Abstract
Purpose of Review
The purpose of this article is to review the recent literature and discuss the new approaches to the diagnosis and treatment of functional dyspepsia (FD).
Recent Findings
According to the recent American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology (CAG) guideline for dyspepsia, Helicobacter pylori (H. pylori) eradication is recommended as a first treatment option, and proton pump inhibitors (PPIs), tricyclic antidepressants, and prokinetics are listed as second-line therapy. On the other hand, in the Japanese guideline for FD, PPIs and prokinetics are recommended as the first-line treatment. In Japan, acotiamide, a recently launched prokinetic, showed significant efficacy in several clinical trials performed either in Japan or Europe. Regarding non-pharmacological treatment, recent topics include acupuncture, electrical stimulation, gastric peroral endoscopic myotomy, and meal and lifestyle modification. These treatments have provided significant efficacy, which provides some insights into the main pathophysiology of this disease.
Summary
Although FD is common among functional gastrointestinal disorders, it is not easy to relieve the dyspeptic symptoms of FD patients. Combinations of pharmacological and non-pharmacological treatment options are expected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional dyspepsia (FD), one of the major functional gastrointestinal disorders, refers to epigastric symptoms that occur without apparent organic disease, based on the premise that gastric dysfunction may be the cause [1••, 2]. FD is a common condition that affects up to 20% of the population [3] and is known to significantly impair patients’ quality of life (QOL). Therefore, FD patients should be appropriately diagnosed and managed. However, the management of FD is challenging. Pharmacological treatment is often used in the clinical setting, yet its effectiveness has been reported to be very low. In this article, the recent literature is reviewed, and the new approaches to diagnosis and treatment of FD are discussed.
Diagnosis of FD
Diagnostic Criteria of FD
FD is diagnosed based on symptoms and defined by the Rome criteria, where typical symptoms of FD are described as bothersome postprandial distention, early satiety, epigastric pain, and epigastric burning.
The Rome criteria for FD were revised in 2016 (Rome IV), and the updated guideline of the American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology (CAG) was published in 2017 [1••, 4••]. In Rome IV, FD is classified as postprandial distress syndrome (PDS) or epigastric pain syndrome (EPS), based on the presence or absence of an association with meals, as well as symptom persistence for 3 of the prior 6 months; these descriptors are similar to those in the Rome III criteria. “Bothersome” symptoms are highlighted in the revised Rome IV criteria more than in the Rome III criteria [5]. As there was an overlap of PDS and EPS in Rome III, in Rome IV, epigastric pain and burning after meal ingestion were included among PDS symptoms to increase specificity and reduce overlap between these categories. In the ACG/CAG guideline, FD is defined by symptoms of dyspepsia (predominantly epigastric pain) that last at least 1 month and are potentially associated with any other upper gastrointestinal symptom, such as epigastric fullness [4••].
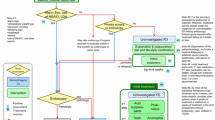
In Japan, Helicobacter pylori gastritis has been covered by medical insurance since 2013; all regimens for eradication of H. pylori infection are covered by national health insurance. The clinical practice guidelines for FD in Japanese and English versions were issued by the Japanese Society of Gastroenterology in 2014 and 2015, respectively [2] (Fig. 1). These guidelines are based on the grading of recommendations assessment, development, and evaluation (GRADE) system, and FD is defined as “a condition chronically presenting symptoms centered in the upper abdomen, such as epigastric pain or discomfort, in the absence of any organic, systemic, or metabolic disease that is likely to explain the symptoms”. In this definition, the symptoms and duration of the disease are not specified, unlike in the Rome IV criteria. These statements are written to be widely distributed and inform primary care physicians of this condition. In fact, it is up to each physician to determine whether the patients’ symptoms are those of FD or not. In another statement in the Japanese guidelines, it is also stated that “FD is not identical to chronic gastritis” [6]. In Japan, FD is a diagnosis recognized by the insurance plan, and the medical fee for this condition is covered by medical insurance. Further recognition of this condition is needed to provide appropriate management for dyspepsia patients without organic causes.
