Abstract
Purpose of Review
Acute right ventricular failure (RVF) is a frequent condition associated with high morbidity and mortality. This review aims to provide a current overview of the pathophysiology, presentation, and comprehensive management of acute RVF.
Recent Findings
Acute RVF is a common disease with a pathophysiology that is not completely understood. There is renewed interest in the right ventricle (RV). Some advances have been principally made in chronic right ventricular failure (e.g., pulmonary hypertension). Due to a lack of precise definition and diagnostic tools, acute RVF is poorly studied. Few advances have been made in this field.
Summary
Acute RVF is a complex, frequent, and life-threatening condition with several etiologies. Transthoracic echocardiography (TTE) is the key diagnostic tool in search of the etiology. Management includes transfer to an expert center and admission to the intensive care unit (ICU) in most severe cases, etiological treatment, and general measures for RVF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute right ventricular failure (RVF) is a frequent and life-threatening condition defined as a clinical syndrome related to structural and/or functional impairment of the right ventricle (RV) decreasing its capacity of blood ejection through the pulmonary circulation [1]. Although historically less studied than the left ventricle (LV), research and clinical interest in the RV have been growing in the past decades. The role of the RV is to keep the right atrium pressure as low as possible by ejecting blood through the pulmonary system which is characterized by low resistances. However, RVF is a frequent pathology associated with a poor prognosis [1, 2] with the same incidence as left ventricular failure (LVF) [1]. Etiologies of RVF are various including myocardial infarction (MI), acute respiratory distress syndrome (ARDS), pulmonary embolism (PE), myocarditis, pericarditis, arrhythmias, and pulmonary hypertension (PH) [3]. The dramatic increase in RV afterload that may occur after PE, tension pneumothorax, or ARDS leads to a dilation of the RV and increased right heart pressures, also known as acute cor pulmonale [4,5,6,7].
During the past few years, there has been a growing interest in the RV. It is now clearly established that the RV plays an essential role in the cardiovascular system and its failure may negatively impact the prognosis [8]. Transthoracic echocardiography (TTE) is the key diagnostic tool in search of the etiology. After some physiological reminders on the RV, this review focuses on the pathophysiological basis, the diagnosis, and the management of acute RVF according to the current state of knowledge on the subject.
Physiology of RV
Definition
Acute RVF is defined as a clinical syndrome related to structural and/or functional impairment of the RV with systemic congestion resulting from impaired RV filling and/or reduced flow output [1, 9]. A clear definition of RVF is lacking but frequently includes RV dilation (e.g., RV/LF ratio > 0.6 on echocardiography) and paradoxical septal motion [1, 2, 9]. Chronic RVF is a consequence of increasing pressure in the RV caused by PH, most frequently observed in pulmonary disease or chronic heart failure (CHF) [10].
Anatomy and Physiology
The right ventricle is a thin muscular structure (its muscle mass is six times less than that of LV) wrapped around the LV. It is triangular in shape, with the base formed by the tricuspid annulus at the top of the tricuspid valve which has three anatomic faces: septal (contacting the interventricular septum), anterior, and posterior. These proceed into the tendinous cords followed by the papillary muscles. The apex, including the sinus, is trabeculated and the outlet or infundibulum includes the conus, which is a tubular muscular structure supporting the pulmonary valve leaflets [11,12,13].
The right ventricle connects the systemic circulation and the pulmonary circulation at low resistance and high compliance [13]. Its role is to keep right atrium pressures as low as possible by ejecting blood continuously into the pulmonary arterial system. This continuous blood ejection is made possible by the specificities of the pulmonary vascular bed, characterized by low pressures and low resistances. Histologically, the RV is formed by trabecular muscles [14]. During its contraction, the RV performs a complex peristaltic motion around the LV from the conus to the infundibulum. The right ventricle shares common muscle fibers with LV [11], explaining the phenomenon of ventricular coupling due to the participation of the LV to the RV contraction [15,16,17].
The vascularization of the RV is complex [13]. Most of the vascularization of the RV is based on the right coronary artery (from the base of RV to the apex) explaining why its occlusion is followed by an extensive ischemia of the RV. [18]
The right ventricular function depends on several factors summarized in Fig. 1.
Myocardial Contractility
Myocardial contractility is defined as the inherent ability of cardiomyocytes to contract independently of loading conditions and corresponds to the force of contraction per unit of time developed by these cells. Although this concept is particularly interesting from a physiological point of view, its measurement is not possible under experimental conditions [18].
