Abstract
Improvement in functional status, long-term survival, and quality of life has always been the goal of therapy in patients with heart failure with reduced ejection fraction. Neurohormonal modulating medications help patients achieve these goals and, in a subgroup of patients, can promote “reverse remodeling” resulting in the recovery of left ventricular systolic function. In the era of durable mechanical support, myocardial recovery that leads to explantation of the ventricular assist device occurs in a minority of cases. Optimal medical therapy appears to be a key component of achieving myocardial recovery, with recovery more likely in patients with a shorter duration of heart failure and a non-ischemic etiology. However, little is known about future management of patients who attain myocardial recovery, either with or without mechanical support. This review explores the epidemiology, physiology, cellular biology, and long-term outcomes for this subgroup of heart failure patients and outlines areas for future study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is traditionally divided into two broad, clinically distinct subtypes. HF with reduced ejection fraction (HFrEF) has been variously categorized as clinical HF with a left ventricular ejection fraction (LVEF) ≤40, <40, or ≤35 % [1–3], whereas HF with preserved ejection fraction (HFpEF) is usually described as clinical HF with LVEF ≥40 or ≥50 % [1–3]. Although improvements in ejection fraction have long been a goal of HF pharmacological therapy, coronary revascularization, cardiac resynchronization, and temporary mechanical circulatory support, it is in the era of durable mechanical circulatory support that the phenomenon of myocardial recovery has attracted widespread attention and investigation. Much of the recent myocardial recovery literature centers on patients with left ventricular assist devices (LVADs), but the potential for achieving “HF with recovered ejection fraction” (HFrecovEF) is well recognized as a possibility within the natural history of HFrEF [4].
In this review, we will describe the epidemiology of HFrecovEF, both in patients receiving pharmacological HF management and those who undergo LVAD support. We will explore the clinical characteristics, outcomes, and cellular biology of those patients that experience myocardial recovery, as compared to those that do not. We will also review the available published experience regarding management options for patients who achieve myocardial recovery and outline future steps in defining optimal treatment of this incompletely understood subgroup of HF patients.
Epidemiology of Myocardial Recovery
HFpEF and HFrEF are often considered as two very distinct clinical entities; compared to HFrEF, HFpEF patients tend to be older and more often female, have a greater prevalence of medical commodities, and respond poorly—if at all—to medications that demonstrate benefit in HFrEF [1]. However, despite the differing demographics and management strategies, the division between HFpEF and HFrEF is not as distinct as had once been thought. It is now recognized that there is fluidity of ejection fraction measurements over time, with 39 % of HFpEF patients having an LVEF <50 and 39 % of HFrEF patients having an LVEF ≥50 % at some point after diagnosis over a mean 5-year follow-up in one community-based cohort [5].
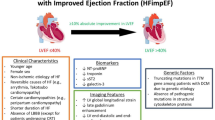
HFrecovEF was relatively prevalent in a single tertiary HF clinic setting [6]. Within a sample of 358 HF patients, 56 were defined as HFpEF, 181 as HFrEF, and 121 as HFrecovEF (LVEF previously <40 but ≥40 % at time of review). Patients with mechanical support were excluded from this report, and the range of HF etiologies in the HFrecovEF group spanned ischemic, valvular, restrictive, toxic, viral, familial, and tachycardia-mediated cardiomyopathies. The patients with recovered LVEF were a clinically distinct group, being younger in age than patients with HFpEF and having a lower prevalence of comorbidities such as hypertension, diabetes, and atrial fibrillation, and larger chamber dimensions. Compared to the HFrEF, the HFrecovEF patients tended to be younger and less likely to have coronary artery disease. In addition, patients with HFrecovEF had milder symptoms, with 73 % of this subgroup designated New York Heart Association (NYHA) I/II and fewer hospital admissions, as compared to patients with HFpEF or HFrEF. The mean LVEF in HFrecovEF patients was 49 ± 7 %, with a mean improvement in LVEF of 23 ± 10 %. There was equal use both of angiotensin-converting enzyme inhibitor/angiotensin receptor blockers in HFrEF and HFrecovEF groups (81 % versus 81 %), and of beta-blockers (87 % HFrEF versus 82 % HFrecovEF, p = 0.35). Conversely, mineralocorticoid antagonist use was significantly less prevalent in the HFrecovEF group (38 versus 19 %, p = 0.001).
