Abstract
Purpose of the Review
Patients with cardiomyopathy and impaired left ventricular (LV) ejection fraction are at risk of sudden cardiac death (SCD). In selected heart failure patients, cardiac resynchronization therapy (CRT) provides LV reverse remodeling and improves the cellular and molecular function leading to a reduced risk of ventricular arrhythmia and SCD. Consequently, some CRT candidates may not need concomitant ICD therapy. This review aimed at focusing on the residual risk of SCD in patients receiving CRT and discussing the requirement of a concomitant ICD therapy in CRT candidates.
Recent Findings
New imaging diagnostic tools may be helpful to accurately predict patient with a residual risk of SCD and who required a CRT-D implantation. Recent data highlighted that cardiac computed tomography (CT) or myocardial scar tissue analysis using contrast-enhanced cardiac magnetic resonance (CMR) was able to predict the occurrence of VA in patients with bi-ventricular pacing.
Summary
Cardiac imaging and specifically myocardial scar analysis seem promising to evaluate the risk of SCD following bi-ventricular pacing and will probably be of great help in the future to accurately identify those who needs concomitant defibrillator’s protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with cardiomyopathy and reduced left ventricular ejection fraction (LVEF) are at risk of heart failure (HF) symptoms and ventricular arrhythmias (VA), leading to sudden cardiac death (SCD) [1, 2]. In selected HF patient with impaired LVEF and wide QRS, cardiac resynchronization therapy (CRT) implantation has been shown to decrease mortality, morbidity, and improve quality of life [3,4,5,6,7,8]. Indeed, CRT responders experience a LV reverse remodeling associated with an improvement of the cellular and molecular function [9, 10].
An additional benefit has also been published with a decreased risk of ventricular arrhythmia. Indeed, some studies demonstrated a reduction of appropriate implantable cardioverter-defibrillator (ICD) therapy, mostly correlated to positive LV remodeling [5, 11,12,13]. These results may suggest that some patients might not necessarily require a concomitant ICD therapy. However, the residual VA risk following CRT implantation is inconsistent, influenced by pre-implantation and post-implantation factors (i.e., etiology of cardiomyopathy, baseline echocardiography, or imaging parameters such as cardiac fibrosis) [14•]. Thus, the decision to implant a CRT-P or a CRT-D is sometimes challenging in clinical practice.
This review aimed at focusing on the residual risk of SCD in HF patients receiving CRT and discussing the requirement of a concomitant ICD implantation in CRT candidates.
The Benefit of Cardiac Resynchronization Therapy and Defibrillator Implantation in Heart Failure Patients
Current guidelines highly recommend CRT implantation for symptomatic HF patients in sinus rhythm with severe LVEF and large left bundle branch block (> 150 ms) (class I, level A) but also with a lower level in patients with QRS duration between 130 and 150 ms (level B). For patients without LBBB the class of recommendation is lower [15]. These recommendations are based on numerous trials establishing the positive impact on mortality and morbidity of CRT provided by CRT pacemakers in severe HF patients (NYHA functional class III/IV) (i.e., MUSTIC, CARE HF, and MIRACLE trials) or CRT-P/CRT-D (Companion) [3,4,5, 16]. Additionally, the benefit of CRT implantation has also been demonstrated in mildly symptomatic HF patients (NYHA II) (i.e., REVERSE, MADIT-CRT, and RAFT trials), mainly with CRT-D with a LV reverse remodeling and a decrease in HF hospitalization [6,7,8]. Among these studies of mild-HF CRT recipients, the RAFT trial with a longer follow-up was the only one to observe a positive impact on mortality, with a 25% risk reduction [8].
Regarding the benefit of ICD implantation, primary prevention indication has been shown to decrease the risk of SCD in HF patients with reduced LVEF [17]. Indeed, in the SCD-HeFT trial including patients with or without ischemic cardiomyopathy, ICD therapy reduced by 23% the risk of mortality compared to placebo in a population of 847 HF patients who remained symptomatic (functional class II-III) with LVEF < 35% after drug treatment optimization [18]. Interestingly, a subgroup analysis of the SCD-HeFT study demonstrated a benefit of ICD implantation to prevent sudden tachyarrhythmic death in patients with either ischemic or non-ischemic cardiomyopathy [19]. Currently, an ICD in primary prevention is recommended to reduce the risk of SCD in ischemic or non-ischemic patients with symptomatic HF (NYHA II/III) and an LVEF < 35% despite an optimal medical therapy beyond 40 days after myocardial infarction in those with ischemic heart disease) [15].
