Abstract
Purpose of the Review
Hyperkalemia is a common electrolyte abnormality that can lead to life-threatening cardiac arrhythmia. Medical management of acute hyperkalemia revolves around three strategies—stabilizing the myocardium, intracellular shifting of serum potassium, and enhancing elimination of total body potassium via urinary or fecal excretion. In this review, we outline the current evidence behind the acute medical management of hyperkalemia.
Recent Findings
Two new oral potassium-binding agents, patiromer and sodium zirconium cyclosilicate, show promise in the management of hyperkalemia. Their role in the acute setting needs further investigation. Recent investigations also suggest that the optimal dosing of intravenous insulin may be lower than previously described.
Summary
Despite its prevalence, there is wide variability in the medical management of hyperkalemia in the acute setting. High-quality evidence demonstrating efficacy is lacking for many medications, though novel oral potassium-binding agents show promise. Overall, more research is necessary to establish optimal dosing strategies to manage hyperkalemia in the acute setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperkalemia is a potentially life-threatening disorder occurring in 1–10% of hospitalized patients [1] and up to 2–3% of patients presenting to the emergency department (ED) [2]. It most commonly occurs in patients with diabetes mellitus (DM), acute renal failure, chronic kidney disease (CKD), and heart failure (HF) [3, 4] due to either the underlying pathology or the therapeutic medications that alter potassium excretion [4, 5]. While the prevalence of hyperkalemia in the overall population in the USA was 1.57% in 2014, the prevalence was 6.35% among patients with CKD and/or heart failure [6].
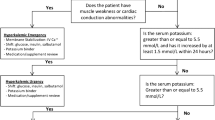
Although there is no internationally agreed upon definition of levels of hyperkalemia, the European Resuscitation Council defines mild hyperkalemia as a plasma level of 5.5–5.9 mmol/L, moderate hyperkalemia as 6.0–6.5 mmol/L, and severe hyperkalemia as > 6.5 mmol/L [7]. Mild hyperkalemia usually presents with nonspecific symptoms of nausea, vomiting, abdominal pain, and generalized weakness [8], while more severe hyperkalemia can cause increased excitability of the myocardium that may lead to significant conduction disorders [9].
Elevated serum potassium has been correlated with increased risk of mortality in multiple patient populations [10, 11]. In 2009, Einhorn et al. performed a retrospective analysis of 245,808 veterans and found an increased risk of mortality within just 1 day of a hyperkalemic event. Notably, this risk was higher for patients who had normal kidney function at baseline [12]. Goyal et al. also demonstrated a “U-shaped” relationship between serum potassium and in-hospital mortality in patients with acute myocardial infarction, where the lowest mortality rates were observed when serum potassium was between 3.5 and 4.5 mmol/L [13]. Currently, acute management of hyperkalemia involves a combination of several strategies: stabilization of the myocardium, intracellular shifting, elimination via urinary or fecal excretion, and definitive care for refractory patients with hemodialysis. This article reviews available efficacy and safety data for each of these strategies for managing hyperkalemia in the acute setting.
Stabilization of the Myocardium
Calcium
Calcium directly antagonizes the toxic effects of hyperkalemia on myocyte membranes. Evidence for this, however, is primarily based on older case series [14,15,16] and expert opinion, as randomized studies are not feasible or ethical. According to the European Council of Resuscitation and the American Heart Association, in the setting of hyperkalemia, calcium is indicated for life-threatening EKG changes including absent P waves, peaked T waves, wide QRS interval, sine-wave pattern, ventricular arrhythmias, and cardiac arrest [7, 17].
Calcium is available in two popular formulations: calcium gluconate and calcium chloride; 1 g of calcium gluconate contains 4.6 mEq of calcium while1 g of calcium chloride contains 13.6 mEq of calcium. Calcium gluconate is given in doses of 1–3 g over 2–5 min, while calcium chloride is given in doses of 500–1000 mg [17, 18]. The protective effect of calcium salts on stabilizing the myocardium in hyperkalemia should be seen within 5 min [19]. Calcium does not have any effect on the serum potassium level and needs to be combined with other therapies to lower serum potassium. Doses can be repeated every 5 min if life-threatening EKG changes persist [7, 17].