Basuray and colleagues recently added insights into the clinical features, biomarker profiles, and clinical prognosis for patients with the HFrecovEF subtype [4]. Their report from the Penn Heart Failure Study (n = 1821 chronic HF patients recruited from tertiary referral clinics) identified 176 subjects with LVEF ≥50 % on cohort enrollment, but with a prior LVEF of <50 %. The median difference between enrollment EF and EF nadir was 28 % (25th and 75th percentiles, 20 and 35 %) during a median time period of 29 months (25th and 75th percentiles, 16 and 53 months). The HFrecovEF subgroup had higher than normal levels of brain natriuretic protein (BNP), troponin I, soluble fms-like tyrosine kinase receptor-1 (sFlt-1), and uric acid although significantly lower than patients with HFrEF and HFpEF. This suggests ongoing abnormalities of salt and water homeostasis and abnormal myocyte biology that may place the patient at increased risk of cardiac events as compared to the general population despite improvement in LV function. As in Punnoose et al., HFrecovEF patients had less severe symptoms, with a greater prevalence of patients in NYHA class I–II than the HFrEF or HFpEF subgroups [4]. The use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers was also common among these HFrecovEF patients (85 versus 90 % in HFrEF, p < 0.001), as was beta-blocker use (88 versus 92 % in HFrEF, p < 0.001). Mineralocorticoid antagonist use was higher in the HFrEF group, as were the use of digoxin and diuretics. Data regarding HF medication strategies in HFrecovEF are presented below, although prospective investigation of the long-term use of the neurohormonal medications in the recovery cohort is lacking.
At a maximum of 8.9 years’ follow-up, all-cause mortality, or the need for cardiac transplantation or mechanical circulatory support, was lower in patients with HFrecovEF when compared to patient with HFrEF and HFpEF: hazard ratio for HFrEF, as compared to HFrecovEF, 4.1, 95 % confidence interval (CI) 2.4–6.8; hazard ratio for HFpEF, 2.3, 95 % CI 1.2–4.5. However, in addition to the persistent abnormalities in biomarkers, the HFrecovEF subgroup still experienced a significant number of hospitalizations for HF, with approximately 50 % of this group having been hospitalized by 6 years.
Select etiologies of cardiomyopathy may have a particular propensity towards myocardial recovery. The recently published results from the Investigations of Pregnancy-Associated Cardiomyopathy (IPAC) study confirmed a high likelihood of recovery in women who develop peripartum cardiomyopathy [7]. Seventy-two percent of participants with complete data available demonstrated an improvement in LVEF to ≥50 % at 12 months of follow-up. Takotsubo, also known as stress cardiomyopathy, is characterized by transient left ventricular dysfunction with an apical ballooning pattern that spontaneously recovers [8]. There may be significant improvements in systolic function in the days and weeks after an acute myocardial infarction [9], as well as the potential for recovery after revascularization for patients with myocardial stunning or hibernation [10].
Epidemiology of Myocardial Recovery with Mechanical Circulatory Support
Ventricular assist devices have revolutionized the management of stage D HF. Continuous-flow LVADs (CF-LVAD) have become the standard of care for patients with advanced HFrEF and refractory symptoms despite optimal medical therapies, and have been demonstrated to improve both quality of life and survival as either a bridge to transplant (BTT) or destination therapy (DT) [11, 12]. Since their advent, ventricular assist devices (pulsatile as well as continuous flow) have been associated with the possibility of myocardial recovery. There are reports in some patients of reverse remodeling accompanied by significant improvement in LVEF over time; however, only in a minority of LVAD patients is this improvement sufficient to allow successful explanation of the mechanical support device [13]. There is also geographic variation in the degree of interest in promoting and monitoring for myocardial recovery during LVAD support, as well as variations in the rates of LVAD explantation. Overall in North America, only approximately 1 % of patients underwent LVAD explantation for myocardial recovery in the latest Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) report [14]. Similarly, in the HeartMate II BTT and DT trials, the rate of myocardial recovery allowing for device explantation was 1.8 % [15]. It is postulated that the powerful mechanical unloading of the LV offered by these devices, accompanied by increases in the doses of HF pharmacotherapy tolerated by patients after LVAD implantation, is a major contributor towards the process of myocardial recovery during mechanical support.