In clinical practice the decision to implant a CRT-P or CRT-D varies widely among countries and the choice of the right device for the right patient is sometime challenging. In the COMPANION trial, patients were randomized to compare CRT with or without ICD vs. optimal medical therapy in a 1:2:2 fashion. Authors clearly showed that both device therapies reduced the endpoints of death and HF hospitalization compared to medical therapy group. However, no difference has been found between CTR-P and CRT-D in these populations, but the study was not powered to compare these two treatments [16].
Impact of CRT Implantation on the Risk of Sudden Cardiac Death
LV Reverse Remodeling Induced by CRT
In HF patients, some parameters (such as impaired LVEF, myocardial adverse remodeling with calcium homeostasis deregulation, or wide intrinsic QRS duration) are associated with the occurrence of ventricular arrhythmia (VA) [20, 21]. Interestingly, CRT positively impacts these factors in selected HF patients. Indeed, molecular remodeling has been evaluated in animal models and later on confirmed in human studies. In a canine model of left ventricular dyssynchrony, bi-ventricular pacing partially restored ryanodine receptor expression leading to more homogeneous diastolic calcium sparks [22]. Similarly, reverse calcium regulatory genes were evaluated in 24 patients scheduled for CRT and responders experienced a significant improvement of the sarcoplasmic reticulum calcium ATPase 2α and phospholamban gene expression, whereas non-responders did not have positive molecular reverse remodeling [23]. Additionally, other human data showed a significant decrease in the pathologic hypertrophic gene expression after CRT [10]. Furthermore, in a canine model, bi-ventricular pacing reduced LV fibrosis and decreased pro-fibrotic factors in both the serum and myocardial tissue [24]. Beyond molecular improvement, echocardiographic reverse remodeling has been described in various studies. In a sub-analysis of 323 patients enrolled in the MIRACLE study, authors demonstrated that after a 6-month follow-up, CRT significantly reduced end-diastolic and end-systolic volumes and increased LVEF [25]. These effects were confirmed in the CARE-HF trial [5]. Similarly, in less severe patients reverse LV remodeling of 262 CRT recipients included in the REVERSE cohort was analyzed. After 2 years of follow-up, the LV end-systolic and LV end-diastolic volume index were reduced by 30% and 20%, respectively. Similar findings were observed in the MADIT-CRT and RAFT trials [7, 8]. A recent CT-guided study has evaluated the impact of CRT on LV wall thickness change in 8 CRT responders. Authors showed that response to CRT is associated with wall thickness normalization (defined as wall thickness > 6 mm) and especially in basal and mid ventricular segments [26].
Inconsistent CRT Response Among Candidates
Currently, a significant decrease in LV end-systolic volume index between 15 and 25% at 6 months, with or without increase in LVEF, defines a positive echocardiographic CRT response [27, 28]. Despite major technical improvements, the benefit of CRT is inconsistent between candidates and around 15% of CRT recipients may present a decline of the LV function over time. Conversely, between 10% and 25% are described as “super-responders” and experience an exceptional improvement with a “normalization” or “near”-normalization of LVEF [29,30,31]. Many factors are associated with response to CRT, such as female gender, lower body mass index, non-ischemic cardiomyopathy, wider QRS or LBBB morphology, and lack of dilated left atria [30]. Interestingly, a specific LBBB-induced cardiomyopathy due to chronic major mechanical dyssynchrony has been described and authors reported a LV dimension normalization after CRT implantation [31]. However, despite a better understanding in cardiac dyssynchrony and a better selection in CRT candidates, 30% still do not respond to therapy. Consequently, pathologic LV remodeling and electrical/structural substrates induced by the cardiomyopathy still persist. As a result, residual risk of SCD seems variable in CRT patients and remains complex to evaluate.