Preload Conditions of RV
Preload conditions correspond to RV end-diastolic pressure (or the level of maximal stretch of myocardial fibers before isovolemic contraction) [13]. Right ventricular preload conditions are influenced [14] by venous return, the volume of the venous system, compliance of the RV, heart rate, LV filling pressures, and intrapericardial pressures.
Afterload Conditions of RV
Defined as the resistive power opposing myocardial fibers during contraction, afterload is the determining factor of myocardial oxygen consumption. Therefore, increased RV afterload induces an increase in RV oxygen requirements [19]. Because the RV myocardium is thin (high compliance), increased afterload conditions will result in RV dilation without an increase in diastolic pressures.
Heart Rate
Heart rate is an important determinant of cardiac output (CO). During tachycardia, RV filling time is reduced (while the systolic ejection time is unchanged) inducing a reduction in the systolic ejection volume.
Ventricular Geometry
The change in LV geometry (anatomic changes, arrhythmias, and conduction disturbances) has direct consequences on RV performance and systolic ejection volume.
Ventricular Coupling
The right and left ventricles are anatomically arranged in series, enclosed in an inextensible pericardial sac, making them dependent on each other [20]. In case of an increased volume of the RV (PE for example), the interventricular septum is displaced toward the LV during diastole, resulting in an increase in left heart pressures and a decrease in systolic ejection volume (Bernheim reverse effect) [18, 20,21,22,23].
Pathophysiology of RVF
The pathophysiology of acute RVF summarized in Fig. 2 is complex and incompletely understood and involves several factors, namely:
-
Reduced RV contractility (RV infarction, right heart cardiomyopathy, post-operative RV failure)
-
Volume overload (tricuspid or pulmonary insufficiency)
-
Pressure overload (PH, PE, left heart cardiomyopathy, ARDS, cardiac tamponade, pneumothorax)
In the event of reduced myocardial contractility, increased pressure, and/or volume overload of the RV, compensatory mechanisms to maintain constant blood ejection of RV will rapidly be set up. The heterometric adaptation initially described by Starling defined as an increased contractility of stretching myocardial fibers is the first compensatory mechanism put in place. Secondly, homomeric adaptation increases the myocardial contraction force by increasing myocardial calcium transient [24]. Despite these rapidly implemented compensatory mechanisms and in the absence of immediate treatment of the cause of RVF, the RV will rapidly dilate (with the appearance of tricuspid regurgitation, (TR)) leading rapidly to a reduction in right and left blood ejection. Precipitating factors of acute RVF include mechanical ventilation (increasing transpulmonary pressure (alveolar-pleural pressure), reduced RV stroke volume, and CO [25, 26]), hypoxemia [27] and hypercapnia [28] (by acting on the state of contraction of the pulmonary vessels, increase vascular resistance and therefore pulmonary artery pressure (PAP)), and an excessive vascular filling (increasing RV dilation, right atrial pressures, and congestion of the upstream organs (liver and kidney)).
Epidemiology of Acute RVF
The prevalence of acute RVF is difficult to estimate mainly due to the lack of a precise and clear definition of this condition.
The occurrence of acute RVF is associated with poor outcomes, namely high in-hospital mortality rates ranging from 5 to 7% according to studies [9]. Etiologies of acute RVF are various. The three main causes of acute RVF includes MI, PE, and ARDS in 30 to 50% of cases [29]. Acute RV myocardial infarction (RVMI) is typically seen in patients with acute inferior MI and is reported to be present in more than 50% [30]. In the SHOCK trial, where patients with MI complicated by cardiogenic shock were enrolled, predominant RVF was observed in 5% of patients [31]. Acute PE can cause acute RV strain because of pressure overload [15]. Acute RVF is reported to be present in 25 to 60% of patients with acute PE [32, 33] [15]. The occurrence of acute RVF during ARDS is a major risk factor of -ICU mortality [34].
Infectious etiologies of acute RVF are various. In a prospective study of patients with myocarditis, the presence of RV dysfunction diagnosed by cardiac magnetic resonance imaging (MRI) was associated with a hazard ratio of 3.4 for death or heart transplantation and was the strongest predictor of death [35]. Arrhythmias such as atrial fibrillation and atrial flutter are often associated with RV pressure overload, and can negatively affect RV filling [3]. Post-surgical acute RVF occurs during or after noncardiac surgery due to myocardial ischemia [15], secondary to hypoxia, microemboli, arrhythmias, and excessive fluid loading [15]. However, its incidence is difficult to determine. More than 20% of patients with implanted isolated left ventricular assist device (LVAD) experience acute RVF, which is a leading cause of premature morbidity and mortality [36,37,38]. The implantation of LVAD increases venous return and RV preload, which leads to a collapse of RV function, RV dilation, TR, leftward shift of the interventricular septum, and a reduced RV stroke volume [15].