The initial reports of myocardial recovery during the pulsatile LVAD era were mixed. Although there was some recognition both in the USA and Germany that LV recovery with mechanical unloading was a possibility [16, 17], this was balanced by concerns that the rate of successful progression to explantation was too low to justify the intensive testing required to ascertain myocardial recovery [18]. There were also preliminary observations that patients with less pre-implantation myocardial fibrosis may be more likely to achieve successful explantation [17]. The most encouraging step forward in this area of investigation came in 2005, when Birks and collogues published their experience with a formal HeartMate I (pulsatile LVAD) weaning protocol [19]. Among 15 patients with severe HF secondary to non-ischemic cardiomyopathy and no histologic evidence of active myocarditis, 11 achieved sufficient myocardial recovery to undergo LVAD explantation. In preparation for explantation, this cohort was managed with maximally tolerated doses of lisinopril, carvedilol, spironolactone, and losartan to enhance reverse remodeling. Once regression of left ventricular dilatation was achieved, the beta 2-adrenergic receptor agonist clenbuterol was added to the regimen. Post-explantation, one patient died due to refractory arrhythmias at 24 h, and a second died of lung cancer at 27 months post-explantation. The cumulative freedom from recurrent HF among the surviving patients was 100 and 88.9 % 1 and 4 years post-explantation, respectively, with an almost-normal quality of life assessment at 3 years. Fifty-nine months post-explantation, the mean left ventricular ejection fraction was 64 ± 12 % and the mean left ventricular end-diastolic diameter was 59.4 ± 12.1 mm. Other centers have also reported sustained recovery and survival exceeding 9 years post-explantation [20, 21], although there is also recognition that severe and potentially fatal HF recurrences can occur even years after a seemingly successful explantation again highlighting the persistence of risk factors, whether genetic, cellular or environmental, in this group of patients.
Dr. Birks furthered her observations regarding the potential for recovery with a prospective study of LVAD weaning in 20 non-ischemic HF patients supported by CF-LVADs (HeartMate II) [22]. The protocol again included high-dose neurohumoral antagonists and the addition of clenbuterol. Twelve patients had sufficiently encouraging echocardiograms, exercise tests, and right heart catheterizations performed at low pump speed settings to proceed to explantation surgery. The estimated survival without HF recurrence was 83.3 % at 1 and 3 years. After a mean 431-day follow-up, surviving explants had an ejection fraction of 58.1 ± 13.8 % and left ventricular end-diastolic diameter of 59.0 ± 9.3 mm. Patients who recovered sufficiently for explantation tended to be younger than those who did not recover. Other groups that have looked at predictors of successful LVAD explantation have identified non-ischemic HF etiology, younger age, shorter HF duration and recovery time, and higher pre-weaning LVEF as predictors of successful LVAD explantation [15, 23]. It has also been suggested that the unloading capacity of the prior era of pulsatile devices may have been superior to the current CF-LVADs, although mechanisms for this observation remain unclear [24, 25]. The Utah group has demonstrated that prolonged continuous-flow LVAD unloading does not induce hypertrophy regression to the point of atrophy and degeneration [26]. There is a growing consensus that given the scarcity of donor hearts, the potential complications of remaining on LVAD support, and the importance of protocolized pharmacological and imaging interventions, it is important to raise the profile of the potential for myocardial recovery during LVAD support [27, 28]. However, many challenges to expanding the recovery pathway remain—chiefly the fact that successful reverse cardiac remodeling does not necessarily lead to sustained clinical myocardial recovery, the reasons for which continue to require further basic and clinical investigation [13].
