Abstract
Purpose of Review
To provide an update on the prevalence, pathophysiology, disease associations, and treatment options for bile acid malabsorption (BAM).
Recent Findings
•Molecular mechanisms—BAs prevent water reabsorption and increase water secretion by intracellular mediators, increasing aquaporin channels and intracellular permeability. •Inflammatory bowel disease—new molecular mechanisms of BAM are identified in patients without ileal disease, including changes in expression of ileal BA transporter and nuclear receptors involved in BA homeostasis. •Microscopic colitis—BAM is one of the mechanisms leading to microscopic colitis. •Diagnostic testing—new diagnostic tests have been launched in the USA (serum C4 and fecal 48-h BA excretion); stimulated FGF19 has higher detection of BAM compared to fasting sample alone. •Treatment—investigational FXR agonists may provide a daily, oral option for treatment of BAM instead of BA sequestrants.
Summary
There is a greater appreciation of the biological role of bile acids across multiple fields of medicine, including gastrointestinal indications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: What Is Already Known About BAM?
Bile acids (BAs) are detergent molecules made in the liver and secreted with meals to emulsify fat and fat soluble vitamins to aid in absorption [1]. BAs retain their cholesterol structure, but differ only by their conjugation, resulting in various functions throughout the gastrointestinal tract. In particular, chenodeoxycholic acid (CDCA) and deoxycholic acid (DCA) both induce increased colonic secretion and motility.
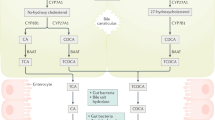
The liver synthesizes the primary BAs, CDCA, and cholic acid (CA). Any free BAs in the ileum are efficiently (~ 95%) absorbed via an active transporter, the apical sodium bile acid transporter (ASBT). Upon reabsorption, BAs stimulate the nuclear farsenoid X receptor (FXR), which increases fibroblast growth factor 19 (FGF19). FGF19 acts as an enteroendocrine hormone and travels to the liver via the hepatic vein to decrease hepatic BA synthesis by decreasing the rate limiting enzyme, 7 α-hydroxy-4-cholesten-3-one (C4) (Fig. 1) [2, 3].
A graphical representation of the enterohepatic circulation. Left panel indicates BA circulation in healthy individuals. BAs are reabsorbed in the ileum, activate FXR and increase FGF19 synthesis. FGF19 then binds to the FGFR4 and klotho β receptors to decrease C4 and subsequent hepatic BA synthesis. Right panel: In BAM, BAs are reabsorbed, but FGF19 remains low, or there are mutations within the FGFR4 or klotho β receptors that do not inhibit hepatic BA synthesis. BAs that enter the colon bind to the GPBAR1 receptor and cause increased colonic transit and secretion. Reproduced with permission from ref. 2, Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol 592: 2967–2980, 2014
Bile acid malabsorption (BAM) is a disease entity characterized by diarrhea and a combination of abdominal discomfort and bloating [4, 5]. Traditionally, there are three types of BAM recognized [6]: type 1 BAM includes ileal disease, such as Crohn’s disease, ileal resection, and radiation ileitis; type 2 BAM is “idiopathic,” with similar presentation as functional diarrhea or IBS-D; type 3 BAM is secondary to diseases, such as chronic pancreatitis and celiac disease. A fourth category (sometimes termed type 4) of patients with BAM consists of those with increased BA synthesis from metformin and hypertriglyceridemia [7].
The bile acid sequestrants (BAS), colestipol, cholestyramine, and colesevelam, are the mainstay of treatment of BAM. Open-label studies have demonstrated an improvement in colonic transit and ease of stool passage with colesevelam in patients with elevated C4 and total fecal BA [8]. Additionally, clinical improvement with BAS has been correlated with BAM severity. Thus, 96% of patients with severe BAM, based on 75SeHCAT retention, improved with BAS; 80% with moderate BAM improved; and 70% with mild BAM improved [9].