Diagnosis of Acute RVF
The diagnosis of RVF is challenging since there is no specific sign of the condition and often various signs co-exist. Of note, TTE is the key exam for the diagnosis and the severity assessment of acute RVF. Biology is often used to detect organ dysfunction and to precise the etiology of acute RVF. Clinical signs, biology, and TTE parameters are summarized in Table 1.
Clinical Signs of Acute RVF
Clinical signs of acute RVF are summarized in Table 1. The clinical presentation of acute RVF is heterogenous, depending on its etiology, of the presence of systemic congestion and organ dysfunction. In the most severe cases, patients with acute RVF can present signs of cardiogenic shock (e.g., tachycardia, hypotension, skin mottling, oliguria/anuria, encephalopathy, cold extremities) [15].
Biology
There is no specific biomarker for the diagnosis of acute RVF [1]. Biological assays are mainly used for etiological diagnosis (e.g., troponinemia in MI). Impaired renal function (with increased serum creatinine and blood urea nitrogen associated with a decreased glomerular filtration rate (GFR)) and liver function (increased serum alanine transaminase and serum aspartate transaminase, increased lactate level, and decreased coagulation factors) are observed in case of severe congestion [9]. Finally, while the B-type natriuretic peptide (BNP) is nonspecific to RVF [39], several studies found an association between BNP levels and outcomes in patients admitted for PAH with acute RVF[26, 40, 41].
Electrocardiographic Evaluation
Acute RVF is often associated with sinus tachycardia. In addition, electrocardiographic signs associated to the etiology (e.g., PE) can be found. Finally, atrial arrhythmias are frequent [15].
Transthoracic Echocardiographic Signs and Parameters
Transthoracic echocardiography is a safe and reproducible exam for the diagnosis and severity assessment of acute RVF. Echocardiographic signs a various and may all not be present (Table 2). A RV dilation (defined by a RV/LV ratio > 0.6 [1, 9, 42]) with loss of the triangular conformation of RV ((its shape rounding at the apical 4-chamber view) associated or not dilation of the inferior vena cava (IVC) is frequently observed [42]. In the case of severe acute RVF with prominent RV dilation, a paradoxical septal motion can be observed [9]. The presence of hypokinesia and/or dyskinesia certifies myocardial damage Bubble study is strongly recommended in the echocardiographic evaluation of acute RVF, notably for the research of a patent foramen ovale, a direct indicator of increased RV filling pressures.[27].
Right Heart Catheterization
Right heart catheterization is the reference exam for evaluation of acute RVF [1] (measurement of systolic PAP, diastolic PAP, mean PAP, peripheral vascular resistance (PVR), CO, pulmonary capillary wedge pressure (PCWP), right atrial pressure (RAP), and stroke volume (SV)). The right heart catheterization also provides indices of tissue perfusion such as mixed venous oxygen saturation (SvO2). However, due to the invasiveness of the procedure, there has been a shift toward echocardiography in recent years although right heart catheterization does have its advantages, namely in critically ill patients (e.g., mechanical ventilator management, post-cardiovascular surgery), where the accuracy of echocardiography is easily compromised [43]. Hemodynamic parameters observed during acute RVF include the following [1]: increased central venous pressure (CVP) greater than 20 mmHg, inversion of CVP-PCWP gradient (CVP > PCWP), increased right atrial pressure (RAP), and low cardiac index (CI; < 2L/min/m2) in the most severe cases.
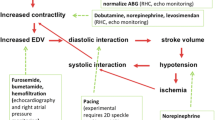
Management of Acute RVF
Given the complexity of the disease and its rapidly fatal course, multidisciplinary management (including cardiologists, pulmonologists, and ICU physicians) in expert centers is often necessary. The emergency is in the etiological diagnosis and severity assessment of the disease to correctly refer the patient. Therapeutic management of acute RVF is based on (Fig. 3):
-
General measures
-
Treatment of consequences of RVF
-
Etiological treatment
General Measures
The goal of the management of acute RVF is to maintain RV function to prevent the evolution to cardiogenic shock. General measures (prevention of increasing RV afterload, maintenance of myocardial contractility, and optimization of RV preload conditions) should therefore be implemented rapidly.
