUR. With this data analyzed, the study concluded that CRT-D devices not only had a shorter runtime by about two years compared to CRT-P devices but were also associated with higher costs. This is significant for cost-effectiveness analyses and reflects the point given above.
Conclusion and actual development
In terms of total costs of different devices, CRT-Ds were found in several studies to be more expensive than all other devices. The increased expense appeared to be mainly in regards to runtime and maintenance costs—including (re)implantation. In studies over past years where CRT-D treatment was compared to ICD alone, CRT-D appeared to be the most cost effective treatment. Further comparison between CRT-D versus CRT-P does not show a clear economic advantage of CRT-D. This appears to be mainly because not enough patients require shock therapy.
Furthermore, a positive health economic effect and higher survival rate is seen in CRT-P full ventricular stimulation versus only right heart stimulation. Studies show, telemedical care has a health economic impact for selected patient groups which extends even to reducing mortality. For HF in both ICD and CRT-D subgroups the given telemonitoring benefit seems to be bigger in higher-risk populations with worse a HF prognosis. All device therapies (CRT-D, CRT-P, ICDs and Pacemakers) are in range of a accepted cost-effectiveness. QALY and ICER calculations provide a nuanced understanding of the economic impact these therapies have in the healthcare landscape. For a more severe state of HF, CRT-D with telemedical care seems the better option from a health economic standpoint.
References
GBD. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2017;392:1789–858.
Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R. the Midlands Research Practices Consortium (MidReC). Prognosis of all-cause heart failure and borderline left ventricu- lar systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J. 2007;28:1128–34.
Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–76.
Nichols GA, Reynolds K, Kimes TM, Rosales AG, Chan WW. Comparison of risk of re- hospitalization, all-cause mortality, and medical care resource utilization in patients with heart failure and preserved versus reduced ejection fraction. Am J Cardiol. 2015;116:1088–92.
Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, ... Heart Failure Association of the ESC (HFA) (2010) EURObservational research programme: the heart failure pilot survey (ESC‐HF Pilot). Europe J Heart Fail 12(10):1076–1084
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–46. https://doi.org/10.1136/hrt.2003.025270.
Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. https://doi.org/10.15420/cfr.2016:25:2.
Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128.
Cowie MR, Anker SD, Cleland JGF, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Fail. 2014;1(2):110–45. https://doi.org/10.1002/ehf2.12005.
Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. https://doi.org/10.1161/CIR.0000000000000350.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. https://doi.org/10.1016/j.jacc.2013.05.019.
McMurray JJV, Adamopoulos S, Anker SD, et al. Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–847. https://doi.org/10.1093/eurheartj/ehs104.
Komajda M, Follath F, Swedberg K, et al. The EuroHeart Failure Survey programme–a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J. 2003;24(5):464–74. https://doi.org/10.1016/s0195-668x(02)00823-0.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Lindmark K, Boman K, Olofsson M, Törnblom M, Levine A, Castelo-Branco A, Schlienger R, Bruce Wirta S, Stålhammar J, Wikström G. Epidemiology of heart failure and trends in diagnostic work-up: a retrospective, population-based cohort study in Sweden. Clin Epidemiol. 2019;11:231–44.
Al-Omary MS, Davies AJ, Evans TJ, Bastian B, Fletcher PJ, Attia J, Boyle AJ. Mortality and readmission following hospitalisation for heart failure in Australia: a systematic review and meta-analysis. Heart Lung Circ. 2018;27:917–27.
Savarese G. Peter Moritz Becher, Lars H Lund, Petar Seferovic, Giuseppe M C Rosano, Andrew J S Coats, Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2022;118(17):3272–87. https://doi.org/10.1093/cvr/cvac013.
Barra S, Providencia R, Narayanan K, Boveda S, Duehmke R, Garcia R, Leyva F, Roger V, Jouven X, Agarwal S, Levy WC, Marijon E. Time trends in sudden cardiac death risk in heart failure patients with cardiac resynchronization therapy: a systematic review. Eur Heart J. 2020;41:1976–86.
Drummond M, Manca A, Sculpher M. Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care. 2005;21(2):165–71. https://doi.org/10.1017/S0266462305050221.
Shah D, et al. Cost-effectiveness analysis of implantable cardiac devices in patients with systolic heart failure: A US perspective using real world data. J Med Econ. 2020. https://doi.org/10.1080/13696998.2020.1746316.
Banka G, Heidenreich PA, Fonarow GC. Incremental cost-effectiveness of guideline-directed medical therapies for heart failure. J Am Coll Cardiol. 2013;61(32):1440.
Joseph P, Dokainish H, McCready T, Budaj A, Roy A, Ertl G, Gomez-Mesa JE, Leong D, Ezekowitz J, Hage C, Lanas F, Maggioni AP, Sliwa K, Zhu J, Rouleau J, Balasubramanian K, Yusuf S, Investigators GC. A multinational registry to study the characteristics and outcomes of heart failure patients: the global congestive heart failure (G-CHF) registry. Am Heart J. 2020;227:56–63.