Risk of Sudden Cardiac Death in Patients Implanted with CRT
VAs are common in HF patients with reduced LVEF. In DAI-PP (Défibrillateur Automatique Implantable-Prévention Primaire) study enrolling up to 5000 ICD recipients implanted primary prevention, > 20% experienced at least one VA episode during 3-year of follow-up [32]. In patients implanted with CRT, the risk of SCD has also been specifically evaluated. Indeed, in the CeRtiTude cohort study, 1705 CRT recipients (535 with CRT-P and 1170 with CRT-D, respectively) were enrolled. Interestingly, patients with CRT-P were sicker at baseline. They were older, less often male. Patients were less likely to have ischemic heart disease, had a wider QRS complex, were more symptomatic, and had more co-morbidities. After 2-year follow-up, authors showed that the overall mortality was 2-fold higher in the CRT-P, but the incidence of SCD and the rate of HF hospitalization were not different between both groups. Importantly, the increased mortality in the CRT-P group was not related to the occurrence of SCD but secondary to HF progression. Investigators concluded that all patients eligible for CRT cannot be “automatically” considered as requiring a CRT-D [33]. These data are consistent with a recent review investigating the SCD incidence among patients implanted with CRT over the last 20 years. Interestingly, there was a gradual decrease in SCD rate since the early 2000s. Notably, the difference in SCD rate between CRT-P and CRT-D recipients decreased considerably over time [34]. These findings suggest that CRT implantation positively impact the risk of VA and could limit the need for concomitant ICD implantation in selected patients.
Beneficial Effect of CRT on the Occurrence of Ventricular Arrhythmia
Various studies have specifically evaluated the impact of CRT on the evolution of VA risk in patients with HF. As previously reported in a population of 31 recipients CRT-D, biventricular pacing was associated with a 66% decrease in appropriate ICD therapies compared to 34 patients implanted with an ICD [35]. Similarly, CRT was associated with a 29% reduction in the occurrence of first VA in patients included in the MADIT-CRT trial. Of note, the beneficial anti-arrhythmic effect of CRT was higher among patients with LBBB with a 2-fold lower risk compared to those with non-LBBB morphology [36].
Previous work showed that the degree of LV reverse remodeling induced by CRT strongly reduced the risk of life-threatening VA. These data are supported by a sub-analysis of the MADIT-CRT trial, which found a decreased VA risk according to the CRT response with a 1-year risk of ICD therapy of 5%, 12%, and 24% in the super-responders, responders, and hypo-responders, respectively [30]. Barsheshet et al. explored the risk of VA between “low” and “high” echocardiographic CRT responders (defined as > 25% reduction of the LV end-systolic volume). After 24 months of follow-up, authors showed that “high” echocardiographic CRT responders had a significantly lower risk of VA compared to those with “low” echocardiographic response or ICD recipients [11]. Recently, the risk of VA has been assessed among the CRT recipients included in the MADIT-CRT trial and who were defined as super-responders. A total of 55 patients achieved a LVEF > 50%, and VA ≥ 200 bpm occurred in only 1 patient, and none received an ICD shock. This result suggests an absolute low risk of VA in CRT super-responders [37]. These data were supported by a multicenter study of 629 CRT-D recipients followed for up to 6 years. A total of 37 patients were super-responder (LVEF > 50%) and experienced a significantly lower 5-year rate of anti-tachycardia pacing and ICD shocks compared to non-super responders (2.7% vs. 22.1% and 2.7% vs. 14.3%, respectively) [12]. A recent meta-analysis including up to 1700 patients assessed a rate of appropriate ICD therapy in patients with LVEF improvement ≥ 45% of 2.3/100 person-years, which was more than 3-fold lower compared to those with LVEF improvement < 45% [38]. Of note, the threshold rate of arrhythmic mortality/100 person-years that is associated with net benefit from ICD has been estimated at 3%, suggesting that some patients at low arrhythmic risk could be implanted with CRT-P rather than CRT-D. To summarize, current data demonstrate that only patients who present complete or near LVEF normalization are at very low risk of VA. These results suggest that reverse remodeling induced by CRT could improve the underlying arrhythmic substrate. Conversely, patients who experienced LVEF improvement > 35% but without normalization of LVEF had a decreased but not eliminated VA risk [39].
How to Predict the Residual Risk of Sudden Cardiac Death in CRT Recipients
In selected HF patients with severe LVEF impairment and prolonged QRS, CRT-P or CRT-D implantation will induce LV reverse remodeling. Currently, many CRT candidates also meet criteria for ICD implantation and up to 80% of patients are implanted with CRT-D in primary prevention in clinical practice [40]. However, among these patients, CRT-P could be self-sufficient to reduce the risk of SCD. Consequently, the challenge is to implant the appropriate device to the appropriate patient and predictors or new diagnostic tools are warranted to identify those at risk of SDC.