Based on advances in knowledge of the prevalence, mechanisms, diagnosis, and treatment of BAM summarized in this article, we propose that consideration of BAM in clinical diagnosis and treatment is ready for prime time.
Symptoms of BAM
Diarrhea is the hallmark symptom associated with BAM. A recent online survey of patients with BAM demonstrated a combination of primary gastrointestinal and systemic symptoms. Of the 1300 participants in an online BAM support group, 100 patients responded to the survey, and 85% reported urgency, 54% abdominal pain, 88% occasional incontinence, and 52% felt the need to remain in close proximity to the bathroom. Secondary to the abdominal discomfort, 40% of patients reported fatigue and at least 60% noted “brain fog” which prevented work efficiency. After treatment with BAS, gastrointestinal and systemic symptoms improved or resolved by at least 50%, and there was a significant improvement in work absences and altered work hours [10••]. Although the study design had weaknesses, including the small sample size of participants and possible inherent bias introduced by self-selection (less than 10% of those eligible participated), the study outlined the extent of physical symptoms and consequences in productivity at work that may be associated with BAM.
Prevalence of BAM Disease in Diverse Conditions
BAM is estimated to affect ~ 1% of the population in western countries [11]. The prevalence of BAM in different conditions has remained largely the same, and the prevalence rates are generally replicated in numerous studies. For example, a retrospective review reported that type 1 BAM was present in 77/87 patients with Crohn’s disease who underwent 75SeHCAT [12], in more than 90% of patients with Crohn’s disease with ileal resection, and in 11–52% of those without ileal resection [13]. Because of the retrospective nature of such studies, it is possible that estimated prevalence is inflated, and additional prospective evaluation is required.
The most valid assessment of the prevalence of type 2 “idiopathic” BAM was a systematic review of 15 prospective studies, most of which were based on 75SeHCAT retention, that reported the prevalence of BAM in patients with functional diarrhea or IBS-D. Thus, severe BAM (< 5% retention) was present in 10%, moderate BAM (< 10% retention) in 32%, and mild BAM (< 15% retention) in 26% [9]. The prevalence of types 3 and 4 BAM is unclear because screening for BAM in these patients is limited.
Advances in Mechanisms of Action of BAs: Pathophysiology of Gastrointestinal Symptoms Due to BAM
BAs cause diarrhea by increasing colonic motility and secretion, and they affect inflammation and the microbiome.
Stimulation of Water and Electrolyte Secretion
Water and electrolyte secretion in response to bile acids is based on several mechanisms: stimulation of intracellular mediators [14,15,16,17,17, 18••, 19], increase in intestinal permeability [20, 21, 22•, 23••], aquaporin channels [24••, 25], enteroendocrine mechanisms [26,27,28], neurocrine mediation [29, 30, 31•], and decrease in sodium and water absorption [32]. Details regarding these mechanisms, mediators and their effects resulting in water and electrolyte secretion are found in Table 1.
Stimulation of Motility
BAs activate the TGR5 on the luminal surface of colonic epithelium; in addition, BAs are passively absorbed to activate enteric neurons to release serotonin, which induces colonic contractions [31•] and chloride secretion [26]. TGR5 mediates the prokinetic actions of intestinal BAs and is required for normal defecation in mice [33]. Recent data have demonstrated that greatest potency for stimulation of TGR5 is with lithocholic acid (LCA) followed by DCA, CDCA, and CA [17].
Two important observations of the effects of BAs on colonic motility in humans are as follows: first, the induction of high amplitude propagating contractions (HAPCs) in the colon with rectal infusion of 1 mM CDCA [34]; and second, the induction of accelerated colonic transit with ileocolonic delivery formulation of Na CDC, 500 to 1000 mg [35]. Assuming the intracolonic volume is ~1 l, this would lead to intracolonic concentration of 1–2.5 mM CDC before colonic dehydroxylation to LCA, which may then stimulate colonic motility through TGR5 receptor activation.