Kocot E, Kotarba P, Dubas-Jakóbczyk K. The application of the QALY measure in the assessment of the effects of health interventions on an older population: a systematic scoping review. Arch Public Health. 2021;79:201. https://doi.org/10.1186/s13690-021-00729-7.
Patenaude BN, Bärnighausen T. Quality-adjusted life year weights and treatment bias: Theory and evidence from cognitive interviews. SAGE Open Med. 2019;12(7):2050312119856986. https://doi.org/10.1177/2050312119856986.PMID:31217971;PMCID:PMC6563399.
Fudim M, Abraham W, von Bardeleben R, et al. Device Therapy in Chronic Heart Failure. J Am Coll Cardiol. 2021;78(9):931–56. https://doi.org/10.1016/j.jacc.2021.06.040.
Brooksbank JA, Albert C. Device-based therapies for decompensated heart failure. Curr Opin Cardiol. 2023;38(2):116–23. https://doi.org/10.1097/HCO.0000000000001026. (Epub 2023 Jan 11 PMID: 36718621).
Oliveira Cardoso Cristiano, Elgalad Abdelmotagaly, Li Ke, Perin Emerson C. Device-based therapy for decompensated heart failure: An updated review of devices in development based on the DRI2P2S classification in Frontiers in Cardiovascular Medicine Vol. 9 2022 https://doi.org/10.3389/fcvm.2022.962839.
Geller JC, Lewalter T, Bruun NE, et al. Implant-based multi-parameter telemonitoring of patients with heart failure and a defibrillator with vs. without cardiac resynchronization therapy option: a subanalysis of the IN-TIME trial. Clin Res Cardiol. 2019;108:1117–27. https://doi.org/10.1007/s00392-019-01447-5.
Veenis JF, Radhoe SP, Hooijmans P, Brugts JJ. Remote monitoring in chronic heart failure patients: is non-invasive remote monitoring the way to go? Sensors (Basel). 2021;21:887. https://doi.org/10.3390/s21030887.
Cichosz SL, Udsen FW, Hejlesen O. The impact of telehealth care on health-related quality of life of patients with heart failure: results from the Danish TeleCare North heart failure trial. J Telemed Telecare. 2020;26:452–61. https://doi.org/10.1177/1357633X19832713.
Niels T B Scholte, Muhammed T Gürgöze, Dilan Aydin, Dominic A M J Theuns, Olivier C Manintveld, Eelko Ronner, Eric Boersma, Rudolf A de Boer, Robert M A van der Boon, Jasper J Brugts, Telemonitoring for heart failure: a meta-analysis, Eur Heart J., Volume 44, Issue 31, 14 2023, Pages 2911–2926, https://doi.org/10.1093/eurheartj/ehad280.
Teimourizad A, Rezapour A, Sadeghian S, et al. Cost-effectiveness of cardiac resynchronization therapy plus an implantable cardioverter-defibrillator in patients with heart failure: a systematic review. Cost Eff Resour Alloc. 2021;19:31. https://doi.org/10.1186/s12962-021-00285-5.
Bertoldi EG, et al. Cost-effectiveness of cardiac resynchronization therapy in patients with heart failure: the perspective of a middle-income country’s public health system. Int J Cardiol. 2013;163(3):309–15.
Mealing S, Woods B, Hawkins N, et al. Cost-effectiveness of implantable cardiac devices in patients with systolic heart failure. Heart. 2016;102:1742–9.
Tomini F, Prinzen F, van Asselt AD. A review of economic evaluation models for cardiac resynchronization therapy with implantable cardioverter defibrillators in patients with heart failure. Eur J Health Econ. 2016 Dec;17(9):1159–1172. https://doi.org/10.1007/s10198-015-0752-3.
Martignani C, Massaro G, Diemberger I, Ziacchi M, Angeletti A, Galiè N, Biffi M. Cost-effectiveness of cardiac resynchronization therapy. J Med Econ. 2020;23(12):1375–8. https://doi.org/10.1080/13696998.2020.1833893.
Funding
The article was not funded in any way.
Author information
Authors and Affiliations
Contributions
All authors contributed by different sections to the manuscript. Christian Elsner was the main writer of the body and did the primary review research, Dennis Häckl reviewed the health economic statement and all others contributed by reviewing, correcting and commenting on the main parts adding single scientific sources from a literature research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
Roland Tilz is a consultant for Boston Scientific, Biotronik, Biosense Webster, Abbott Medical, he received speaker`s honoraria from Boston Scientific, Biotronik, Biosense Webster, Abbott Medical, Lifetech and research grants from Abbott, Biosense Webster, Lifetech.
Human and animal rights statement
This article does not contain any studies with human or animal subjects performed by any of the authors. All studies cited in this article fulfilled the above guidelines.
Informed consent statement
This article did not process any data that needs an informed consent, nor was new data generated, which needs an additional ethics confirmation. All studies cited in this article fulfilled the above guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elsner, C., Bettin, S., Tilz, R. et al. Economic Considerations of Cardiovascular Implantable Electronic Devices for The Treatment of Heart Failure. Curr Heart Fail Rep 21, 186–193 (2024). https://doi.org/10.1007/s11897-024-00664-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-024-00664-y