Clinical Predictors
Identifying patients with residual risk of SCD after CRT implantation is crucial, and previous works described clinical predictor to determine patients at risk of SCD following CRT implantation. Killu et al. found 3 predictors pre-CRT implantation of appropriate ICD therapy in CRT-D recipients: male gender, baseline LV, end-systolic volume and LVEF [12]. Notably, female CRT super-responders have a 5-year incidence of appropriate ICD therapy of 0%. Ruwald et al. described 6 predictors of LVEF normalization (> 50%) after CRT implantation: female gender, no history of myocardial infarction, LBBB, baseline LVEF > 30%, baseline LV end-systolic volume ≤ 170 ml, and baseline left atrial volume index ≤ 45 ml/m2. In this study, a total of 42 patients had all factors and none experienced VAs during follow-up [31]. Additionally, in a cohort of 196 CRT recipients, super-responders experienced a lower rate of VA compared to the non-super-responders (5.9% vs. 24.4%, respectively). Authors also reported four predictors of VA following CRT: baseline QRS duration > 160 ms, amiodarone, previous history of VA, and non-super-responder patients (LVEF < 45%) [41].
Regarding the impact of the underlying cardiomyopathy etiology on the risk of VA following CRT implantation, discrepancy results have been published so far. Indeed, in a cohort of 115 patients with CRT, those with an ischemic cardiomyopathy surprisingly presented a 2-fold decreased risk of appropriated ICD therapies [42]. Controversial data have been published in a population of 689 patients with CRT. The cohort was divided in four groups: patients with ischemic cardiomyopathy vs. non-ischemic dilated cardiomyopathy and those implanted with an ICD vs. CRT-D. Results showed that ischemic patients with CRT-D have a same cumulative probability of appropriate ICD therapy compared to those with only ICD. However, non-ischemic patients with CRT-D have a 2-fold lower of 2-year appropriate ICD incidence compared to the ICD group (24.7% vs. 41.6%, respectively) [43]. Authors deservedly suggest that CRT strongly reduced the occurrence of appropriate ICD therapies in patients with non-ischemic. Similarly, a study enrolling more than 1500 CRT recipients (CRT-D, n = 551, and CRT-P, n = 999) demonstrated that CRT-D was associated with a lower total mortality and hospitalizations for MACE in patients with ischemic cardiomyopathy. However, no differences were found between CRT-D and CRT-P in non-ischemic patients [44]. These works suggest that the cardiomyopathy etiology could be an interesting parameter for helping physicians to define patients who need a CRT-P or CRT-D. However, in a cohort of 795 patients with CRT, investigators showed a trend for improved survival in ischemic patients with CRT-D, but this result was not significant [45]. Nevertheless, further studies are needed to identify subpopulations of patients with ischemic or non-ischemic cardiomyopathy with indications for CRT who may benefit from CRT-D.
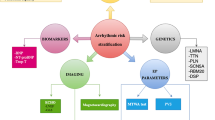
Currently, in the light of the literature, it seems complex to predict the residual risk of VA after CRT implantation, which is mainly driven by predictors of super-response (Fig. 1). On the other hand, besides clinical characteristics, predictors of super-response are based on echocardiography parameters associated with inter-physician’s variability.
Focus on Left Bundle Branch Block-Induced Cardiomyopathy
LBBB has been proved to change the myocardial activation sequence leading to an intra-LV mechanical dyssynchrony, functional myocardial ischemia, and in some cases HF with LVEF impairment [46, 47]. Data from animal models and clinical observations in humans support the existence of LBBB-induced cardiomyopathy representing an optimal target for CRT implantation without concomitant ICD [48]. Two clinical situations can lead to LBBB-dyssynchronopathy: [1] the chronic situation of isolated LBBB on presumed healthy hearts and [2] the acute iatrogenic situation of new LBBB, mainly after aortic valve interventions. Indeed, previous work supports the hypothesis of LBBB cardiomyopathy potentially reversible by bi-ventricular pacing. Vaillant et al. reported a series of patients with a history > 5 years of LBBB without heart disease who developed progressive LV dysfunction (LVEF < 40%) with symptomatic HF (NYHA II to IV). All patients were described as super-responders to CRT with complete resolution of symptoms and regression of LV dysfunction [28]. This pattern of cardiomyopathy may also occur in patients experiencing new iatrogenic LBBB after transcatheter aortic valve replacement (TAVR). Indeed, new LBBB occurred in 12 to 22% in patients scheduled for TAVR [49] and numerous studies reported deleterious effects of new-LBBB among TAVR-recipients leading to LV dysfunction [50, 51]. These data suggest that CRT-P could potentially improve LVEF in patients with de-novo LBBB following TAVR and who experienced LV dysfunction. Nonetheless, this hypothesis deserves proper evaluation in further studies.