Assessment and Optimization of Volemia
The failing RV is sensitive to the fluid status during acute RVF. Hypovolemia worsens myocardial ischemia and perfusion of organs already impaired by upstream congestion of failing RV. Hypervolemia results in an increased RV volume, with a risk of aggravation of the paradoxical septal motion in systole. In this case, volume overload (assessed by TTE), decongestion by diuretics, or even renal replacement therapy (RRT) should be initiated without delay. Loop diuretics play a key role for this indication. Continuous infusion after loading doses rather than bolus injection alone of loop diuretics provides the prompt achievement of a steady state of plasma loop diuretic concentration [1]. Furthermore, a combination of the use of loop diuretics with thiazide-like diuretics is indicated whenever diuretic resistance is suspected [1]. In case of abnormal volume status, a close biological (looking for worsening of renal function) and TTE monitoring (regular assessment of volume status, measurement of CO) is necessary. If hypovolemia is suspected, in addition to an echocardiographic examination, fluid challenge associated with right heart catheterization could be considered to estimate the patient’s volume status. In case of hypotension associated to hypovolemia, a careful fluid challenge may be tested [44]. If necessary (and only when PCWP is normal), conservative infusion may be required, to restore RV end-diastolic volume and CO. The benefit of infusion when PCWP exceeds 30 mmHg is still controversial [45]. Thus, caution is recommended regarding infusion in patients with suspected RVF.
Mechanical Ventilation
In severe shock or ARDS, airway control and mechanical ventilation are often necessary. The increase in transpulmonary pressure (alveolar pressure—pleural pressure) induced by mechanical ventilation can reduce RV stroke volume (and CO) by increasing RV afterload conditions. If necessary, mechanical ventilation should be adjusted with low tidal volume (6 ml/kg of ideal weight), low positive expiratory pressure, and limited auto-PEEP by reducing the respiratory rate and increasing expiratory time [7, 46,47,48]. Inspired fractional of oxygen should be adapted to patient objectives, and to maintain sufficient tissue oxygenation, and to limit the occurrence of hypoxemia (a strong pulmonary arterial vasoconstrictor) but also, to limit the occurrence of denitrogenation atelectasis (responsible for an increase in RV afterload conditions).
Finally, maintenance of sinus rhythm in RVF patients is a critical issue: atrial contraction accounts for 40% of the RV filling (or even more in RVF patients), and thus maintenance of sinus rhythm ensures effective filling [1]. In case of impaired LV ejection fraction with nonsinusal rhythm, amiodarone is the drug of choice. Electric shock must be considered in case of hemodynamic instability [2].
Reducing RV Afterload Conditions
Reducing RV afterload can be achieved by pharmacologic measures and by controlling the vasoconstrictive state of the pulmonary vessels (hypoxemia and hypercapnia as discussed above). Inhaled nitric oxide (NO) and prostacyclin have a direct and selective effect on pulmonary vascular smooth muscle. Inhaled NO has a rapid onset of action and a short half-life. Especially, using NO may be useful in critically ill patients with PH and/or hypoxemia. Following its prolonged administration, a rebound effect on PH has been described upon abrupt discontinuation of NO [49]. Despite its beneficial hemodynamic effects, no association between NO use and prognosis has been found during acute RVF [50].
An alternative to inhaled NO is inhaled prostacyclin. In addition to its vasodilatory agents, prostacyclin is a potent antiplatelet agent. No rebound effect has been described to date. The latest guidelines for the diagnosis and treatment of pulmonary hypertension recently published in 2022 recommend class IIa the use of prostacyclin analogues [51]. Epoprostenol needs continuous intravenous administration and was associated with reduced mortality [52]. Inhaled iloprost also demonstrated a decrease in pulmonary vascular resistance (PVR), which directly affects RV function, in a study in which patients of severe PAH and chronic thromboembolic PH (New York Heart Association functional class III or IV) [53]. Treprostinil is available for subcutaneous, intravenous, inhaled, and oral administration. Subcutaneous, intravenous, and inhaled administration have been shown to improve the symptoms of PAH patients [54,55,56,57]. On the other hand, for oral administration, primary endpoint (6-min walk distance) has not shown significant improvement in PAH patients on background therapy with other medications (bosentan and/or sildenafil) [58, 59], and studies are inconclusive.
Improving Right Coronary Perfusion
In case of hemodynamic instability and hypotension, norepinephrine is the vasopressor of choice to restore right coronary perfusion. Norepinephrine dosages should be tailored to the clinical situation. At high doses, norepinephrine has positive inotropic effects to improve myocardial contractility and CI [60]. Few data are currently available on other catecholamines in acute RVF [61].