Interaction of Bile Acids With Microbiome
Previous studies have demonstrated that patients with IBS-D have a higher proportion of Escherichia coli and decreased Clostridium leptum and Bifidobacterium [36]. Patients with BAM have a higher proportion of fecal primary BAs, particularly CDCA, a secretory BA [4, 36]. Although there is greater understanding of the effects of the microbiota on BAM, there are still gaps in knowledge; therefore, before we can modulate the microbiome to alter fecal BAs and vice versa to improve symptoms, further understanding is needed.
Other Effects of TGR5 Activation by Bile Acids
In addition to the effects on intestinal secretion and motility, TGR5 activation results in two other sets of beneficial effects:
-
A.
Inhibition of damage caused by inflammation
-
(i)
In experimental dextran sodium sulfate colitis [20, 37], BAs activated TGR5 receptors, interfering with cytokine responses and restoring barrier integrity.
-
(ii)
In murine colitis [38], TGR5 activation restored barrier integrity or altered macrophage phenotype to reduce inflammatory damage.
-
(iii)
In Crohn’s disease, TGR5 activation resulted in inhibition of TNF-α production by isolated intestinal macrophages [39].
-
(iv)
In obstructive jaundice, e.g., in bile duct ligated rats, CDCA promoted intestinal epithelial cell proliferation, and decreased mucosal injury [40].
-
B.
Stimulation of enteroendocrine cells to secrete amines and peptides, including glucagon-like peptide 1 and peptide YY; these effects are relevant to the changes in glycemic control and appetite following bariatric surgery [41].
Clinical Associations
Inflammatory Bowel Disease (IBD)
Although the true prevalence of BAM in IBD requires further investigation, BAM is more frequently found in Crohn’s disease as compared to ulcerative colitis. The obvious etiology for BAM in Crohn’s disease is either ileal resection or ileal disease. The longer the segment of ileum involved or resected, the greater the likelihood and/or severity of BAM and responsiveness to BAS [42, 43]. The extent of ileal involvement determines whether the patient experiences diarrhea (< 100 cm diseased) or combination of diarrhea and steatorrhea (> 100 cm involved or resected) [44]. Interestingly, there have been reports of exclusively colonic disease resulting in BAM [45]; indeed, in vitro uptake of BAs by normal ileal biopsies from patients with Crohn’s colitis was reduced, indicating an alternative cause of BAM in certain IBD patients. Given that ~ 75% of BAs reaching the colon are passively absorbed by the colonic epithelium [32], it is conceivable that extensive inflammation caused by colitis would impair this absorption and result in increased fecal BA concentration. In fact, mucosal expression studies have shown that ulcerative pancolitis (but not in exclusively left-sided colitis) was associated with broad changes, including altered expression of major BA transporters (multi-drug resistance associated protein 3 and 4, multi-drug resistance gene 1, organic solute transporter α/β) and nuclear receptors [pregnane X receptor (PXR), vitamin D receptor] in the descending colon. In contrast, Crohn’s ileitis was associated with selective changes in intestinal BA transporter expression [46].
Patients with Crohn’s disease (n = 16; one with evidence of ileal inflammation) had a decrease in ASBT expression to 69% on ileal biopsies compared to healthy controls. ASBT expression and co-expression of glucocorticoid receptor increased by 15–20-fold when exposed to corticosteroids in rats [47••]. This study indicates that BAM may be present in patients without ileal inflammation in Crohn’s disease. However, further analysis is required to determine if a decrease in ASBT expression in patients with Crohn’s disease results in BAM.
PXR is a hepatic nuclear receptor that is activated by excess hepatocyte BAs and aids in alternative methods of BA export from the hepatocyte. In BAM associated with Crohn’s disease, decreased BA absorption from decreased ASBT expression may lead to decreased PXR activation; the degree of BAM was closely associated with deactivation of PXR, particularly in Crohn’s disease [48•]. Patients with PXR mutations and decreased PXR activation have a higher susceptibility to develop IBD [49]. Conversely, in human studies, rifaximin (a PXR activator) decreased inflammation and improved mucosal healing at 12 weeks of treatment [50], possibly secondary to microbiota changes, PXR activation, or decreased inflammation [51]. Thus, glucocorticoids may be potential therapeutic targets of BAM in Crohn’s disease to increase ASBT expression, and rifaximin may be used to activate PXR.