Cardiac Imaging Tools
Currently, the prediction of super-response is mostly based on baseline characteristics and echocardiography parameters. However, new imaging diagnostic tools may be helpful to accurately predict patient with an expected super-response or those at risk of residual SCD and who require protection from VA. Recent data highlighted that cardiac computed tomography (CT) or myocardial scar tissue analysis using contrast-enhanced cardiac magnetic resonance (CMR) are correlated to the response to CRT and the risk of VA in patients with bi-ventricular pacing. A recent work demonstrated in a cohort of 54 CRT recipients that the total area of LV with reduced wall thickness (i.e., < 6 mm) measured with CT would help to stratify response to CRT. The authors showed that CRT candidates with a low percentage of LV wall thickness < 6 mm significantly improved their clinical status and echocardiography parameters compared to the groups with a larger proportion of reduced LV wall thickness [52]. The utility of cardiac CT in prognosticating the risk of VA in non-ischemic patients with CRT has also been evaluated [53]. In this study, authors demonstrated that the total percentage of reduced LV wall thickness (i.e., < 6 mm) was an independent predictor of VA after CRT implantation in non-ischemic patients. About 40% of reduced wall thickness differentiated patients at risk of VA and potential candidates to CRT-D implantation. In this work, it has been suggested that the burden of reduced wall thickness measured at baseline may mirror the extent of LV fibrosis and VT substrate. This hypothesis is supported by a cohort of 14 HF patients who underwent electro-anatomical mapping, and thinner LV was associated with a decrease of local conduction velocity, reduced bipolar/unipolar voltage, and larger LV electrograms. These data demonstrated the potential relationship between reduced wall thickness measured with CT and occurrence of VT during follow-up [54].
The analysis of myocardial scar tissue by contrast-enhanced CMR could also be an interesting tool to differentiate CRT-P from CRT-D candidates. Indeed, a recent work investigated the benefit of CRT-P or CRT-D implantation depending on the absence/presence of LV midwall fibrosis detected by CMR. Authors showed that in non-ischemic patients without LV midwall fibrosis, CRT-D implantation did not confer a better survival compared to CRT-P [55]. Furthermore, the analysis of myocardial scar tissue by contrast-enhance CMR was correlated to the occurrence of VA in CRT candidates, regardless of the etiology of the cardiomyopathy. In another work, the characteristic of the myocardial scar was accurately analyzed among a cohort of 78 patients. The presence of a myocardial scar > 16% and a border zone > 9.5 g were independent predictors of appropriate ICD therapy and SCD, respectively, associated with a 7.8-fold and 4. 6-fold increased risk. Notably, a scar mass > 16% had 100% sensitivity and 81% specificity, for 1-year prediction of appropriate ICD therapy, while a border zone mass > 9.5 g had a 100% sensitivity and 93% specificity for 1-year appropriate ICD therapy. This work highlights that CMR seems promising to identify a very low-risk subgroup or CRT patients that will not benefit from a back-up defibrillator [56]. These data are supported by a larger study enrolling 217 CRT candidates. Pre-procedural scar analyzed using ce-CMR was performed to assess the residual risk of VAs and SCD among this population. A total of 92 patients had no myocardial scar and none experienced ICD therapies or SCD. Among 125 patients with late gadolinium enhancement a total of 25 patients had appropriate ICD therapy or SCD events. Total scar mass, core mass, and border zone mass were significantly greater in this subgroup of patients with arrhythmic events. Furthermore, border zone channel mass was higher in patients with VAs or SCD [57•]. Interestingly, authors developed two algorithms to identify patient without ICD therapy or SCD events during follow-up. These algorithms are based on scar mass and the presence of border channel for the first one and based on scar and border zone mass for the second one. Both algorithms identified patients at high risk of SCD/ICD therapy with 100% sensitivity, 81.3% specificity, and 36.2% positive predictive value for the first algorithm (scar mass + channels) and 100% sensitivity, 79.3% specificity, and 33.3% positive predictive value for the second algorithm (scar mass + border zone mass). Importantly, these algorithms can be used in ischemic and non-ischemic patients [57•]. Recently, the cardiac function measured using CMR has been evaluated to stratify the arrhythmic risk after CRT. In this work, the circumferential strain dyssynchrony parameter analyzed with CMR strain imaging combined with the Seattle HF model score provide promising result to identify CRT-P or CRT-D candidates [58].