Side effects of calcium salts include peripheral vasodilation, hypotension, and bradycardia. Tissue necrosis is also seen more frequently with extravasation of calcium chloride than calcium gluconate, and administration via central veins are preferred for calcium chloride [20]. Calcium should not be given with bicarbonate-containing solutions as calcium carbonate may precipitate. There are also concerns regarding the use of calcium salts in digoxin toxicity causing an irreversible non-contractile state [21]. This is of particular importance in hyperkalemia as severe digoxin toxicity may be accompanied by hyperkalemia [22]. Furthermore, patients on digoxin, namely patients with chronic heart failure, are more prone to hyperkalemia. Animal models have shown that calcium increases digoxin toxicity, but only at non-physiologically high calcium concentrations [23]. More recent data from human studies have not demonstrated worsened outcomes [24, 25]. Levine et al. reviewed 159 cases of digoxin toxicity, of which 23 received calcium. No life-threatening dysrhythmias occurred within 1 h of calcium administration, and mortality was similar between those that received calcium (22%) and those who did not (22%) [25].
Shifting Potassium Intracellularly: Insulin, Albuterol, and Sodium Bicarbonate
Insulin
Insulin binds to glucose transporter type 4 receptor on skeletal muscles, activating sodium-potassium adenosine triphosphatase (ATPase) and leading to potassium transfer from the extracellular space to the intracellular space [26]. Regular insulin has been shown to be effective at decreasing serum potassium, with the onset of action within 15 min, peak activity around 45–60 min, and duration of action between 4 and 6 h [27, 28]. Harel et al. performed a meta-analysis of 11 studies investigating the optimal dosing regimen of insulin [28]. Dosing strategies included 10 units regular insulin IV as a bolus or infusion over 15 or 30 min and 20 units over 60 min. They found that, on average, intravenous (IV) insulin decreased serum potassium by about 0.8 mmol/L at 1 h. Due to the heterogeneity in clinical practice and research methodology, they were unable to determine an optimal dose.
The main side effect of insulin is hypoglycemia, which can be offset with the administration of IV dextrose. Even with the administration of IV dextrose, the systematic review by Harel et al. found that about 20% of patients experience at least one episode of hypoglycemia [28]. The use of weight-based insulin dosing of 0.1 units/kg (maximum 10 units) has been shown to decrease the rates of hypoglycemia while being equally effective at lowering serum potassium [29, 30]. Multiple studies have also investigated using a standardized dose of 5 units of insulin for hyperkalemia with mixed results on efficacy compared to 10 units [31,32,33]. Regardless of insulin dosing strategy, given the risk of hypoglycemia, serum glucose needs to be monitored closely during treatment. Interestingly, transient worsening of hyperkalemia with IV dextrose administration has been described [27, 28].
Albuterol
Albuterol is a beta-2 agonist that promotes intracellular shifting of potassium both through the release of endogenous insulin and independent activation of sodium potassium ATPase of muscle and liver cells [34,35,36]. For decreasing serum potassium, albuterol has an onset within 15–30 min with a duration of action of at least 2 h [27, 36]. A 10-mg dose of nebulized albuterol has been shown to be as effective as 10 units of IV insulin for hyperkalemia, causing a maximal decrease in serum potassium of approximately 1 mmol/L [27, 37,38,39]. The effects of insulin and albuterol in treating hyperkalemia may also be additive [27]. Albuterol administered as a nebulizer treatment or IV infusion has similar decreases in potassium, but infusion has been shown to have increased severity of tachycardia [38]. Common side effects include anxiety, tachycardia, palpitations, and headache. Mild hyperglycemia and a transient, minor increase in serum potassium following albuterol administration have also been demonstrated [27, 40]. Levalbuterol, the isolated R-enantiomer of racemic albuterol, has not been shown to be better than albuterol in lowering serum potassium, though it may have fewer adverse effects [41].
Sodium Bicarbonate
Overall, the data supporting the use of sodium bicarbonate as a treatment for hyperkalemia are controversial. In 1959, Schwarz et al. reported that an infusion of between 144 and 408 mmol of sodium bicarbonate over 2–4 h lowered sodium potassium by 2–3 mmol/L in 4 patients with severe acidosis [42]. Since then, numerous studies have been performed showing little effect on serum potassium in stable hemodialysis patients [27, 37, 43]. It has been suggested that the effect of sodium bicarbonate may be limited to patients with hyperkalemia with concomitant metabolic acidosis. Side effects of sodium bicarbonate administration include hypernatremia and volume overload [1].