Radiation Enteritis and Cancer Chemotherapy
Radiation therapy associated ileitis has long been recognized as a cause for type 1 BAM. It is reported that 44–57% of patients undergoing pelvic radiotherapy develop BAM [52]. Even with technology advances and the field of radiation exposure narrowed, a recent study still reported 13/20 patients (65%) undergoing radiation therapy for cervical or bladder cancer developed BAM [53], possibly because the terminal ileum is immobile relative to other parts of the small bowel and suffers recurrent exposure with each radiation treatment.
It is estimated that BAM is the cause in 40–70% of patients who develop diarrhea after pelvic radiation therapy [54]. There may be an additional contribution by chemotherapy, with sensitization to radiation damage. In addition, the finding that ~ 40% of patients with breast, hematologic and esophagogastric malignancy, and 70% of patients with multiple myeloma develop BAM after treatment suggests that chemotherapy may lead to BAM [54]. For example, lenalidomide, a medication used for multiple myeloma and myelodysplastic syndrome, is associated with diarrhea (~ 10% of patients) [55]. A recent case series demonstrated that 12 consecutive patients referred for progressive diarrhea after initiation of lenalidomide had evidence of BAM [56].
Post-Cholecystectomy BAM
A systematic review evaluated 25 studies that included 3388 patients who had undergone cholecystectomy and determined that only 9.1% of patients developed diarrhea after cholecystectomy; of the patients with diarrhea, 65.5% were diagnosed with BAM [57]. In 125 consecutive patients who underwent laparoscopic cholecystectomy, 25.2% developed diarrhea at 1 week after surgery and 5.7% had diarrhea at 3 months [58•].
Primary BAM: What Causes It?
At present, the most likely cause of primary BAM is reduced production of FGF19 by ileal enterocytes, which is associated with low circulating levels of fasting serum FGF19 and reciprocal increase in serum 7α-C4, a surrogate for the hepatic synthesis of BAs [59]. Walters et al. have also provided preliminary evidence of reduced FGF19 and ASBT mRNA expression in ileal biopsies from patients with BAM [60]. Thus, 75SeHCAT retention significantly correlated with the basal ileal transcript expression of FGF19 and ASBT and with the degree of stimulation by CDCA at 6 h for transcripts of FGF19 and ileal BA binding protein.
Other factors associated with the proteins involved in the enterohepatic circulation have been examined through studies in patients with diarrhea associated with functional gastrointestinal disorders (IBS-D and functional diarrhea). These studies focused on genetic variants that impact ileal BA absorption, feedback inhibition, hepatic BA synthesis, and BA colonic receptors. There were significant associations between acceleration of colonic transit and genetic variations in the GPBAR1 rs11554825, klotho β rs17618244, and fibroblast growth factor receptor 4 rs351855 in patients with IBS-D or functional diarrhea [61, 62].
Microscopic Colitis
Several investigators have studied the role of BAM in microscopic colitis. The cause of BAM may be related to the villous atrophy, inflammation, and collagen deposition in the ileum reported in patients with microscopic colitis [63, 64].
A study from Spain reported BAM (based on 75SeHCAT retention test) in 43% of patients with microscopic colitis, more commonly with lymphocytic (60%) than collagenous (27%) colitis; 86% of patients with BAM responded to cholestyramine [65]. Findings in other studies of BAM in microscopic colitis have been conflicting [66,67,68], and it is also noted that diarrhea patients without BAM may respond to a BAS [69]. Hence, a therapeutic response to BAS does not prove BAM in such patients, and screening for BAM (as with fasting serum C4 measurement) should be undertaken instead of empiric trial of therapy in patients with microscopic colitis.
Diagnosis
BAM is a condition of impaired ileal BA absorption, decreased hepatic feedback inhibition, and increased hepatic BA synthesis. Current BAM diagnostic methods target one of these pathways.