Current literature suggests that myocardial substrate is crucial to predict the risk of VA after CRT implantation and probably more relevant than baseline patient characteristics or echocardiography itself. These results are summarized in Fig. 2. However, randomized studies are required to confirm the usefulness of scar characterization for the identification of CRT candidates that could benefit from adding defibrillator or not to the CRT device.
Conclusion
CRT implantation has massively improved the prognosis of HFrEF. LV reverse remodeling and especially super-responders who experienced LVEF normalization are incontestably associated with a reduction of SCD risk. Data suggest that CRT-P is a self-sufficient tool to protect selected HF patients from ventricular arrhythmias. However, identifying CRT candidates who do not need concomitant SCD protection remains challenging in clinical practice. Cardiac imaging and specifically myocardial scar analysis seem promising to evaluate the risk of SCD following bi-ventricular pacing and will probably be of great help in the future to accurately identify those who need concomitant defibrillator’s protection. Currently, the RESET-CRT (Re-evaluation of Optimal Re-synchronisation Therapy in Patients With Chronic Heart Failure) study is enrolling more than 2000 HF patients with optimal medical therapy who are randomized to receive CRT-D or CRT-P device. This trial will probably provide important new information for improving the choice of CRT-P or CRT-D device implantation (NCT03494933).
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- CRT:
-
Cardiac resynchronization therapy
- CT:
-
Computed tomography
- HF:
-
Heart failure
- ICD:
-
Implantable cardioverter defibrillator
- LBBB:
-
Left bundle branch block
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- SCD:
-
Sudden cardiac death
- VA:
-
Ventricular arrhythmia
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Khand A, Gemmel I, Clark AL, Cleland JGF. Is the prognosis of heart failure improving? J Am Coll Cardiol. 2000;36:2284–6.
Kreuz J, Horlbeck F, Linhart M, Mellert F, Fimmers R, Schrickel J, et al. Independent predictors of mortality in patients with advanced heart failure treated by cardiac resynchronization therapy. Europace. 2012;14:1596–601.
Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344(12):873–80.
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–53.
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–49.
Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54(20):1837–46.
Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329–38.
Birnie DH, Ha A, Higginson L, Sidhu K, Green M, Philippon F, et al. Impact of QRS morphology and duration on outcomes after cardiac resynchronization therapy: results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail. 2013;6(6):1190–8.
Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, et al. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119:1220–30.
Iyengar S, Haas G, Lamba S, Orsinelli DA, Babu GJ, Ferketich AK, et al. Effect of cardiac resynchronization therapy on myocardial gene expression in patients with nonischemic dilated cardiomyopathy. J Card Fail. 2007;13:304–11.
Barsheshet A, Wang PJ, Moss AJ, Solomon SD, Al-Ahmad A, McNitt S, et al. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2011;57(24):2416–23.
Killu AM, Mazo A, Grupper A, Madhavan M, Webster T, Brooke KL, et al. Super-response to cardiac resynchronization therapy reduces appropriate implantable cardioverter defibrillator therapy. Europace. 2018;20:1303–11.
Van der Heijden AC, Höke U, Thijssen J, Borleffs CJ, van Rees JB, van der Velde ET, et al. Super-responders to cardiac resynchronization therapy remain at risk for ventricular arrhythmias and benefit from defibrillator treatment. Eur J Heart Fail. 2014;16(10):1104–11.
• Leyva F, Zegard A, Acquaye E, Gubran C, Taylor R, Foley PWX, et al. Outcomes of cardiac resynchronization therapy with or without defibrillation in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2017;70(10):1216–27. This study provides important information about the impact of myocardial mid wall fibrosis on outcomes in non-ischemic patients receiving CRT.
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281–329.
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50.
Theuns DAMJ, Smith T, Hunink MGM, Bardy GH, Jordaens L. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: a systematic review and meta-analysis. Europace. 2010;12:1564–70.
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37.
Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120:2170–6.
Santangeli P, Rame JE, Birati EY, Marchlinski FE. Management of ventricular arrhythmias in patients with advanced heart failure. J Am Coll Cardiol. 2017;69(14):1842–60.