There is little evidence to suggest that sodium bicarbonate has a role in the management of hyperkalemia. Small studies have shown that sodium bicarbonate monotherapy does not acutely lower potassium when compared to other potassium-lowering agents [37, 44]. One study of 70 patients with acute hyperkalemia demonstrated, however, that treatment with insulin, albuterol, and sodium bicarbonate was more effective than any combination of two of the three medications [35]. Given the limited evidence for sodium bicarbonate, the United Kingdom (UK) Renal Association and European Resuscitation Council do not recommend the routine use of IV sodium bicarbonate for the acute treatment of hyperkalemia in the stable patient [7, 45].
The role of sodium bicarbonate in the management of the unstable or critically ill patient with hyperkalemia has also been investigated. A recent retrospective study on patients with hyperkalemia and cardiac arrest showed that the administration of sodium bicarbonate and calcium was associated with an increased odds ratio for the sustained return of spontaneous circulation (ROSC) [46]. Another study evaluated the utility of sodium bicarbonate in adult ICU patients with severe acidemia (pH ≤ 7.2, bicarbonate ≤ 20) and found that it lowered incidence of hyperkalemia, the need for renal replacement therapy (RRT), and duration on RRT [47]. The European Resuscitation Council recommends 50 mEq sodium bicarbonate IV push for hyperkalemic patients with severe acidosis or renal failure, while the American Heart Association (AHA) recommends 50 mEq sodium bicarbonate IV push for hyperkalemic patients in the setting of cardiac arrest [7, 17].
Elimination of Potassium
Diuretics
Though many diuretics enhance urinary excretion of potassium from the body, loop diuretics are most commonly used in the acute management of hyperkalemia. Loop diuretics inhibit sodium-potassium-chloride cotransporter (NKCC2) channels in the loop of Henle and thick ascending limb. This blocks the tubular reabsorption of sodium at these sites, increasing sodium delivery to the distal tubule, and stimulating potassium excretion [48, 49]. The natriuretic and kaliuretic effects of loop diuretics are overall dose-dependent, but can be unpredictable and may worsen acute kidney injury (AKI). Studies of the inpatient setting have demonstrated that they can be used alone or in combination with thiazide diuretics to produce synergistic effects on diuresis and potassium excretion [50].
Loop diuretics should only be used in adequate volume resuscitated patients, not in patients who are hypovolemic [48, 49]. Interestingly, in 2013, Chawla et al. report the utility of a furosemide stress test to determine which patients with AKI will not respond to furosemide. With 77 patients, they demonstrated that a urine output of < 200 mL in the first 2 h after 1.0 or 1.5 mg/kg of furosemide had a sensitivity of 87.1% and specificity of 84.1% for predicting patients who would progress to worsening AKI [51].
Acetazolamide increases delivery of bicarbonate to the distal nephron, which can result in increased potassium excretion, and it has also been shown to be effected in enhancing urinary excretion of potassium [52, 53]. Acetazolamide alone, however, is not recommended due to its potential for causing metabolic acidemia which may subsequently increase serum potassium [54].
Sodium Polystyrene Sulfonate
Oral potassium resins, specifically sodium polystyrene sulfonate (SPS), have been around for several decades as adjunctive therapy for treatment of hyperkalemia. SPS, also known as kayexalate, was approved by the FDA in 1958 for the treatment of hyperkalemia. It binds potassium primarily in the large intestine in exchange for sodium. It can be given PO or as an enema and is often given with sorbitol to promote diarrhea [55]. One gram of SPS exchanges about 0.5–1 mEq of potassium in vivo [56], and early clinical trials demonstrated the potential of SPS resin to lower potassium [57, 58]. These trials, however, were generally low quality and not well controlled.
More recent data has supported the utility of SPS in decreasing serum potassium. A randomized control trial (RCT) by Lepage et al. in 2015 in 33 outpatients with CKD and serum potassium between 5.0–5.9 mmol/L showed that 30-g oral SPS per day for 7 days resulted in a decrease of serum potassium of 1.04 mmol/L compared to placebo [59•]. Nasir and Ahmad investigated 97 patients with CKD and hyperkalemia and demonstrated that 15-g oral SPS per day reduced the average serum potassium from 5.8 to 4 .8mmol/L after 3 days [60•]. Other retrospective studies have demonstrated similar decreases in serum potassium with SPS [61,62,63]. SPS has also been showed to have a dose-dependent response [55]. The role of SPS in the management of hyperkalemia in the acute setting is highly limited and less clear. A retrospective study by Mistry et al. demonstrated the efficacy of SPS for decreasing serum potassium based on repeat potassium sometime between 2 and 12 h after administration of SPS. Calcium polystyrene sulfonate has also been shown to be effective in decreasing serum potassium [63, 64], though perhaps not as effective as SPS [65].