Improvement of Myocardial Contraction
In case of reduced CO due to impaired myocardial contractility during acute RVF, inotropes should be rapidly considered to restore peripheral perfusion. Dobutamine does not increase pulmonary vascular resistance at low doses (< 5 g/kg/min) but may rapidly induce tachycardia and hypotension which may in turn result in increased myocardial ischemia [9]. Dobutamine should therefore be combined with norepinephrine to prevent the occurrence of hypotension [1]. Milrinone is a phosphodiesterase III (PDE3) inhibitor that prevents the degradation of cyclic adenosine monophosphate (cAMP), increasing the available intracellular calcium supply [1]. Milrinone improves myocardial contractility but is responsible for peripheral vasodilation that can potentially worsen myocardial ischemia, thus limiting its use for RVF. Levosimendan is a positive inotropic agent that combined PDE3 and calcium sensitizer, acting by increasing the sensitivity of myofilaments to calcium, but has the characteristic of not increasing the cytosolic calcium concentration and is therefore without a negative effect on diastolic function [62]. Furthermore, it does not increase myocardial oxygen consumption in patients with heart failure [62, 63]. In addition, levosimendan induces pulmonary, systemic, and coronary vasodilation through its action on adenosine triphosphate-dependent acid potassium channels [63]. It can therefore potentially lead to a decrease in myocardial perfusion, but due to the dilation of the coronary network, myocardial perfusion is actually increased [63]. This inotropic treatment cannot be considered without initially restoring adequate right coronary perfusion. Further studies are needed to investigate inotropes in acute RVF.
Treatment of Consequences of RVF
Right ventricular failure is associated with an increased risk of developing organ dysfunction, namely liver and kidney failure, due to systemic congestion and hypoperfusion (through a reduced CO). Screening for the impact of congestion on these organs involves monitoring liver function (PT, factor V associated with liver enzymes) and kidney function (serum creatinine, BUN, and GFR assessment). The management of the consequences of RVF includes the avoidance of all potentially hepatotoxic (e.g., paracetamol) and nephrotoxic treatments (e.g., aminoglycosides). There are few specific treatments, and treatment for RVF is the best treatment for these consequences.
Etiological Treatment
Etiological treatment of RVF is a major point in the management of acute RVF and should be initiated as soon as possible. Coronary angiography and reperfusion need to be considered for myocardial infarction, anticoagulation or interventional thrombectomy for pulmonary embolism, and pericardiocentesis for cardiac tamponade. Surgery or catheter-based interventions are quickly needed in case of valvular lesions.
Mechanical Circulatory Support
Knowledge on the benefit of mechanical circulatory support in acute RVF remains limited and no recommendation is available to date. The initiation of mechanical circulatory support in acute RVF can reasonably be proposed when the etiology is reversible (bridge to recovery) or while waiting for a heart transplant (bridge to transplantation). The time to implantation of mechanical assistance before the occurrence of organ failure is the main prognostic factor [64]. Clinical trials comparing the efficacy of these devices with other management are still needed.
Ongoing Trials and Perspectives
Table 2 summarizes ongoing trials related to acute RVF. A study (NCT04792879) exploring the detective power of pulsed Doppler ultrasound of the common femoral vein for acute RVF in the setting of PH may support the diagnosis of ARHF by echocardiography in a more convenient and rapid way. Although a higher risk of occurrence of RVF has been reported in patients after LVAD implantation [65, 66], few studies have studied long-term follow-up with echocardiography. In this regard, an ongoing study (NCT03552679) involving 600 patients will add new insight into the prognosis of post-LVAD implantation patients at high risk of occurrence of RVF. One study (NCT01757522) is attempting to propose the two-dimension (2D) strain as new echocardiographic parameters for the detection of RV dysfunction in patients with ARDS. Assessment of myocardial strain may point to earlier right ventricular problems. In addition, regional contractility patterns may vary with underlying disease [13], so maybe one means of approaching the complex pathogenesis of RVF. In patients with ARDS, the adverse effects of mechanical ventilators on RVF are particularly problematic. Finally, another study (NCT03202641) compares two different strategies for PEEP: a group using a low PEEP/high FiO2 table (PEEP ARDSnet) and a group managed with pulmonary recruitment maneuver followed by PEEP guided by transpulmonary pressure (PEEP LRM).