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37.
Vanderheyden M, Mullens W, Delrue L, Goethals M, de Bruyne B, Wijns W, et al. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51(2):129–36.
Wang J, Gong X, Chen H, Qin S, Zhou N, Su Y, et al. Effect of cardiac resynchronization therapy on myocardial fibrosis and relevant cytokines in a canine model with experimental heart failure. J Cardiovasc Electrophysiol. 2017;28(4):438–45.
Wang J, Gong X, Chen H, et al. Effect of cardiac resynchronization therapy on myocardial fibrosis and relevant cytokines in a canine model with experimental heart failure. J Cardiovasc Electrophysiol. 2017;28(4):438–45.
St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107(15):1985–90.
Galand V, Ghoshhajra B, Szymonifka J, Leclercq C, Truong QA, Singh JP. Computed tomography-guided assessment of response to cardiac resynchronization therapy. JACC Clin Electrophysiol. 2019;5(8):987–9.
Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608–16.
Prinzen FW, Vernooy K, Auricchio A. Cardiac resynchronization therapy: state-of-the-art of current applications, guidelines, ongoing trials, and areas of controversy. Circulation. 2013;128(22):2407–18.
Stankovic I, Belmans A, Prinz C, et al. The association of volumetric response and long-term survival after cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2017;18(10):1109–17.
Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, et al. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) study. J Am Coll Cardiol. 2012;59(25):2366–73.
Vaillant C, Martins RP, Donal E, Leclercq C, Thébault C, Behar N, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol. 2013;61(10):1089–95.
Amara N, Boveda S, Defaye P, et al. Implantable cardioverter-defibrillator therapy among patients with non-ischaemic vs. ischaemic cardiomyopathy for primary prevention of sudden cardiac death. Europace. 2018;20(1):65–72.
Marijon E, Leclercq C, Narayanan K, Boveda S, Klug D, Lacaze-Gadonneix J, et al. Causes-of-death analysis of patients with cardiac resynchronization therapy: an analysis of the CeRtiTuDe cohort study. Eur Heart J. 2015;36(41):2767–76.
Barra S, Providência R, Narayanan K, Boveda S, Duehmke R, Garcia R, et al. Time trends in sudden cardiac death risk in heart failure patients with cardiac resynchronization therapy: a systematic review. Eur Heart J. 2019. https://doi.org/10.1093/eurheartj/ehz773.
Arya A, Haghjoo M, Dehghani MR, Alasti M, Alizadeh H, Kazemi B, et al. Effect of cardiac resynchronization therapy on the incidence of ventricular arrhythmias in patients with an implantable cardioverter-defibrillator. Heart Rhythm. 2005;2(10):1094–8.
Ouellet G, Huang DT, Moss AJ, Hall WJ, Barsheshet A, McNitt S, et al. Effect of cardiac resynchronization therapy on the risk of first and recurrent ventricular tachyarrhythmic events in MADIT-CRT. J Am Coll Cardiol. 2012;60(18):1809–16.
Ruwald MH, Solomon SD, Foster E, Kutyifa V, Ruwald AC, Sherazi S, et al. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2014;130(25):2278–86.
Chatterjee NA, Roka A, Lubitz SA, Gold MR, Daubert C, Linde C, et al. Reduced appropriate implantable cardioverter-defibrillator therapy after cardiac resynchronization therapy-induced left ventricular function recovery: a meta-analysis and systematic review. Eur Heart J. 2015;36(41):2780–9.
Zhang Y, Guallar E, Blasco-Colmenares E, Butcher B, Norgard S, Nauffal V, et al. Changes in followup left ventricular ejection fraction associated with outcomes in primary prevention implantable cardioverter-defibrillator and cardiac resynchronization therapy device recipients. J Am Coll Cardiol. 2015;66:524–31.
García-Lunar I, Castro-Urda V, Toquero-Ramos J, Mingo-Santos S, Moñivas-Palomero V, Daniela Mitroi C, et al. Ventricular arrhythmias in super-responders to cardiac resynchronization therapy. Rev Esp Cardiol (Engl Ed). 2014;67(11):883–9.
García-Lunar I, Castro-Urda V, Toquero-Ramos J, Mingo-Santos S, Moñivas-Palomero V, Daniela Mitroi C, et al. Ventricular arrhythmias in super-responders to cardiac resynchronization therapy. Rev Esp Cardiol (Engl Ed). 2014;67(11):883–9.