75Selenium HomotauroCholic Acid Test
75SeHCAT is the gold standard for BAM diagnosis. It measures the retention of radiolabeled BAs 7 days after ingestion. The lower the retention of radiolabeled BAs indicates that BAs are lost in the feces, and the lowest levels of retention reflect more severe BAM. Current cut-offs for mild, moderate, and severe BAM are < 15, < 10, and < 5% retention respectively at 7 days. 75SeHCAT is a simple, noninvasive measurement of BAM, but it requires specialized equipment, exposes patients to radiation, and is not available in many countries, including the USA.
BA ileal absorption efficiency varies widely from day to day, from 49 to 86% [70], and it also depends on BA recycling rates, since the number of times the BAs enter the enterohepatic circulation can vary from 4 to 16 per day [71]. With this variability, even a healthy individual with BA recycling ≥ 8 cycles per day may have abnormal 75SeHCAT retention. Conversely, a patient with a lower rate of BA recycling may have equivocal or normal 75SeHCAT retention [72]. 75SeHCAT is an excellent tool for BAM diagnosis where available, but evaluation of BA recycling rates may improve its accuracy.
48-Hour Fecal Bile Acid Test
In places without access to 75SeHCAT, a 48-h fecal BA test is the best current option. Patients consume a high-fat diet (100 g/day) for 4 days and collect stool for the last 48 h. Total and primary fecal BA levels have demonstrated a significant positive association with 75SeHCAT retention [73, 74].
Although the stool collection is cumbersome, a 48-h fecal BA allows direct total and individual BA measurements without radiation exposure. The current cut-off for a diagnosis of BAM is total fecal BAs ≥ 2337 μmol/48 h [5]. Patients with IBS-D have shown significantly higher levels of primary BAs (CDCA and CA) compared to healthy controls (< 5%), which correlated with stool frequency and consistency [4]. The percentage of primary and secretory (CDCA and DCA) BAs was significantly associated with colonic transit at both 24 and 48 h. Based on this data and the secretory functions of BAs, BAM diagnosis may be made in patients with total fecal BAs of ≥ 2337 μmol/48 h or primary BAs (CDCA + CA)/total fecal BAs ≥ 5%. However, lower levels of fecal BA excretion, especially when associated with high fecal primary BAs or secretory BAs (CDCA and DCA), are associated with faster colonic transit, suggesting that the combination may reflect a contribution of BAs to the diarrhea, even if the total fecal BA excretion is < 2337 μmol/48 h [75••].
Fasting Serum 7 α-Hydroxy-4-Cholesten-3-One (C4)
Serum C4 is the rate limiting step associated with BA synthesis in the liver. Thus, C4 is a direct measure of BA synthesis, and a higher value is indicative of BAM. C4 has been validated against both 75SeHCAT and fecal BAs. When comparing against 75SeHCAT, C4 had sensitivity of 90% and specificity of 79% at a cut-off value of 48.4 ng/mL [76].
A recent study evaluated 184 healthy participants to determine the optimal cut-off to diagnose BAM. Serum C4 < 17 ng/mL indicates no evidence of BAM; C4 > 52.5 ng/mL is diagnostic for BAM; and C4 values in the 17–52.5 ng/mL range are equivocal and require confirmation as with 48-h fecal BA [77]. With these new cut-offs, performance characteristics were analyzed. This study demonstrated a significant association between replicate C4 samples; the majority of the replicate samples remained < 52.5 ng/mL and had 85% specificity and negative predictive value compared to 48-h fecal BAs, indicating that fasting serum C4 is an efficient and convenient method to rule out BAM [78].
Fasting serum C4 was also utilized in patients with IBD. Patients with Crohn’s disease had higher C4 levels compared to healthy controls, and high C4 was associated with ileal disease or resection and non-bloody diarrhea. Baseline C4 levels in ulcerative colitis were similar to healthy controls [79]. Thus, fasting serum C4 may be beneficial to aid in the diagnosis of BAM in IBD, particularly in Crohn’s disease; BAM cut-off values need to be further investigated in this population.