Algorithm for diagnosis and treatment of FD in Japan (ref. 2). FD, functional dyspepsia
New Motility Tests for the Diagnosis of FD
In the pathophysiology of FD, gastric motility disorders and visceral hypersensitivity are important factors that are directly associated with symptom deterioration [7,8,9,10]. According to previous reports, adaptive relaxation disorders and delayed gastric emptying are particularly associated with upper gastrointestinal symptoms. Barostat testing is generally regarded as the gold standard for measurement of gastric adaptive relaxation, but it has been used only for research purposes at a limited number of institutions because of its invasiveness as a test. A new measurement of the adaptive relaxation response of the stomach using high-resolution manometry has attracted attention as a new noninvasive technique that can even be used in children [11•, 12]. Regarding the evaluation of gastric emptying, the 13-C urea breath test (UBT) is currently often used. There is overlap in the dyspeptic symptoms of idiopathic gastroparesis and FD, but only a minority of FD patients have delayed gastric emptying, suggesting that UBT is of particular value in distinguishing between idiopathic gastroparesis and functional dyspepsia. However, these tests are only regarded as auxiliary methods for making the diagnosis.
Biomarkers of FD
No biomarker of FD has been identified. Although differences in ghrelin, cholecystokinin, serotonin, leptin, gastrin, calcium, and various genetic polymorphisms have been reported, no definite diagnostic indicator has been used in the diagnosis of FD. Nuclear magnetic resonance (NMR)-based analytical approaches to metabolomics have been used to identify changes in levels of glutamine, alanine, proline, high-density lipoprotein, β-glucose, α-glucose, low-density lipoprotein, and very low-density lipoprotein in patients with FD [13].
Treatment of FD
Generally, treatment of FD should be based on the etiology of the condition. However, as is well known, FD is a multifactorial and complex disease, and treatment based on its pathogenesis is difficult. Therefore, the current strategy for pharmacological treatments is empirical. In many guidelines or treatment recommendations, Helicobacter pylori (H. pylori) eradication is used as the treatment for infection-positive patients. Among many drugs besides H. pylori eradication treatment, acid inhibitory drugs, such as proton pump inhibitors (PPIs), are considered as the first treatment option, following the use of psychotropic agents and prokinetics. In the recently published ACG/CAG guideline, H. pylori eradication is also recommended as a first-line treatment option, and PPIs, tricyclic antidepressants (TCAs), and prokinetics are listed as second-line therapy (Figs. 2 and 3). Among the prokinetic agents, acotiamide, which has shown significant efficacy in several clinical trials performed either in Japan or Europe, has been introduced in Japan.
Algorithm for the management of undiagnosed dyspepsia according to the ACG/CAG clinical guideline (ref. 4). ACG/CAG, American College of Gastroenterology and Canadian Association of Gastroenterology
Algorithm for the management of dyspepsia according to the ACG/CAG clinical guideline (ref. 4). ACG/CAG, American College of Gastroenterology and Canadian Association of Gastroenterology
Recent non-pharmacological treatments include acupuncture, electrical stimulation, gastric peroral endoscopic myotomy (G-POEM), and meal and lifestyle modification. These treatments have provided significant efficacy, and they provide insight into the main pathophysiology of this disease. However, more evidence is necessary to establish them as treatments for FD (Fig. 3).
H. pylori Eradication Therapy
Eradication of H. pylori has been reported as both effective and ineffective for dyspepsia. However, based on the meta-analyses, H. pylori infection is thought to be associated with the onset of dyspepsia [14, 15]. H. pylori is considered to be a trigger of dyspepsia, but not in all cases of FD. In addition, eradication therapy is at least likely to reduce the risk of peptic ulcer or gastric cancer. The Kyoto global consensus meeting concluded the following: the disappearance or improvement of dyspepsia at 6–12 months after eradication of H. pylori defines H. pylori-associated dyspepsia and should be regarded as different from FD [16]. This consensus is also in the Rome IV criteria [1••]. Given the above, it is thought that eradication therapy should be performed because it is clearly effective for symptoms, although it is not significantly effective for FD. The recent ACG/CAG guideline also recommends eradication of H. pylori as first-line treatment for patients with FD aged 60 years and older who are diagnosed as H. pylori-positive after organic diseases are ruled out by esophagogastroduodenoscopy [4••] (Fig. 1). In addition, for patients under the age of 60 years, noninvasive testing is recommended, and patients should be treated for H. pylori infection if positive.