Mechanical Support Myocardial Recovery Protocols
Myocardial recovery during LVAD support still requires detailed prospective studies to identify not only the pharmacologic and testing strategies that maximize potential for explanation, but also to define optimal management beyond device explantation. The left ventricular volume overload of advanced heart failure is responsible for the initial compensatory chamber dilatation. This remodeling process is increasingly deleterious over time and leads to a vicious cycle of neurohormonal, histological, and clinical changes that manifest as progressive worsening of heart failure. Hemodynamically, LVADs can be very successful in unloading the failing left ventricle. Improved ventricular geometry and function has been demonstrated with both pulsatile and continuous-flow devices, and in some cases halting and even reversing remodeling of the left ventricle [29]. On occasion, this “reverse remodeling” phenomenon is sufficient to translate into meaningful clinical recovery of the left ventricle, allowing for LVAD explantation as described above.
Thus far, the mixed success with the “bridge to recovery” strategy may in part be due to the lack of standardized LVAD weaning protocols and patient selection for potential recovery, as well as heterogeneity in clinical and echocardiographic markers of LVAD recovery, lack of consensus for medical therapy pre- and post-explantation, and a paucity of prospective data supporting the recovery approach. Standardized LVAD weaning protocols continue to be developed and prospectively studied. The ongoing Remission From Stage D Heart Failure (RESTAGE-HF) trial is a multi-center, prospective study of subjects with non-ischemic cardiomyopathy and a HeartMate II LVAD device [30]. This trial is studying a standardized protocol of pharmacologic therapy and device weaning procedures, with optimization of pump speed for maximal unloading from early after implantation. At 4, 6, 9, and 12–18 months post-implantation, echocardiography is performed at a low LVAD speed of 6000 rpm, with a 6-min walk test at 6 weeks, 4, 6, 8, and 12–18 months post-implantation. Preliminary results are promising thus far, with five subjects of a total of 22 enrolled successfully explanted at a median of 265 days post-explantation. Subjects who are successfully explanted remain on the high-dose HF medications after pump removal.
Beyond evaluating which tests best assess the heart’s ability to withstand LVAD explantation, there is also interest in determining whether ventricular reconditioning maneuvers may promote a sustained recovery. There is some experience with designing weaning protocols that incorporate periods of withdrawal of LVAD support by decreasing LVAD speed while closely monitoring the left ventricular end-diastolic dimension (LVEDD) and left ventricular end-systolic dimension (LVESD), aortic valve opening time, and ejection fraction. Dr. Frazier and colleagues at the Texas Heart Institute prospectively studied 30 LVAD patients for weaning and explantation, the majority of whom were supported by HeartMate II devices [31]. NYHA functional class I patients, who tended to be younger and who were treated with optimal HF medical therapy, were selected for the reconditioning protocol. This protocol incorporated a period of ventricular unloading, achieved by increasing the pump speed to decrease the LVEDD to the upper limit of normal and correct or minimize mitral regurgitation. Initially, this was only achieved when the aortic valve remained closed throughout the cardiac cycle. If the patient was clinically stable, the device speed was briefly decreased to 6000 rpm with close measurement of the aortic valve opening time. Once the opening time exceeded 10 % of the cardiac cycle, the speed was gradually reduced as long as the LV dimensions remained normal and MR was controlled. The reconditioning phase was advanced at every outpatient visit. Device explantation was considered once the aortic valve opening time was normal at 6000 rpm with LVEDD <6 cm. Subsequent exercise testing with bicycle stress or dobutamine was performed to determine the appropriateness of explantation. Twenty-seven patients successfully proceeded to explantation with one patient requiring re-implantation 2.7 years post-explant. The remaining patients remained in NYHA classes I and II with medical therapy alone with a mean survival of 1172 ± 948 days. These results promote consideration of the merits of LVAD programming that could reduce the pump speeds automatically at programed intervals to “exercise” the heart to promote myocardial recovery.