Serum Fasting Fibroblast Growth Factor 19 (FGF19)
BAs reabsorbed within the ileal epithelium are potent stimulators of the nuclear FXR receptors, which induce FGF19 transcription. FGF19 is an enteroendocrine hormone that reaches the liver via the portal circulation and provides negative feedback for hepatic BA synthesis, thereby impacting the size of the BA pool. Recent literature demonstrates that CDCA is the most potent FXR agonist, followed by CA (81%), DCA (40%), and LCA (4%) relative to CDCA’s potency [80].
Serum FGF19 is inversely correlated with serum C4 [59], and this has been replicated in subsequent studies in IBS-D [5] and IBS-C [81]. A low serum FGF19 may reflect BAM. Based on previous studies, there is no evidence of genetic abnormalities in the FXR gene (NR1H4) in this patient population [62]. Based on the 5th percentile in healthy volunteers, the proposed cut-off value for BAM is ≤ 61.7 pg/mL. Performance characteristics of FGF19 demonstrated a nearly significant correlation (p = 0.053) with replicate samples, 75% specificity and 79% negative predictive value, and the majority of values remained within the normal range with replicate samples [78]. This indicates that a single, fasting serum FGF19 is helpful to rule out BAM.
A CDCA-stimulated FGF19 test may increase the test’s sensitivity to diagnose BAM, particularly in patients with primary BAM. Similar to cardiac stress tests or endocrine stimulation tests, stressing the system may be the best method to detect disease, particularly in patients with less overt symptoms or pathophysiology, for example, to detect BAM in IBS-D patients. A meal stimulus alone did not demonstrate a significant change in pre- and postprandial FGF19 in diarrhea controls or patients with proven BAM [82]. However, a stimulus of a meal with CDCA demonstrated a significant increase in postprandial FGF19 compared to preprandial levels in healthy controls, but stable pre- and postprandial FGF19 in the BAM cohort [83••]. As FGF19 is an enteroendocrine hormone, accuracy of this test may be enhanced with a stimulus. Further confirmatory studies are required.
Treatment
Dietary Modifications
A low-fat diet with < 20% of total dietary intake added to a BAS treatment (e.g., colesevelam in 44% of a 114-patient cohort with 75SeHCAT < 20%) significantly improved abdominal discomfort, distension, urgency, and stool consistency and frequency [84].
Bile Acid Sequestrants
There are three major BAS available in either powder or tablet formulations: cholestyramine, colestipol, and colesevelam. Patients are instructed to take these medications with meals in order to bind free BAs and prevent the colonic effects of increased colonic motility and secretion. Two recent double-blinded, placebo-controlled, randomized trials with a BAS, showed colesevelam in Crohn’s disease diagnosed with BAM (elevated serum C4) was associated with a significantly higher number of patients with > 30% reduction of liquid stool and reduction of median number of liquid stools from 5 to 2 per week compared to placebo [85], and a second trial that compared cholestyramine with hydroxypropyl cellulose in patients with chronic water diarrhea (though only some with 75SeHCAT < 15%) showed that a mean percent decrease in watery stools was significantly higher in the cholestyramine group, but there was no difference in the primary aim of the proportion with mean ≤ 3 liquid bowel movements per week [86]. However, hydroxypropyl cellulose actually binds BAs, and was shown to improve stool frequency, consistency, urgency, and incontinence after 6 weeks’ treatment in patients with idiopathic BAM and Crohn’s disease with ileal resection [87].
After BAS treatment, patients have higher total fecal BA loss and increased serum C4 compared to baseline. The increase in measured total fecal BAs is the result of the methanol extraction step to which the stool sample is subjected. This frees the fecal BAs from the binding resin and provides a paradoxical increase in total fecal BAs. Because of the loss of fecal BAs in the stool, hepatic BA synthesis and, thus, serum C4 are increased [88].