Proton Pump Inhibitors
PPIs are considered first-line treatment for patients who are diagnosed with FD. Although many studies and meta-analyses have reported the effectiveness of PPIs, only around 14% of patients with dyspepsia show improvement [17•, 18]. Generally, there is no difference in acid-secretion function between FD and controls. However, it has been reported that postprandial acid exposure in the duodenum is greater in some patients with FD [19]. Patients with FD who have strong acid exposure in the duodenum may experience more severe symptoms than patients with FD who have normal acid exposure. It has been reported that motility and clearance in the duodenum are reduced by instillation of acid into the duodenum, and various upper abdominal symptoms may be more severe in patients with FD [20, 21]. Recent data suggest that low-grade inflammation is present in the duodenum of FD patients. The focus of research in the field has moved to gastric motility, low-grade inflammation, and mucosal permeability of the duodenum [22,23,24]. A recent study reported that PPIs suppress the duodenal eosinophilia of FD patients [25].
Prokinetic Agents (Acotiamide)
Although meta-analyses have reported the relatively high effectiveness of prokinetic agents, reports are inconsistent. It is likely that publication bias occurred. Moreover, previous studies used cisapride, which has been withdrawn due to cardiovascular side effects. Therefore, many specialists have questioned the efficacy of prokinetic agents. Acotiamide was released in 2013 in Japan, and it improves enterokinesis by acting directly on peripheral synaptic clefts to increase acetylcholine levels, while other prokinetic agents such as mosapride and trimebutine act on the nerve plexus in the gastrointestinal tract. Large-scale, double-blind, placebo-controlled trials have proven that acotiamide is effective for patients who are classified as having PDS-type FD [26, 27]. A recent study using gastric scintigraphy reported that acotiamide significantly improved gastric accommodation and emptying, thereby relieving abdominal fullness in patients with PDS-type FD [28] (Figs. 4 and 5). In addition, chronic administration of acotiamide appears to be safe and effective and improves patients’ QOL [29••]. In recent reports, a PPI combined with acotiamide reduced upper abdominal postprandial distention and early satiety more significantly in patients with FD and heartburn. Therefore, greater therapeutic efficacy may result from proper treatment based on a correct understanding of patients’ complaints [30].
Effect of acotiamide on gastric motility using gastric scintigraphy. Representative images of the gastric scintigraphy of Japanese FD patients of pre and post medication of acotiamide. The gastric radioactivity was apparently increased after 2 weeks of dosing of acotiamide, suggesting that gastric accommodation was clearly improved. This is a representative image of the patients who participated in our clinical study (ref. 28). FD, functional dyspepsia
Effect of acotiamide on GSRS in Japanese FD patients. The abdominal pain and dyspepsia score was significantly improved after study drug administration of acotiamide (abdominal pain: pre, 2.4 ± 1.3; post, 1.8 ± 0.6; p = 0.04, dyspepsia: pre: 2.8 ± 0.8, post: 2.3 ± 0.8, p = 0.0003). The total GSRS score was also significantly improved in the acotiamide group (pre, 2.5 ± 0.6; post, 2.1 ± 0.6, p = 0.0007) (ref. 28). GSRS, Gastrointestinal Symptom Rating Scale; FD, functional dyspepsia
Tricyclic Antidepressants
The ACG/CAG guideline recommended the use of TCAs for FD following a trial of PPIs, and TCAs should be used prior to prokinetic agents when PPIs are not effective (Fig. 2). The effects of psychoactive agents on FD were examined in 13 randomized controlled trials (RCTs), and their usefulness was reported, with a number needed to treat (NNT) of six [31•, 32, 33]. In addition, three trials reported the usefulness of TCAs [34]. RCTs showed no apparent advantage of selective serotonin reuptake inhibitors (SSRIs) for symptoms of FD. In the ACG/CAG guideline, TCA is recommended as second-line therapy following use of PPIs.
Kampo Medicines
In the Japanese FD guidelines, some Kampo medicines are recommended for patients who do not respond to PPIs and prokinetic agents. Although the effectiveness of some Kampo medicines, such as hangekobokuto and rikkunshito, has been reported, no high-quality or well-organized reports have been published. A recent RCT using placebo and rikkunshito showed significant improvement of dyspeptic symptoms in patients with PDS-type FD [35•, 36] (Fig. 5). Recent advances in making placebo with similar smell and taste to rikkunshito could make the evidence along these lines more reliable; it is hoped that further high-quality evidence will become available (Fig. 6).