Cellular Mechanisms of Recovery
Cardiac remodeling is a complex, typically pathological state, in which cellular and extracellular changes take place altering the geometry and kinetics of the heart muscle, resulting in clinical heart failure. Cellular remodeling incorporates changes in cardiomyocyte size and shape, as well as molecular and signaling abnormalities that result in fetal gene expression, modification to excitation-contraction coupling, alterations in cell-survival signaling, and derangements in myofilament function and cellular metabolism. Extracellular changes observed in the remodeling heart include alterations in the composition of the fibrous and vascular elements of the cardiac extracellular matrix leading to deformation of the cardiac chamber. The phenotype of the pathologically remodeled heart depends greatly on the inciting event. For example, when volume overload takes place, the cardiomyocytes, which are typically thin and elongated, ensue a formation in series. In pressure overload, cardiomyocytes become shortened and bulky, aligning themselves in parallel [32].
“Reverse remodeling”—the process of cardiac chamber volume (particularly end-systolic volume) decreasing, often accompanied by improved beta-adrenergic responsiveness—has long been a secondary endpoint of HF pharmacotherapy trials. Renin-angiotensin-aldosterone inhibition, beta-blockade, cardiac resynchronization, and ventricular assist devices all have the potential to achieve reverse remodeling, and to date, every therapy with mortality benefits in HFrEF is capable of inducing reverse remodeling [33]. At a cellular level, reverse remodeling reduces myocyte size and interstitial fibrosis, increases microvascular density, inhibits fetal gene expression, and upregulates sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) gene expression, thus improving cellular calcium cycling [32]. However, not every patient exposed to these therapies achieves reverse remodeling, and the reverse remodeling response is not always durable. There have been a number of observations regarding which patients are more likely to successfully reverse remodel, as well as efforts to identify new molecular targets for the inhibition or reversal of maladaptive cardiac remodeling.
Patients with LVADs undergo a similar process of reverse remodeling that, as described above, can extend as far as myocardial recovery in some cases. As described by Drakos et al., the unloading of the failing left ventricle with an LVAD results in endothelial cell activation, increased density of the microvasculature, and increased fibrosis [26]. Whether this cascade of events could lead to eventual myocardial atrophy or degeneration is controversial. Drakos el al. found that the increased interstitial and total collagen content of the heart after unloading is not associated with hypertrophy regression to the point of atrophy or degeneration [26]. Wohlschlager et al. reported a marked decline in the deoxyribonucleic acid (DNA) content, nuclear size, and the number of nuclei in the myocytes of patients with LVADs [34]. In addition, LVADs are associated with a decline in polyploidy and an increase in the number of diploid cells suggesting a decline in the process of protein synthesis as a result of unloading [34]. Whether these cellular changes are durable is unknown and requires ongoing, prospective investigation. In addition, the role of chronic, heart failure pharmacotherapy in LVAD patients is not clearly understood and is often underutilized, likely at least partially due to the historically low rates of recovery that have been previously reported.
Interesting insights into optimizing myocardial energetics have come from the cardiac resynchronization (CRT) field. Unlike inotropic therapy, CRT appears to energetically advantage the failing heart enabling it to achieve improved cardiac output for a lower level of substrate consumption. Components of this pathway include redox modifications to the myocardial mitochondria that enhance the efficiency of ATP generation [35, 36], as well as improvements in the physical alignment of ryanodine receptors in relation to SERCA to optimize excitation-contraction coupling [37]. Mechanical unloading with LVAD placement also has the potential to improve excitation-contraction coupling [38]. Understanding which patients have the greatest potential to achieve these subcellular reverse remodeling changes will be a key component in pursuing clinically meaningful myocardial recovery.
Post-recovery Management
There is still a paucity of prospective data to guide the management of patients who experience myocardial recovery. The persistent abnormalities in biomarker profile despite a high prevalence of ongoing neurohumoral antagonist therapies noted by Basuray and colleagues lends weight to the hypothesis that HF pharmacotherapy should be continued to maximize the likelihood of a sustained LVEF and clinical response. However, the data to support this approach is scarce. Two reports from Korea have retrospectively studied management strategies and the risk of HFrEF recurrence [39, 40]. Moon and colleagues retrospectively studied 42 patients with non-ischemic cardiomyopathy who had achieved LVEF recovery (LVEF ≥40 % and a ≥10 % increase in absolute value). The LVEF subsequently fell below 40 % in 19 % of the cohort; patients who experienced deterioration in LVEF were more likely to have discontinued HF medications (62.5 versus 5.9 %, p < 0.05).