Conclusion
Right ventricular failure is a clinical syndrome affecting the systemic and pulmonary circulation. Multidisciplinary treatment is needed and transfer to an expert center should be considered. The primary goal of management is to identify the cause and to assess the severity of the disease. Transthoracic echocardiography plays a central role in the management of acute RVF. Therapeutic management of acute RVF is challenging and includes general measures to maintain RV function and to prevent cardiogenic shock, treatment of the etiology, and consideration of mechanical circulatory support in the most severe cases. However, data are clearly lacking, and further studies are needed to better understand the pathophysiology and to optimize therapeutic management.
Data Availability
The dataset generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Grignola JC, Domingo E. Acute right ventricular dysfunction in intensive care unit. Biomed Res Int. 2017;2017:8217105. https://doi.org/10.1155/2017/8217105.
Grignola JC, Domingo E, López-Meseguer M, Trujillo P, Bravo C, Pérez-Hoyos S, et al. Pulmonary arterial remodeling is related to the risk stratification and right ventricular-pulmonary arterial coupling in patients with pulmonary arterial hypertension. Front Physiol. 2021;12:631326. https://doi.org/10.3389/FPHYS.2021.631326.
Arrigo M, Huber LC, Winnik S, Mikulicic F, Guidetti F, Frank M, et al. Right ventricular failure: pathophysiology, diagnosis and treatment. Card Fail Rev. 2019;5:140. https://doi.org/10.15420/CFR.2019.15.2.
Aubry A, Paternot A, Vieillard-Baron A. Cor pulmonale. Rev Mal Respir. 2020;37:257–66. https://doi.org/10.1016/J.RMR.2019.10.012.
Jardin F. Ventricular interdependence: how does it impact on hemodynamic evaluation in clinical practice? Intensive Care Med. 2003;29:361–3. https://doi.org/10.1007/S00134-003-1643-0.
Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol. 1985;1999(87):1644–50. https://doi.org/10.1152/JAPPL.1999.87.5.1644.
Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med. 2001;29:1551–5. https://doi.org/10.1097/00003246-200108000-00009.
Schmeißer A, Rauwolf T, Groscheck T, Fischbach K, Kropf S, Luani B, et al. Predictors and prognosis of right ventricular function in pulmonary hypertension due to heart failure with reduced ejection fraction. ESC Heart Fail. 2021;8:2968. https://doi.org/10.1002/EHF2.13386.
Harjola VP, Mebazaa A, Čelutkiene J, Bettex D, Bueno H, Chioncel O, et al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016;18:226–41. https://doi.org/10.1002/EJHF.478.
Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–71. https://doi.org/10.1002/EJHF.873.
Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart. 2006;92(Suppl):1. https://doi.org/10.1136/HRT.2005.077875.
Goor DA, Lillehei GW. Congenital malformations of the heart. 1st ed. New York: Grunne & Stratton. 1975.
Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1463–82. https://doi.org/10.1016/J.JACC.2018.12.076.
Duperret S, Schmitt Z, Bonnet A, Wallon G, Aubrun F. Dysfonction cardiaque droite. Le Congrès Médecins Urgences vitales. 2013. https://sofia.medicalistes.fr/spip/IMG/pdf/Dysfonctioncardiaquedroite.pdf. Accessed 10 May 2013.
Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137:e578-622. https://doi.org/10.1161/CIR.0000000000000560.
Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, et al. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;198:e15-43. https://doi.org/10.1164/RCCM.201806-1160ST.
Damiano RJ, la Follette P, Cox JL, Lowe JE, Santamore WP. Significant left ventricular contribution to right ventricular systolic function. Am J Physiol 1991;261. https://doi.org/10.1152/AJPHEART.1991.261.5.H1514.
Martin CRBVB. Physiologie humaine appliquée. Paris: Arnette; 2017.
Crystal GJ, Pagel PS. Right ventricular perfusion: physiology and clinical implications. Anesthesiology. 2018;128:202–18. https://doi.org/10.1097/ALN.0000000000001891.
Visner MS, Arentzen CE, O’Connor MJ, Larson EV, Anderson RW. Alterations in left ventricular three-dimensional dynamic geometry and systolic function during acute right ventricular hypertension in the conscious dog. Circulation. 1983;67:353–65. https://doi.org/10.1161/01.CIR.67.2.353.
Guyton A HJ. Précis de physiologie médicale. Piccin. 2003.
Redington AN, Rigby ML, Shinebourne EA, Oldershaw PJ. Changes in the pressure-volume relation of the right ventricle when its loading conditions are modified. Br Heart J. 1990;63:45–9. https://doi.org/10.1136/HRT.63.1.45.