Effect of rikkunshito on overtreatment efficacy after for 4- and 8-week treatment. The incidence of extremely improved and improved after 8-week treatment of rikkunshito was 8.2% and 29.5% that was significantly higher than those of placebo group (1.8% and 21%) (p = 0.019) (ref. 35)
Acupuncture
Many articles have been published on the use of acupuncture for dyspepsia, mainly in China. However, the sample size was small in all articles, and few reports provided high-level evidence for an active or placebo effect. RCTs comparing acupuncture and sham treatment reported that acupuncture significantly improved dyspeptic symptoms. However, a meta-analysis comparing acupuncture and prokinetic agents found no significant differences in the improvement of dyspepsia [37, 38]. A recent RCT comparing percutaneous electrical and normal acupuncture found that the former was effective for dyspepsia in patients with refractory FD [39]; however, further detailed examination is required because of a faulty study design.
Electrical Stimulation
Gastric electrical stimulation using a laparoscopically implanted device in the abdomen reportedly improves gastric emptying and dyspepsia in patients with gastroparesis [40, 41]. Continuous electrical stimulation may act directly on the gastric myenteric plexus and the vagus nerve (nausea and vomiting are induced by vagus stimulation). This may become a new therapy for refractory gastroparesis.
Gastric Peroral Endoscopic Myotomy
Peroral endoscopic myotomy (POEM) is a novel therapy for esophageal achalasia. G-POEM has reportedly been found to be useful for gastroparesis [42,43,44]. G-POEM improves gastric emptying by forming a tunnel in the mucosa, similar to that in POEM, and an incision through the muscular layers of the pyloric region enables passage through the pyloric ring. Gastric emptying was significantly improved, and clinical improvement was achieved in 70–80% of patients. G-POEM also improved symptoms of dyspepsia (e.g., nausea, early satiety, and distention) and QOL in the early period after surgery. Although the indications, safety, and efficacy of G-POEM remain controversial, this treatment may prove useful for intractable dyspepsia.
Meal and Lifestyle Modification
RCTs have reported that a high-fat FODMAP (fermentable oligosaccharide, disaccharide, monosaccharide, and polyols) diet and gluten-containing food contribute to the onset of dyspepsia. Wheat protein, milk protein, fruit juice, peppers, chilies, coffee, and alcohol affect sensation and motility in the gastrointestinal tract and induce dyspeptic symptoms [45]. However, some RCTs and meta-analyses showed that ingestion of peppermint, caraway seeds, and STW 5 herbal extracts is effective [46]. FD symptoms may be affected by individual lifestyles, and an improved lifestyle may relieve dyspeptic symptoms. Therefore, patients whose symptoms persist after treatment should attempt lifestyle modification.
Conclusion
This article provided an update of the diagnosis and treatment of FD based on current topics and knowledge. FD is now covered by national health insurance in Japan, and basic concepts of diagnosis and treatment of FD are being established. However, medical doctors and staff have not widely used this disease name, FD. It is expected that dyspeptic symptoms will be appropriately managed based on the concept for the diagnosis and treatment of FD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–92 This paper is an updated Rome classification of FD.
Miwa H, Kusano M, Arisawa T, Oshima T, Kato M, Joh T, et al. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol. 2015;50(2):125–39.
Oshima T, Miwa H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J Neurogastroenterol Motil. 2015;21(3):320–9.
•• Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112(7):988–1013 This paper is an updated ACG and CAG guidelines of FD.
Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79 Review.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004. 1490;328(7454):19.
Vanheel H, Carbone F, Valvekens L, et al. Pathophysiological abnormalities in functional dyspepsia subgroups according to the Rome III criteria. Am J Gastroenterol. 2017;112:132140.
Asano H, Tomita T, Nakamura K, Yamasaki T, Okugawa T, Kondo T, et al. Prevalence of gastric motility disorders in patients with functional dyspepsia. J Neurogastroenterol Motil. 2017;23(3):392–9.
DiBaise JK, Patel N, Noelting J, Dueck AC, Roarke M, Crowell MD. The relationship among gastroparetic symptoms, quality of life, and gastric emptying in patients referred for gastric emptying testing. Neurogastroenterol Motil. 2016;28:234–42.
Simrén M, Törnblom H, Palsson OS, van Tilburg MAL, Van Oudenhove L, Tack J, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67(2):255–62.