Park et al. retrospectively evaluated a cohort of 85 consecutively enrolled subjects (62 males, mean age 57 years), whose LVEF had recovered to >45 % accompanied by an incremental LVEF change of ≥10 % compared with the initial LVEF. Outcomes were reviewed for a mean of 50 ± 33 months after LVEF recovery with no changes in baseline medical pharmacotherapies. Thirty-three patients developed a recurrence of LV systolic dysfunction. When divided by the presence or absence of recurrence of systolic dysfunction, both groups had high prevalence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (100 versus 92 %, respectively, p = 0.62). However, there was a non-significant trend towards lower beta-blocker and mineralocorticoid antagonist use in patients who had a recurrence of systolic dysfunction: beta-blocker use 18 versus 29 %, respectively, p = 0.100 and mineralocorticoid antagonist use 24 versus 35 %, respectively, p = 0.105. On multivariate analysis, older age, diabetes, and larger LV end-diastolic dimension at presentation were all independent predictors of systolic dysfunction recurrence. The authors concluded that patients in these groups should be more intensively managed after an improvement in LVEF. A recent systematic review of HF medication withdrawal within mixed populations of chronic stable HF and HFrecovEF also concluded that there were risks associated with the withdrawal of renin-angiotensin-aldosterone system inhibitors or beta-blockers [41].
Although suggestive, these retrospective studies do not prove causality and may be confounded by other reasons that independently increase the risk of recurrence. For example, patients who have blood pressure or renal intolerance of HF medications are most likely to have their medications discontinued, and may also have the highest risk of future clinical deterioration. Regardless, in the absence of more robust prospective data, these studies suggest potential benefit of HF medication continuation in the HFrecovEF population. Should the patient and/or provider opt to withdraw goal-directed medical therapy following recovery of function, these data would suggest that ongoing screening of LV function at periodic intervals is reasonable to ensure stability of cardiac function. Society practice guidelines are yet to make recommendations on management after LVEF improvement, probably owing to the paucity of data in this area. Recalling the persistent abnormalities of biomarker profile observed by Basuray et al., the ongoing use of neurohumoral antagonism in HFrecovEF seems to be a sensible approach, given the safety profile of beta-blockers and ACE inhibitors with careful clinical monitoring.
There is also limited data to guide the ongoing need for implantable cardioverter-defibrillator (ICD) therapy in the setting of HFrecovEF. Among 91 patients undergoing ICD generator exchanges, 25 had LVEF improvement of at least 10 % to greater than 35 % [42]. The incidence of appropriate ICD shocks was the same between individuals with or without LVEF recovery. A subgroup of the Defibrillators in Non-ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial population received serial LVEF assessments; 56 of the 187 with a follow-up LVEF had a measurement >35 % on their last assessment during the first 2 years of the trial. There were four arrhythmic events in subjects with a recovered LVEF versus 24 events in those with an LVEF <35 % (p = 0.006). Likewise in a cohort of 231 Veterans Affairs patients, 26 % no longer met guideline indications for ICD therapy at the time of generator exchange [43]. Subjects without ongoing ICD indications received a smaller number of appropriate ICD therapies than patients with indications (2.8 versus 10.7 % annually, p < 0.001), but once again, appropriate shocks were delivered in the HFrecovEF group. Most recently, the Prospective Observational Study of Implantable Cardioverter-Defibrillators (PROSE-ICD) study prospectively studied the outcomes of 538 subjects undergoing primary prevention ICD placement [44]. During the 4.9-year follow-up, 126 subjects had an LVEF improvement to >35 %. Of these, only four subjects received an appropriate ICD shock. Therefore, although the incidence of life-threatening ventricular arrhythmias is lower in individuals with an improvement in LVEF, as compared to those who continue to meet ICD indications, the risk is not completely eliminated. Conversely, the MADIT-CRT investigators observed only one ventricular arrhythmia event among 55 subjects with LVEF recovery >50 % and therefore suggested these patients could be considered for downgrade from CRT-defibrillator to CRT-pacemaker at the time of battery depletion, if ventricular arrhythmias have not been detected [45].