Adverse events with SPS include electrolyte disturbances such as hypokalemia, hypomagnesemia, and hypocalcemia, and gastrointestinal symptoms such as nausea, vomiting, constipation, and diarrhea [59•]. Severe gastrointestinal adverse effects such as ulceration, bleeding, ischemic colitis, and perforation can also occur with SPS [61, 63]. The incidence of ischemic colitis and intestinal perforation is between 0.27 and 1.8% as quoted in the literature [56, 66, 67] with a mortality rate as high as 36% [56]. A systematic review by Harel et al. in 2013 found that these severe gastrointestinal events due to SPS may occur anywhere along the gastrointestinal tract, with the large bowel being the most common site [68]. Patients with decreased transit time seem to be more at risk for the severe gastrointestinal side effects of SPS, and the FDA currently recommends against the use of SPS in patients with abnormal bowel function or who are at risk for constipation or impaction [67].
Patiromer
Patiromer has recently been approved by the FDA as an oral potassium resin. Patiromer binds potassium in exchange for calcium; it is an inorganic, smooth, and spherical compound that is not systemically absorbed [69]. Like SPS, the site of action of patiromer in the gastrointestinal tract is the colon [70]. One gram of patiromer can bind to more than 8 mEq of potassium under physiologic conditions [69].
The efficacy and safety of patiromer for the treatment of chronic hyperkalemia has been demonstrated across three randomized control trials totaling approximately 700 patients. The first of which is the PEARL-HF trial in 2011 that studied 120 adult patients with chronic heart failure and serum potassium between 4.3–5.1 mmol/L. Patients were given 15-g oral patiromer or placebo twice daily. Patients in the patiromer group had a significantly lower mean serum potassium by day 3, which remained true for the remainder of the 28-day study period [71•]. The AMETHYST-DN trial in 2015 was a phase 2 dose-ranging study of 306 patients with type II diabetes, CKD, and hyperkalemia. Using doses ranging from 4.2- to 16.8-g oral patiromer twice daily, it demonstrated a dose-dependent reduction in serum potassium with patiromer compared to placebo. Patients with increasing severity of hyperkalemia also had a greater absolute reduction in serum potassium [72•]. Finally, the OPAL-HK trial in 2015 was a phase 3 study of 243 patients with CKD and hyperkalemia. It again demonstrated the dose-dependent efficacy of patiromer in the chronic treatment of hyperkalemia with a mean reduction in serum potassium of 1.01 mEq/L noted by day 3 of treatment [73•]. A separate subgroup analysis of the OPAL-HK trial of patients ≥ 65 years old showed lower rates of recurrent hyperkalemia in this age group [74].
Overall patiromer appears to be well tolerated with low rates of adverse events. The most common side effects include mild to moderate constipation, diarrhea, hypokalemia, and hypomagnesemia. Few serious adverse events occurred in the above trials, and no serious adverse events were attributed to patiromer [71•, 72•, 73•].
While the above three randomized control trials provide strong evidence that patiromer is effective for the chronic management of hyperkalemia and may allow patients to be more adherent to guideline renin-angiotensin-aldosterone system inhibitor (RAASi) therapies, the role of patiromer in the acute management of hyperkalemia is an area that needs further investigation. A single study by Bushinsky et al. did show that patiromer significantly reduced serum potassium by 0.21 mmol/L at 7 h in the inpatient setting, though the sample size was small [75].
Sodium Zirconium Cyclosilicate
In 2018, zirconium silicate (ZS-9) was approved by the FDA for the treatment of hyperkalemia [76]. ZS-9 is an inorganic, microporous zirconium silicate compound that selectively binds potassium in the intestines. It is not systemically absorbed, and 1 g of ZS-9 binds approximately 3 mEq of potassium [77].
The efficacy of ZS-9 in reducing serum potassium has been demonstrated across multiple randomized clinical trials totaling approximately 1100 patients. In a phase 2 study, in 2015, Ash et al. investigated 90 adult patients with CKD and hyperkalemia. They showed a dose-dependent decrease in serum potassium with oral ZS-9 compared to placebo. Interestingly, 10 g three times daily decreased serum potassium by 0.11 mmol/L within just 1 h, reaching a maximum reduction in serum potassium by 0.92 mmol/L by 38 h [78•].