• Carbone F, Tack J, Hoffman I. The intragastric pressure measurement: a novel method to assess gastric accommodation in functional dyspepsia children. J Pediatr Gastroenterol Nutr. 2017;64(6):918–24 This paper showed new measurement methods of adaptive relaxation response of the stomach using high-resolution manometry.
Carbone F, Vanuytsel T, Tack J. The effect of mirtazapine on gastric accommodation, gastric sensitivity to distention, and nutrient tolerance in healthy subjects. Neurogastroenterol Motil 2017;29(12).
Wu Q, Zou M, Yang M, Zhou S, Yan X, Sun B, et al. Revealing potential biomarkers of functional dyspepsia by combining 1H NMR metabonomics techniques and an integrative multi-objective optimization method. Sci Rep. 2016;6:18852.
Moayyedi P. Helicobacter pylori eradication for functional dyspepsia: what are we treating?: comment on “Helicobacter pylori eradication in functional dyspepsia”. Arch Intern Med. 2011;171(21):1936–7.
Mazzoleni LE, Sander GB, Francesconi CF, Mazzoleni F, Uchoa DM, De Bona LR, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med 2011;28;171(21):1929–1936.
Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67.
• Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017; This paper is a systematic review to evaluate whether PPI therapy provides symptomatic relief in FD.
Wang WH, Huang JQ, Zheng GF, Xia HH, Wong WM, Liu XG, et al. Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials. Clin Gastroenterol Hepatol. 2007;5(2):178–85.
Lee KJ, Demarchi B, Demedts I, Sifrim D, Raeymaekers P, Tack J. A pilot study on duodenal acid exposure and its relationship to symptoms in functional dyspepsia with prominent nausea. Am J Gastroenterol. 2004;99(9):1765–73.
Oshima T, Okugawa T, Tomita T, Sakurai J, Toyoshima F, Watari J, et al. Generation of dyspeptic symptoms by direct acid and water infusion into the stomachs of functional dyspepsia patients and healthy subjects. Aliment Pharmacol Ther. 2012;35(1):175–82.
Ishii M, Kusunoki H, Manabe N, Kamada T, Sato M, Imamura H, et al. Evaluation of duodenal hypersensitivity induced by duodenal acidification using transnasal endoscopy. J Gastroenterol Hepatol. 2010;25(5):913–8.
NJ WMM, Aro P, Ronkainen J, Storskrubb T, Hindley LA, Harmsen WS, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:11751183.
Wang X, Li X, Ge W, Huang J, Li G, Cong Y, et al. Quantitative evaluation of duodenal eosinophils and mast cells in adult patients with functional dyspepsia. Ann Diagn Pathol. 2015;19(2):50–6.
Du L, Shen J, Kim JJ, Yu Y, Ma L, Dai N. Increased duodenal eosinophil degranulation in patients with functional dyspepsia: a prospective study. Sci Rep. 2016;6:34305.
Potter MDE, Wood NK, Walker MM, Jones MP, Talley NJ. Proton pump inhibitors and suppression of duodenal eosinophilia in functional dyspepsia. Gut. 2018.
Matsueda K, Hongo M, Tack J, Saito Y, Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut. 2012;61(6):821–8.
Altan E, Masaoka T, Farré R, Tack J. Acotiamide, a novel gastroprokinetic for the treatment of patients with functional dyspepsia: postprandial distress syndrome. Expert Rev Gastroenterol Hepatol. 2012;6(5):533–44.
Nakamura K, Tomita T, Oshima T, Asano H, Yamasaki T, Okugawa T, et al. A double-blind placebo controlled study of acotiamide hydrochloride for efficacy on gastrointestinal motility of patients with functional dyspepsia. J Gastroenterol. 2017;52(5):602–10.
•• Tack J, Pokrotnieks J, Urbonas G, Banciu C, Yakusevich V, Bunganic I, et al. Long-term safety and efficacy of acotiamide in functional dyspepsia (postprandial distress syndrome)-results from the European phase 3 open-label safety trial. Neurogastroenterol Motil. 2018;30(6):e13284 This paper indicated the long-term safety and efficacy of acotiamide in FD.
Yamawaki H, Futagami S, Kawagoe T, Maruki Y, Hashimoto S, Nagoya H, et al. Improvement of meal-related symptoms and epigastric pain in patients with functional dyspepsia treated with acotiamide was associated with acylated ghrelin levels in Japan. Neurogastroenterol Motil. 2016;28(7):1037–47.