Conclusions and Future Directions
Recovery of myocardial function is not uncommon in patients with reduced ejection fraction. This is a primary goal of medical therapy and increasingly sought in LVAD patients. However, it is also recognized that patients who do recover are at ongoing risk for future deterioration and adverse events, which raises concerns about the safety of HF medication discontinuation in the HFrecovEF cohort. Prospective studies are needed to further explore the pathophysiology of HFrecovEF—both to optimize clinical outcomes for these patients and also to aid in the development of therapies that target novel pathways of myocardial recovery.
References
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239.
Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1–194.
McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Bohm M, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Eur Heart J. 2012;14:803–69.
Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, et al. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129(23):2380–7.
Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5(6):720–6.
Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17(7):527–32.
McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66(8):905–14.
Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Int Med. 2004;141(11):858–65.
Sjöblom J, Muhrbeck J, Witt N, Alam M, Frykman-Kull V. Evolution of left ventricular ejection fraction after acute myocardial infarction: implications for implantable cardioverter-defibrillator eligibility. Circulation. 2014;130(9):743–8.
Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117(1):103–14.
Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–43.
Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–96.
Drakos SG, Kfoury AG, Stehlik J, Selzman CH, Reid BB, Terrovitis JV, et al. Bridge to recovery: understanding the disconnect between clinical and biological outcomes. Circulation. 2012;126(2):230–41.
Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33(6):555–64.
Goldstein DJ, Maybaum S, MacGillivray TE, Moore SA, Bogaev R, Farrar DJ, et al. Young patients with nonischemic cardiomyopathy have higher likelihood of left ventricular recovery during left ventricular assist device support. J Card Fail. 2012;18(5):392–5.
Frazier OH, Benedict CR, Radovancevic B, Bick RJ, Capek P, Springer WE, et al. Improved left ventricular function after chronic left ventricular unloading. Ann Thorac Surg. 1996;62(3):675–82.
Müller J, Wallukat G, Weng YG, Dandel M, Spiegelsberger S, Semrau S, et al. Weaning from mechanical cardiac support in patients with idiopathic dilated cardiomyopathy. Circulation. 1997;96(2):542–9.
Mancini DM, Beniaminovitz A, Levin H, Catanese K, Flannery M, DiTullio M, et al. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation. 1998;98(22):2383–9.
Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355(18):1873–84.
Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB, Hetzer R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation. 2005;112(9 Suppl):I37–45.
Segura AM, Dris L, Massin EK, Clubb FJ, Buja LM, Frazier OH, et al. Heart failure in remission for more than 13 years after removal of a left ventricular assist device. Tex Heart Inst J. 2014;41(4):389–94.
Birks EJ, George RS, Hedger M, Bahrami T, Wilton P, Bowles CT, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123(4):381–90.
Dandel M, Weng Y, Siniawski H, Potapov E, Drews T, Lehmkuhl HB, et al. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118(14 Suppl):S94–105.
Pruijsten RV, Lok SI, Kirkels HH, Klöpping C, Lahpor JR, De Jonge N. Functional and haemodynamic recovery after implantation of continuous-flow left ventricular assist devices in comparison with pulsatile left ventricular assist devices in patients with end-stage heart failure. Eur J Heart Fail. 2012;14(3):319–25.
Krabatsch T, Schweiger M, Dandel M, Stepanenko A, Drews T, Potapov E, et al. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann Thorac Surg. 2011;91(5):1335–40.
Diakos NA, Selzman CH, Sachse FB, Stehlik J, Kfoury AG, Wever-Pinzon O, et al. Myocardial atrophy and chronic mechanical unloading of the failing human heart: implications for cardiac assist device-induced myocardial recovery. J Am Coll Cardiol. 2014;64(15):1602–12.
Adler E, Silva Enciso J. Functional improvement after ventricular assist device implantation: is ventricular recovery more common than we thought? J Am Coll Cardiol. 2013;61(19):1995–7.
Selzman CH, Madden JL, Healy AH, McKellar SH, Koliopoulou A, Stehlik J, et al. Bridge to removal: a paradigm shift for left ventricular assist device therapy. Ann Thorac Surg. 2015;99(1):360–7.