Dexter L. Atrial septal defect. Br Heart J. 1956;18:209–25. https://doi.org/10.1136/HRT.18.2.209.
Cingolani HE, Pérez NG, Cingolani OH, Ennis IL. The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol 2013;304. https://doi.org/10.1152/AJPHEART.00508.2012.
Santamore WP, Gray LA. Left ventricular contributions to right ventricular systolic function during LVAD support. Ann Thorac Surg. 1996;61:350–6. https://doi.org/10.1016/0003-4975(95)01056-4.
Noveanu M, Breidthardt T, Potocki M, Reichlin T, Twerenbold R, Uthoff H, et al. Direct comparison of serial B-type natriuretic peptide and NT-proBNP levels for prediction of short- and long-term outcome in acute decompensated heart failure. Crit Care. 2011;15. https://doi.org/10.1186/CC9398.
Naija W, Gayat E, Lortat-Jacob B, Mebazaa A. Anaesthesia and right ventricular failure. Ann Fr Anesth Reanim. 2009;28:1007–14. https://doi.org/10.1016/J.ANNFAR.2009.07.091.
Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, et al. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging. 2010;3:10–8. https://doi.org/10.1016/J.JCMG.2009.09.017.
Grignola JC, Domingo E. Acute right ventricular dysfunction in intensive care unit. 2017.https://doi.org/10.1155/2017/8217105
Kakouros N, Cokkinos DV. Right ventricular myocardial infarction: pathophysiology, diagnosis, and management. Postgrad Med J. 2010;86:719–28. https://doi.org/10.1136/PGMJ.2010.103887.
Jacobs AK, Leopold JA, Bates E, Mendes LA, Sleeper LA, White H, et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol. 2003;41:1273–9. https://doi.org/10.1016/S0735-1097(03)00120-7.
Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Crit Care. 2011;15. https://doi.org/10.1186/CC10119.
Konstantinides SV, Meyer G, Bueno H, Galié N, Gibbs JSR, Ageno W, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. https://doi.org/10.1093/EURHEARTJ/EHZ405.
Zochios V, Parhar K, Tunnicliffe W, Roscoe A, Gao F. The right ventricle in ARDS. Chest. 2017;152:181–93. https://doi.org/10.1016/J.CHEST.2017.02.019.
Caforio ALP, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–33. https://doi.org/10.1093/EURHEARTJ/EHM076.
Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–24. https://doi.org/10.1016/J.JTCVS.2009.11.020.
Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015;34:1123–30. https://doi.org/10.1016/J.HEALUN.2015.06.015.
Soliman OII, Akin S, Muslem R, Boersma E, Manintveld OC, Krabatsch T, et al. Derivation and validation of a novel right-sided heart failure model after implantation of continuous flow left ventricular assist devices: the EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation. 2018;137:891–906. https://doi.org/10.1161/CIRCULATIONAHA.117.030543.
Cepkova M, Kapur V, Ren X, Quinn T, Zhuo H, Foster E, et al. Clinical significance of elevated B-type natriuretic peptide in patients with acute lung injury with or without right ventricular dilatation: an observational cohort study. Ann Intensive Care. 2011;1:18. https://doi.org/10.1186/2110-5820-1-18.
Pirracchio R, Salem R, Mebazaa A. Use of B-type natriuretic peptide in critically ill patients. Biomark Med. 2009;3:541–7. https://doi.org/10.2217/BMM.09.45.
Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, et al. The REVEAL Registry Risk Score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–62. https://doi.org/10.1378/CHEST.11-0676.
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F. Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med. 2002;166:1310–9. https://doi.org/10.1164/RCCM.200202-146CC.
Cochran JM, Alam A, Guerrero-Miranda CY. Importance of right heart catheterization in advanced heart failure management. Rev Cardiovasc Med. 2022;23. https://doi.org/10.31083/J.RCM2301012.
Ait-Hamou Z, Teboul JL, Anguel N, Monnet X. How to detect a positive response to a fluid bolus when cardiac output is not measured? Ann Intensive Care. 2019;9. https://doi.org/10.1186/S13613-019-0612-X.
Sibbald WJ, Driedger AA. Right ventricular function in acute disease states: pathophysiologic considerations. Crit Care Med. 1983;11:339–45. https://doi.org/10.1097/00003246-198305000-00004.
Harjola VP, Parissis J, Brunner-La Rocca HP, Čelutkienė J, Chioncel O, Collins SP, et al. Comprehensive in-hospital monitoring in acute heart failure: applications for clinical practice and future directions for research. A statement from the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2018;20:1081–99. https://doi.org/10.1002/EJHF.1204.