Two-phase three trials showed similar reductions in serum potassium. The HARMONIZE trial in 2015 was a phase 3 dose-ranging trial that investigated the efficacy of ZS-9 in 237 ambulatory patients with a history of hyperkalemia. ZS-9 significantly reduced serum potassium within 1 h (− 0.2 mmol/L compared to placebo), with a difference of − 1.1 mmol/L at 48 h. Median time to normalization of serum potassium was 2.2 h. Much like patiromer, reductions in serum potassium were dose-dependent, and patients with higher baseline potassium experienced greater reductions in serum potassium [79•]. Furthermore, another phase 3 trial by Packham et al. demonstrated the efficacy of ZS-9 for the management of chronic hyperkalemia, though the study only lasted 2 weeks [80]. Adverse events of ZS-9 include edema, gastrointestinal symptoms, and hypokalemia. Overall, it appears to be well tolerated with few serious adverse events reported by the prior studies.
Conclusion
Hyperkalemia is a life-threatening illness as it can lead to dangerous cardiac dysrhythmias and possibly death. Elevated serum potassium has been associated with increased risk of morality, even within 1 day of an episode of hyperkalemia. Current acute management of hyperkalemia relies on three major strategies:
-
1.
Stabilization of the myocardial membrane with calcium salts—calcium gluconate 1–3 g IV or calcium chloride 500–1000 mg IV should be given every 5 min for life-threatening EKG changes including absent P waves, peaked T waves, wide QRS interval, sine-wave pattern, and ventricular arrhythmias. Calcium salts should also be given in cardiac arrest situations when hyperkalemia is present. Calcium salts may precipitate hypotension and bradycardia. Avoid giving calcium chloride through a peripheral line due to the increased risk of tissue necrosis with extravasation.
-
2.
Intracellular shifting of serum potassium with insulin and albuterol—regular acting insulin 5–10 units IV should be administered along with dextrose and frequent glucose monitoring to decrease the risk of hypoglycemia. Nebulized albuterol 10 mg should also be given for further reduction of serum potassium. Albuterol can be given intravenously, but this route has been associated with increased severity of tachycardia.
-
3.
Enhanced elimination via urine and feces with diuretics and oral potassium resins, respectively. Though the evidence for the acute management of hyperkalemia is limited, we recommend giving a loop diuretic and sodium polystyrene sulfonate to help eliminate potassium. Diuretics may precipitate or worsen AKI in patients who are not adequately volume resuscitated. Sodium polystyrene sulfonate should be used with caution given the association with severe gastrointestinal side effects such as ulceration, bleeding, colonic ischemia/necrosis, and intestinal perforation.
The utility of sodium bicarbonate continues to be controversial in the management of hyperkalemia. Sodium bicarbonate should not be routinely administered for the acute treatment of hyperkalemia, though it should be considered for patients with severe metabolic acidosis and in cardiac arrest. Two new oral potassium binders, patiromer and zirconium silicate, have had highly promising results for the management of hyperkalemia, with greater evidence of efficacy and safety when compared to sodium polystyrene sulfonate. Their roles in the acute management of hyperkalemia remain to be determined.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Mahoney BA, Smith WAD, Lo D, Tsoi K, Tonelli M, Clase C. Emergency interventions for hyperkalemia. Cochrane Database Syst Rev 2009;1–54.
Singer AJ, Thode HC, Peacock WF. A retrospective study of emergency department potassium disturbances: severity, treatment, and outcomes. Clin Exp Emerg. 2017;4(2):73–9.
Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–21.
Bandak G, Sang YY, Gaspirini A, Chang AR, Ballew SH, Evans M, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm creatinine measurements (SCREAM) project. J Am Heart Assoc. 2017;6:1–13.
Chang A, Sang YY, Leddy J, Yahya T, Kirchner HL, Inker LA, et al. Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension. 2017;67:1181–8.
Betts KA, Woolley JM, Mu F, McDonald E, Tang W, Wu EQ. The prevalence of hyperkalemia in the United States. Curr Med Res Op. 2018;34(6):971–8.
Truhlar A, Deakin C, Soar J, Khalifa GEA, Alfonzo A, Bierenes JJLM, et al. European resuscitation council guidelines for resuscitation 2015. Resuscitation. 2015;95:148–201.