Dandel M, Weng Y, Siniawski H, Stepanenko A, Krabatsch T, Potapov E, et al. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: criteria for weaning from ventricular assist devices. Eur Heart J. 2011;32(9):1148–60.
Birks EJ, Drakos S, Selzman C, Starling R, Cunningham C, Slaughter M, et al. Remission from stage D heart failure (RESTAGE-HF): early results from a prospective multi-center study of myocardial recovery. J Heart Lung Transplant. 2015;34(4):S40–1.
Frazier OH, Baldwin ACW, Demirozu ZT, Segura AM, Hernandez R, Taegtmeyer H, et al. Ventricular reconditioning and pump explantation in patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015;34(6):766–72.
Koitabashi N, Kass DA. Reverse remodeling in heart failure—mechanisms and therapeutic opportunities. Nat Rev Cardiol. 2012;9(3):147–57.
Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2010;56(5):392–406.
Wohlschlaeger J, Levkau B, Brockhoff G, Schmitz KJ, von Winterfeld M, Takeda A, et al. Hemodynamic support by left ventricular assist devices reduces cardiomyocyte DNA content in the failing human heart. Circulation. 2010;121(8):989–96.
Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, et al. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet. 2010;3(1):78–87.
Wang S-B, Foster DB, Rucker J, O’Rourke B, Kass DA, Van Eyk JE. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ Res. 2011;109(7):750–7.
Sachse FB, Torres NS, Savio-Galimberti E, Aiba T, Kass DA, Tomaselli GF, et al. Subcellular structures and function of myocytes impaired during heart failure are restored by cardiac resynchronization therapy. Circ Res. 2012;110(4):588–97.
Ibrahim M, Navaratnarajah M, Siedlecka U, Rao C, Dias P, Moshkov AV, et al. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca(2+)-induced Ca(2+)release in a rodent model of heart failure. Eur J Heart Fail. 2012;14(6):571–80.
Moon J, Ko Y-G, Chung N, Ha J-W, Kang S-M, Choi E-Y, et al. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can J Cardiol. 2009;25(5):e147–50.
Park J-S, Kim J-W, Seo K-W, Choi B-J, Choi S-Y, Yoon M-H, et al. Recurrence of left ventricular dysfunction in patients with restored idiopathic dilated cardiomyopathy. Clin Cardiol. 2014;37(4):222–6.
Hopper I, Samuel R, Hayward C, Tonkin A, Krum H. Can medications be safely withdrawn in patients with stable chronic heart failure? Systematic review and meta-analysis. J Card Fail. 2014;20(7):522–32.
Naksuk N, Saab A, Li J-M, Florea V, Akkaya M, Anand IS, et al. Incidence of appropriate shock in implantable cardioverter-defibrillator patients with improved ejection fraction. J Card Fail. 2013;19(6):426–30.
Kini V, Soufi MK, Deo R, Epstein AE, Bala R, Riley M, et al. Appropriateness of primary prevention implantable cardioverter-defibrillators at the time of generator replacement: are indications still met? J Am Coll Cardiol. 2014;63(22):2388–94.
Zhang Y, Guallar E, Blasco-Colmenares E, Butcher B, Norgard S, Nauffal V, et al. Changes in follow-up left ventricular ejection fraction associated with outcomes in primary prevention implantable cardioverter-defibrillator and cardiac resynchronization therapy device recipients. J Am Coll Cardiol. 2015;66(5):524–31.
Ruwald MH, Solomon SD, Foster E, Kutyifa V, Ruwald A-C, Sherazi S, et al. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2014;130(25):2278–86.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Johny S Kuttab, Michael S Kiernan, and Amanda R Vest declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Epidemiology of Heart Failure
Rights and permissions
About this article
Cite this article
Kuttab, J.S., Kiernan, M.S. & Vest, A.R. Epidemiology of “Heart Failure with Recovered Ejection Fraction”: What do we do After Recovery?. Curr Heart Fail Rep 12, 360–366 (2015). https://doi.org/10.1007/s11897-015-0274-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-015-0274-4