Jardin F, Vieillard-Baron A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med. 2003;29:1426–34. https://doi.org/10.1007/S00134-003-1873-1.
Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862–70. https://doi.org/10.1007/S00134-015-4141-2.
Christenson J, Lavoie A, O’Connor M, Bhorade S, Pohlman A, Hall JB. The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med. 2000;161:1443–9. https://doi.org/10.1164/AJRCCM.161.5.9806138.
Bhorade S, Christenson J, O’Connor M, Lavoie A, Pohlman A, Hall JB. Response to inhaled nitric oxide in patients with acute right heart syndrome. Am J Respir Crit Care Med. 1999;159:571–9. https://doi.org/10.1164/AJRCCM.159.2.9804127.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–731. https://doi.org/10.1093/EURHEARTJ/EHAC237.
Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:993. https://doi.org/10.1056/NEJM199602013340504.
Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. https://doi.org/10.1056/NEJMOA020204.
Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. https://doi.org/10.1164/AJRCCM.165.6.2106079.
Bourge RC, Waxman AB, Gomberg-Maitland M, Shapiro SM, Tarver JH, Zwicke DL, et al. Treprostinil administered to treat pulmonary arterial hypertension using a fully implantable programmable intravascular delivery system: results of the DelIVery for PAH Trial. Chest. 2016;150:27–34. https://doi.org/10.1016/J.CHEST.2015.11.005.
Richter MJ, Harutyunova S, Bollmann T, Classen S, Gall H, Gerhardt MDF, et al. Long-term safety and outcome of intravenous treprostinil via an implanted pump in pulmonary hypertension. J Heart Lung Transplant. 2018;37:1235–44. https://doi.org/10.1016/J.HEALUN.2018.06.006.
McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–22. https://doi.org/10.1016/J.JACC.2010.01.027.
Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142:1383–90. https://doi.org/10.1378/CHEST.11-2212.
Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144:952–8. https://doi.org/10.1378/CHEST.12-2875.
Martin C, Perrin G, Saux P, Papazian L, Gouin F. Effects of norepinephrine on right ventricular function in septic shock patients. Intensive Care Med. 1994;20:444–7. https://doi.org/10.1007/BF01710657.
Ghignone M, Girling L, Prewitt RM. Volume expansion versus norepinephrine in treatment of a low cardiac output complicating an acute increase in right ventricular afterload in dogs. Anesthesiology. 1984;60:132–5. https://doi.org/10.1097/00000542-198402000-00009.
Haikala H, Nissinen E, Etemadzadeh E, Levijoki J, Lindén IB. Troponin C-mediated calcium sensitization induced by levosimendan does not impair relaxation. J Cardiovasc Pharmacol. 1995;25:794–801. https://doi.org/10.1097/00005344-199505000-00016.
Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, et al. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther. 2000;68:522–31. https://doi.org/10.1067/MCP.2000.110972.
Kapur NK, Paruchuri V, Jagannathan A, Steinberg D, Chakrabarti AK, Pinto D, et al. Mechanical circulatory support for right ventricular failure. JACC Heart Fail. 2013;1:127–34. https://doi.org/10.1016/J.JCHF.2013.01.007.
Jabagi H, Nantsios A, Ruel M, Mielniczuk LM, Denault AY, Sun LY. A standardized definition for right ventricular failure in cardiac surgery patients. ESC Heart Fail. 2022;9:1542. https://doi.org/10.1002/EHF2.13870.
Bellavia D, Iacovoni A, Scardulla C, Moja L, Pilato M, Kushwaha SS, et al. Prediction of right ventricular failure after ventricular assist device implant: systematic review and meta-analysis of observational studies. Eur J Heart Fail. 2017;19:926–46. https://doi.org/10.1002/EJHF.733.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Pr. Mebazaa reports personal fees from Orion, Roche, Adrenomed, and Fire 1 and grants and personal fees from 4TEEN4, Abbott, Roche, and Sphyngotec. Dr. Deniau was invited to a meeting in Henningsdorf by 4TEEN4 Pharmaceuticals GmbH. The remaining authors declared no potential conflicts of interest with respect to the research authorship and/or publication of this article. The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asakage, A., Bækgaard, J., Mebazaa, A. et al. Management of Acute Right Ventricular Failure. Curr Heart Fail Rep 20, 218–229 (2023). https://doi.org/10.1007/s11897-023-00601-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-023-00601-5