Medford-Davis L, Zubaid R. Derangements of potassium. Emerg Med Clin N Am. 2014;32:329–47.
Pfennig CL, Slovis CM. Electrolyte disorders. Rosen's emergency medicine: concepts and clinical practice: Elsevier; 2018. p. 1516–32.
Khanagavi J, Gupta T, Aronow WS, Shah T, Garg J, Ahn C, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251–7.
An JN, Lee JP, Jeon HJ, Kim DH, Oh YK, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16(6):225.
Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–62.
Goyal A, Spertus J, Gosch K, Venkitachalam L, Jones PG, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307(2):157–64.
Merril JP, Levin HD, Somerville W, Smith S. Clinical recognition and treatment of acute potassium intoxication. Ann Intern Med. 1950;33:797–830.
Chamberlain MJ. Emergency treatment of hyperkalemia. Lancet Lond Engl. 1964;33:464–7.
Meroney W, Herndon R. The management of acute renal insufficiency. JAMA. 1954;155:877–83.
Vanden Hoek TL, Morrison LJ, Shuster M, Donnino M, Sinz E, Lavonas EJ, et al. 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care, part 12. Cardiac arrest in special situations. Circulation. 2010;122:829–61.
Rossignol P, Legrand M, Kosiborod M, Hollenberg SM, Peacock WF, Emmett M, et al. Emergency management of severe hyperkalemia: guideline for best practice and opportunities for the future. Pharmacol Res. 2016;113:585–91.
Alfonso AVM, Isles C, Geddes C, Deighan C. Potassium disorders - clinical spectrum and emergency management. Resuscitation. 2006;70:10–25.
Moss J, Syrengelas A, Antaya R, Lazova R. Calcinosis cutis: a complication of intravenous administration of calcium gluconate. J Cutan Pathol. 2006;33:60–2.
Erickson CP, Olson KR. Case files of the medical toxicology fellowship of California poison control system - San Francisco: calcium plus digoxin - more taboo than toxic? J Med Toxicol. 2008;4(1):33–9.
Manini AF, Nelson LS, Hoffman RS. Prognostic utility of serum potassium in chronic digoxin toxicity. Am J Cardiovasc Drugs. 2016;11(3):173–8.
Nola GT, Pope S, Harrison DC. Assessment of the synergistic relationship between serum calcium and digitalis. Am Heart J. 1970;79:499–507.
Fenton F, Smally AJ, Laut J. Hyperkalemia and digoxin toxicity in a patient with kidney failure. Ann Emerg Med. 1996;28:440–1.
Levine M, Nikkanen H, Pallin D. The effects of intravenous calcium in patients with digoxin toxicity. J Emerg Med. 2011;40(1):21–46.
Ho K. A critically swift response: insulin-stimulated potassium and glucose transport in skeletal muscle. Clin J Am Soc Nephrol. 2011;6:1513–6.
Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38:869–72.
Harel Z, Kamel K. Optimial dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One. 2016:1–12;11(5):e0154963.
Brown K, Setji TL, Hale SL, Cooper A, Hong B, Herbst R, et al. Assessing the impact of an order panel utilizing weight-based insulin and standardized monitoring of blood glucose for patients with hyperkalemia. Am J Med Qual. 2018;33(6):598–603.
Wheeler DT, Scafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia: hyperkalemia treatment and hypoglycemia. J Hosp Med. 2016;11:355–7.
McNicholas BA, Pham MH, Carli K, Chen CH, Colobong-Smith N, Anderson AE, et al. Treatment of hyperkalemia with a low-dose insulin protocol is effective and results in reduced hypoglycemia. Int Soc Nephrol. 2018;3(2):328–36.
Garcia J, Pintens M, Morris A, Takamoto P, Baumgartner L, Tasaka CL. Reduced versus conventional dose insulin for hyperkalemia treatment. J Pharm Pract. 2018;6:089719001879922.
LaRue HA, Peksa GD, Shah SC. A comparison of insulin doses for the treatment of hyperkalemia in patients with renal insufficiency. Pharmcotherapy. 2017;37:1516–22.
Putcha N, Allon M. Management of hyperkalemia in dialysis patients. Semin Dial. 2007;20:431–9.
Ngugi NN, McLigeyo SO, Kayima JK. Treatment of hyperkalemia by altering the transcellular gradient in patients with renal failure: effect of vrious therapeutic approaches. East Afr Med J. 1997;74:503–9.