• Ford AC, Luthra P, Tack J, Boeckxstaens GE, Moayyedi P, Talley NJ. Efficacy of psychotropic drugs in functional dyspepsia: systematic review and meta-analysis. Gut. 2017;66(3):411–420. Review This paper is a systematic review of psychotropic drug in FD.
Braak B, Klooker TK, Wouters MM, Lei A, van den Wijngaard RM, Boeckxstaens GE. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlledstudy. Aliment Pharmacol Ther. 2011 Sep;34(6):638–48.
Talley NJ, Locke GR, Saito YA, Almazar AE, Bouras EP, Howden CW, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized controlled study. Gastroenterology. 2015 Aug;149(2):340–9.e2.
Tan VP, Cheung TK, Wong WM, Pang R, Wong BC. Treatment of functional dyspepsia with sertraline: a double-blind randomized placebo-controlled pilot study. World J Gastroenterol. 2012;18(42):6127–33.
• Tominaga K, Sakata Y, Kusunoki H, Odaka T, Sakurai K, Kawamura O, et al. Rikkunshito simultaneously improves dyspepsia correlated with anxiety in patients with functional dyspepsia: a randomized clinical trial (the DREAM study). Neurogastroenterol Motil. 2018;30(7):e13319 This paper is a randomized, placebo-controlled, double-blind clinical trial to determine the efficacy of rikkunshito in FD patients.
Suzuki H, Matsuzaki J, Fukushima Y, Suzaki F, Kasugai K, Nishizawa T, et al. Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia--a multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol Motil. 2014;26(7):950–61.
Kim KN, Chung SY, Cho SH. Efficacy of acupuncture treatment for functional dyspepsia: a systematic review and meta-analysis. Complement Ther Med. 2015;23(6):759–66.
Lan L, Zeng F, Liu GJ, Ying L, Wu X, Liu M, et al. Acupuncture for functional dyspepsia. Cochrane Database Syst Rev. 2014;10:CD008487.
Zheng H, Xu J, Sun X, Zeng F, Li Y, Wu X, et al. Electroacupuncture for patients with refractory functional dyspepsia: a randomized controlled trial. Neurogastroenterol Motil. 2018;30(7):e13316.
Laine M, Sirén J, Koskenpato J, Punkkinen J, Rantanen T, Typpö I, et al. Outcomes of high-frequency gastric electric stimulation for the treatment of severe, medically refractory gastroparesis in Finland. Scand J Surg. 2018;107(2):124–9.
Davis BR, Sarosiek I, Bashashati M, Alvarado B, McCallum RW. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21(2):222–7.
Mekaroonkamol P, Dacha S, Wang L, Li X, Jiang Y, Li L, et al. Gastric peroral endoscopic pyloromyotomy reduces symptoms, increases quality of life, and reduces health care use for patients with gastroparesis. Clin Gastroenterol Hepatol 2018
Xue HB, Fan HZ, Meng XM, Cristofaro S, Mekaroonkamol P, Dacha S, et al. Fluoroscopy-guided gastric peroral endoscopic pyloromyotomy (G-POEM): a more reliable and efficient method for treatment of refractory gastroparesis. Surg Endosc. 2017;31(11):4617–24.
Malik Z, Kataria R, Modayil R, Ehrlich AC, Schey R, Parkman HP, et al. Gastric per oral endoscopic myotomy (G-POEM) for the treatment of refractory gastroparesis: early experience. Dig Dis Sci 2018 22.
Duncanson KR, Talley NJ, Walker MM, Burrows TL. Food and functional dyspepsia: a systematic review. J Hum Nutr Diet. 2018;31(3):390–407.
Acker BW, Cash BD. Medicinal foods for functional GI disorders. Curr Gastroenterol Rep. 2017;19(12):62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the author.
Additional information
This article is part of the Topical Collection on Neurogastroenterology and Motility Disorders of the Gastrointestinal Tract
Rights and permissions
About this article
Cite this article
Tomita, T., Oshima, T. & Miwa, H. New Approaches to Diagnosis and Treatment of Functional Dyspepsia. Curr Gastroenterol Rep 20, 55 (2018). https://doi.org/10.1007/s11894-018-0663-4
Published:
DOI: https://doi.org/10.1007/s11894-018-0663-4