Loughlin G, Avila P, Martinez-Ferrer JB, Alzueta J, Vinolas X, Brugada J, et al. Association of cardiac resynchronization therapy with the incidence of appropriate implantable cardiac defibrillator therapies in ischaemic and non-ischaemic cardiomyopathy. Europace. 2017;19(11):1818–25.
Fernández-Armenta J, Berruezo A, Mont L, Sitges M, Andreu D, Silva E, et al. Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace. 2012;14(11):1578–86.
Leyva F, Zegard A, Umar F, Taylor RJ, Acquaye E, Gubran C, et al. Long term clinical outcomes of cardiac resynchronization therapy with or without defibrillation: impact of the aetiology of cardiomyopathy. Europace. 2018;20(11):1804–12.
Drozd M, Gierula J, Lowry JE, Paton MF, Joy E, Jamil HA, et al. Cardiac resynchronization therapy outcomes in patients with chronic heart failure: cardiac resynchronization therapy with pacemaker versus cardiac resynchronization therapy with defibrillator. Cardiovasc Med (Hagerstown). 2017;18(12):962–7.
Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109(9):1133–9.
Skalidis EI, Kochiadakis GE, Koukouraki SI, Parthenakis FI, Karkavitsas NS, Vardas PE. Phasic coronary flow pattern and flow reserve in patients with left bundle branch block and normal coronary arteries. J Am Coll Cardiol. 1999;33(5):1338–46.
Auffret V, Martins RP, Daubert C, Leclercq C, Le Breton H, Mabo P, et al. Idiopathic/iatrogenic left bundle branch block-induced reversible left ventricle dysfunction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(24):3177–88.
Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;136:1049–69.
Testa L, Latib A, De Marco F, et al. Clinical impact of persistent left bundle-branch block after transcatheter aortic valve implantation with CoreValve revalving system. Circulation. 2013;127:1300–7.
Urena M, Mok M, Serra V, Dumont E, Nombela-Franco L, DeLarochellière R, et al. Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon-expandable valve. J Am Coll Cardiol. 2012;60:1743–52.
Galand V, Ghoshhajra B, Szymonifka J, Das S, Orencole M, Barré V, et al. Left ventricular wall thickness assessed by cardiac computed tomography and cardiac resynchronization therapy outcomes. Europace. 2020;22(3):401–11.
Galand V, Ghoshhajra B, Szymonifka J, Das S, Leclercq C, Martins RP, et al. Utility of computed tomography to predict ventricular arrhythmias in patients with nonischemic cardiomyopathy receiving cardiac resynchronization therapy. Am J Cardiol. 2020;125(4):607–12.
Ustunkaya T, Desjardins B, Liu B, Zahid S, Park J, Saju N, et al. Association of regional myocardial conduction velocity with the distribution of hypoattenuation on contrast-enhanced perfusion computed tomography in patients with post-infarct ventricular tachycardia. Heart Rhythm. 2019;16(4):588–94.
Leyva F, Taylor RJ, Foley PW, Umar F, Mulligan LJ, Patel K, et al. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2012;60(17):1659–67.
Fernández-Armenta J, Berruezo A, Mont L, et al. Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace. 2012;14(11):1578–86.
• Acosta J, Fernández-Armenta J, Borràs R, Anguera I, Bisbal F, Martí-Almor J, et al. Scar characterization to predict life-threatening arrhythmic events and sudden cardiac death in patients with cardiac resynchronization therapy: the GAUDI-CRT Study. JACC Cardiovasc Imaging. 2018;11(4):561–72. This study provides an extensive description of how myocardial characterization using MRI can predict the risk SDC after CR implantation.
Bilchick KC, Auger DA, Abdishektaei M, Mathew R, Sohn MW, Cai X, et al. CMR DENSE and the Seattle Heart Failure Model inform survival and arrhythmia risk after CRT. JACC Cardiovasc Imaging. 2020;13(4):924–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The article is part of the Topical Collection on Devices
Rights and permissions
About this article
Cite this article
Galand, V., Martins, R.P., Behar, N. et al. CRT-Pacemaker Versus CRT-Defibrillator Who Needs Sudden Cardiac Death Protection?. Curr Heart Fail Rep 17, 116–124 (2020). https://doi.org/10.1007/s11897-020-00465-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-020-00465-z