Montoliu J, Lens S, Revert L. Potassium-lowering effect of albuterol for hyperkalemia in renal failure. Arch Intern Med. 1987;147:713–7.
Blumberg A, Wiedman P, Shaw S, Gnadinger M. Effect of various therapeutic approaches on plasma potassium and major regulating factors in terminal renal failure. Am J Med. 1988;85:507–12.
Liou HH, Chiang SS, Wu SC, Huang TP, Campese VM, Smorgorzewski M, et al. Hypokalemic effects of intravenous infusion or nebulization of salbutamol in patients with chronic renal failure: comparative study. Am J Kidney Dis. 1994;23(2):266–71.
Lens XM, Montoliu J, Cases A, Campistol JM, Revert L. Treatment of hyperkalemia in renal failure: salbutamol v. insulin. Nephrol Dial Transplant. 1989;4:228–32.
Mandelberg A, Krupnik Z, Houri S, Smetana S, Gilad E, Matas Z, et al. Salbutamol mtered-dose inhaler with spacer for hyperkalaemia. How fast? How safe? Chest. 1999;115:617–22.
Pancu D, LaFlamme M, Evans E, Reed J. Levalbuterol is as effective as racemic albuterol in lowering serum potassium. J Emerg Med. 2003;25:13–6.
Schwarz KC, Cohen BD, Lubash GD, Rubin AL. Severe acidosis and hyperpotassemia treated with sodium bicarbonate infusion. Circulation. 1959;19:215–20.
Gutierrez R, Schlessinger F, Oster JR, Rietberg B, Perez GO. Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium concentration in patients with end-stage renal disease. Miner Electrolyte Metab. 1991;17:297–302.
Allon M, Shanklin N. Effect of bicarbonate administration on plasma potassium in dialysis patients: interactions with insulin and albuterol. Am J Kidney Dis. 1996;28:508–13.
Alfonzo A, Soar J, MacTier R, Fox J, Shillday I, et al. Treatment of acute hyperkalaemia in adults. 2014.
Wang CH, Huang CH, Chang WT, Tsai MS, Yu PH, Wu YW, et al. The effects of calcium and sodium bicarbonate on severe hyperkalemia during cardiopulmonary resuscitation: a retrospective cohort study of adult in-hospital cardiac arrest. Resuscitation. 2016;98:105–11.
Jaber S, Paugam C, Futier E, Lefrant JY, Sigismond L, et al. Sodium bicarbonate therapy for patients with severe metabolic acodisis in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392:31–40.
Reyes A. Effects of diuretics on renal excretory function. Eur Heart J. 1992;13:15–21.
Reyes A. Renal excretory profiles of loop diuretics: consequences for therapeutic application. J Cardiovasc Pharmacol. 1993;22:S11–23.
Channer KS, McLean KA, Lawson-Matthew P, Richardson M. Combination diuretic treatment in severe heart failure: a randomised controlled trial. Br Heart J. 1994;71:146–50.
Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17(5):R207.
Carlisle EJ, Donnelly SM, Ethier JH, et al. Modulation of the secretion of potassium accompanying anions in humans. Kidney Int. 1991;39:1206–12.
Kamel KS, Ethier JH, Quaggin S, Levin A, Albert S, Carlisle EJ, et al. Studies to determine the basis for hyperkalemia in recipients of a renal transplant who are treated with cyclosporine. J Am Soc Nephrol. 1992;2:1279–84.
Weisberg L. Management of severe hyperkalemia. Crit Care Med. 2008;36:3246–51.
Kessler C, Ng J, Valdez K, Xie H, Geiger B. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J Hosp Med. 2011;6:136–40.
McGowan CE, Saha S, Chu G, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493–7.
Flinn RB, Merrill JP, Welzant WR. Treatment of the oliguric patient with a new sodium-exchange resin and sorbitol. N Engl J Med. 1961;264:111–5.
Scherr L, Ogden DA, Mead AW, Spritz N, Rubin AL. Management of hyperkalemia with a cation-exchange resin. N Engl J Med. 1961;264:115–9.
• Lepage L, Dufour AA, Doiron J, Handfield K, Desforges K, Bell R, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10:2136–42 One of two randomized control trials demonstrating the efficacy of sodium polystyrene sulfonate in the management of hyperkalemia with a significant reduction in serum potassium by seven days.