Although BAS are effective in improving abdominal symptoms and stool characteristics, this treatment option does not target the underlying pathophysiology. When diagnostic tests are not available, empiric treatment with BAS is advocated for patients with suspected BAM. However, many patients do not tolerate current therapies. After 5 years of follow-up among patients with primary BAM treated with BAS, ~ 60–70% of the patients discontinued cholestyramine or colesevelam as a result of adverse effects, particularly nausea. Since multiple studies have demonstrated that measuring the severity of BAM can predict the response to treatment, there is strong rationale to measure BAM rather than just empiric treatment [9, 89].
FXR Agonists
BAs reabsorbed within the ileum activate the FXR receptor, with CDCA the most potent activator. A synthetic FXR agonist, obeticholic acid, is 100 times more potent than CDCA [90]. Although initially developed for cholestatic liver diseases such as primary biliary cirrhosis, two studies have demonstrated the efficacy of obeticholic acid in BAM. FXR agonists attenuated calcium and cAMP dependent chloride secretion on colonic epithelium [91]. The second study was a 2-week trial of obeticholic acid daily in patients with primary and secondary BAM and chronic diarrhea. Patients with primary and secondary BAM noted a significant improvement in stool frequency, form, and total diarrhea index, with corresponding increase in FGF19 and decrease in C4 and fecal BAs. In patients with chronic diarrhea, there were no clinical changes despite changes in FGF19, C4, and total fecal Bas [92].
FXR agonists target the underlying mechanism of primary BAM without the adverse effect of nausea and the requirement of multiple doses per day with BAS. Thus, FXR agonists may provide an effective alternative treatment for BAM.
Conclusions
In the past 5 years, the BA field has expanded, and its relevance to multiple fields of medicine is appreciated outside diseases like the cholangiopathies. We anticipate that the greatest impact will be in clinical diagnosis of unexplained diarrhea in patients with IBS-D, microscopic colitis, and even IBD without ileal inflammation or resection. This has been facilitated by the validation of screening serum tests and fecal BA excretion. Novel therapeutic approaches targeting FXR and TGR5 receptors might open new avenues for treatment of intestinal and cholestatic liver diseases, including primary sclerosing cholangitis [93]. BAs may induce DNA damage in colon cells, including mutations and genomic instability, and their potential role in colon cancer development will continue to be a focus of research [94].
Abbreviations
- ASBT:
-
Apical sodium bile acid transporter
- BA:
-
Bile acid
- BAM:
-
Bile acid malabsorption
- BAS:
-
Bile acid sequestrants
- C4:
-
7 α-hydroxy-4-cholesten-3-one
- CA:
-
Cholic acid
- CDCA:
-
Chenodeoxycholic acid
- DCA:
-
Deoxycholic acid
- FGF19:
-
Fibroblast growth factor 19
- FXR:
-
Farsenoid X receptor
- GPBAR1/TGR5:
-
G protein coupled bile receptor 1
- HAPC:
-
High amplitude propagating contractions
- IBS:
-
C—constipation predominanat irritable bowel syndrome
- IBS:
-
D—diarrhea predominant irritable bowel syndrome
- IBD:
-
Inflammatory bowel disease
- LCA:
-
Lithocholic acid
- PXR:
-
Pregane X receptor
- 75SeHCAT:
-
75Selenium homotaurocholic acid test
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hofmann AF, Small DM. Detergent properties of bile salts: correlation with physiological function. Ann Rev Med. 1967;18(1):333–76. https://doi.org/10.1146/annurev.me.18.020167.002001.
Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol. 2014;592(14):2967–80. https://doi.org/10.1113/jphysiol.2014.270892.
Hofmann AF. The syndrome of ileal disease and the broken enterohepatic circulation: cholerheic enteropathy. Gastroenterology. 1967;52(4):752–7.
Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(10):1270–5, e1. https://doi.org/10.1016/j.cgh.2013.04.020.
Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10(9):1009–15, e3. https://doi.org/10.1016/j.cgh.2012.05.006.