• Nasir K, Ahmad A. Treatment of hyperkalemia in patients with chronic kidney disease: a comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll Abbottabad. 2014;26(4):455–8 One of two randomized control trials demonstrating the efficacy of sodium polystyrene sulfonate in the management of hyperkalemia with a significant reduction in serum potassium by three days.
Hunt TV DeMott JM, Ackerbauer KA, Whittier WL, Peksa GD. Single-dose sodium polystyrene sulfonate for hyperkalemia in chronic kidney disease or end-stage renal disease. Clin Kidney J 2018;1–6.
Mistry M, Shea A, Giguere P, Nguyen ML. Evaluation of sodium polystyrene sulfonate dosing strategies in the inpatient management of hyperkalemia. Ann Pharmacother. 2016;50(6):455–62.
Hagan AE, Farrington CA, Wall GC, Belz MM. Sodium polystyrene sulfonate for the treatment of acute hyperkalemia: a retrospective study. Clin Nephrol. 2016;85(1):38–43.
Yu MY, Yeo J, Park JS, Lee CH, Kim GH. Long-term efficacy of oral calcium polystyrene sulfonate for hyperkalemia in CKD patients. PLoS One. 2017;12(3):e0173542.
Nakamura T, Fujisaki T, Miyazono M, Yoshihara M, Jinnouchi H, Fukunari K, et al. Risks and benefits of sodium polystyrene sulfonate for hyperkalemia in patients on maintenance hemodialysis. Drugs RD. 2018;18:231–5.
Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with post-operative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159–61.
United States Food and Drug Administration. Kayexalate (sodium polystyrene sulfonate) powder label - FDA. 2010.
Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126:264.e9–24.
Li L, Harrison SD, Cope MJ, Park C, Lee L, Salaymeh F, et al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther. 2016;21(5):456–65.
Patiromer. Package insert. Redwood City, CA, USA: Relypsa, LLC; 2015.
• Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patiehts with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–8 First randomized control trial demonstrating efficacy and safety of patiromer in the management of chronic hyperkalemia. Demonstrated that 15g oral patiromer twice daily significantly lower mean serum potassium by day 3.
• Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Effect of Patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151–61 Phase II trial demonstrating efficacy and safety of patiromer in the management of chronic hyperkalemia. A major contribution of this study is that AMETHYST-DN was a dose ranging study that established efficacy of multiple doses ranging from 4.2g to 16.8g oral patiromer twice daily led to a dose dependent response in the reduction of serum potassium.
• Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patriomer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. NEJM. 2015;372(3):211–21 Phase III trial demonstrating efficacy and safety of patiromer in the management of chronic hyperkalemia. It also demonstrated that cessation of patiromer would to larger increases in serum potassium and higher rates of recurrent hyperkalemia when compared with placebo.
Weir MR, Bushinsky DA, Benton WW, Woods SD, Mayo MR, Arthur SP, et al. Effect of patiromer on hyperkalemia recurrence in older chronic kidney disease patients taking RAAS inhibitors. Am J Med. 2018;131:555–64.
Bushinsky DA, Williams GH, Pitt B, Weir MR, Freeman MW, Garza D, et al. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int. 2015;88:1427–33.
Novel Drug Approvals for 2018. 2018.
Stavros F, Yang A, Leon A, Nuttal M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014;9(12):e114686.
• Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88:404–11 Phase II trial demonstrating the efficacy and safety of ZS-9 in the management of chronic hyperkalemia in a dose dependent manner. Significant reduction were also seen within one hour, suggesting a role for ZS-9 in the acute management of hyperkalemia.
• Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 38 days among outpatients with hyperkalemia. JAMA. 2015;312(21):2223–33 Phase III trial demonstrating the efficacy and safety of ZS-9 in the management of chronic hyperkalemia in a dose dependent manner. This trial also suggests a role for ZS-9 in the acute management of hyperkalemia with a median time of 2.2 hours for the normalization of serum potassium.
Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. NEJM. 2015;372(3):222–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mengyang Liu declares no potential conflicts of interest.
Zubaid Rafique is a principal investigator and consultant for AstraZeneca and Vifor Pharma.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Emergency Medicine
Rights and permissions
About this article
Cite this article
Liu, M., Rafique, Z. Acute Management of Hyperkalemia. Curr Heart Fail Rep 16, 67–74 (2019). https://doi.org/10.1007/s11897-019-00425-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-019-00425-2