Fromm H, Malavolti M. Bile acid-induced diarrhoea. Clin Gastroenterol. 1986;15(3):567–82.
Scarpello JH, Hodgson E, Howlett HC. Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabetic Med. 1998;15(8):651–6. https://doi.org/10.1002/(SICI)1096-9136(199808)15:8<651::AID-DIA628>3.0.CO;2-A.
Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8(2):159–165.e5. https://doi.org/10.1016/j.cgh.2009.10.020.
Wedlake L, A'Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30(7):707–17. https://doi.org/10.1111/j.1365-2036.2009.04081.x.
•• Bannaga A, Kelman L, O'Connor M, Pitchford C, Walters JR, Arasaradnam RP. How bad is bile acid diarrhoea: an online survey of patient-reported symptoms and outcomes. BMJ Open Gastroenterol. 2017;4(1):e000116. An online survey which evaluated the breath of symptoms association with BAM. https://doi.org/10.1136/bmjgast-2016-000116.
Walters JR, Pattni SS. Managing bile acid diarrhoea. Ther Adv Gastroenterol. 2010;3(6):349–57. https://doi.org/10.1177/1756283X10377126.
Borghede MK, Schlütter JM, Agnholt JS, Christensen LA, Gormsen LC, Dahlerup JF. Bile acid malabsorption investigated by selenium-75-homocholic acid taurine (75SeHCAT) scans: causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur J Intern Med. 2011;22(6):e137–e40. https://doi.org/10.1016/j.ejim.2011.08.013.
Barkun AN, Love J, Gould M, Pluta H, Steinhart H. Bile acid malabsorption in chronic diarrhea: pathophysiology and treatment. Can J Gastroenterol. 2013;27(11):653–9. https://doi.org/10.1155/2013/485631.
Conley DR, Coyne MJ, Bonorris GG, Chung A, Schoenfield LJ. Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. Am J Dig Dis. 1976;21(6):453–8. https://doi.org/10.1007/BF01072128.
Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl(−) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am J Phys. 2013;305(4):C447–56. https://doi.org/10.1152/ajpcell.00416.2012.
Domingue JC, Ao M, Sarathy J, Rao MC. Chenodeoxycholic acid requires activation of EGFR, EPAC, and Ca2+ to stimulate CFTR-dependent Cl- secretion in human colonic T84 cells. Am J Phys. 2016;311(5):C777–92. https://doi.org/10.1152/ajpcell.00168.2016.
Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–40. https://doi.org/10.1074/jbc.M209706200.
•• Borg JF, Yde J, Wu Q, Lajczak N, Keely S, Fenton RA, et al. Regulated expression of the Na+/K+-ATPase pump in colonic epithelium by bile acids. FASEB J. 2017;31(Suppl. 1):856. 10-10. This study details that another mechanism diarrhea in patient s with BAM is the lack of electrolyte and water absorption because of changes in the basolateral sodium/potassium ATPase channels.
Suhail M. Na(+), K(+)-ATPase: ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions. J Clin Med Res. 2010;2(1):1–17. https://doi.org/10.4021/jocmr2010.02.263w.
Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6(10):e25637. https://doi.org/10.1371/journal.pone.0025637.
Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94(5):661–74.
• Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: structure-activity relationships. Am J Phys. 2007;292:G290–7. This study describes the mechanism of colonic chloride and water secretion in the setting of BAM.
•• Sarathy J, Detloff SJ, Ao M, Khan N, French S, Sirajuddin H, et al. The Yin and Yang of bile acid action on tight junctions in a model colonic epithelium. Physiologic Rep. 2017;5(10):e13294. This paper discusses methods of increased water secretion into the colon via decrease in tight junction and increased paracellular movement and an increase in aquaporin channels. https://doi.org/10.14814/phy2.13294.
•• Yde J, Borg J, Fenton RA, Moeller HB. Altered expression of aquaporin water channels in a rat model of chronic diarrhea due to bile acid malabsorption. FASEB J. 2017;31(Suppl. 1):703. 14-14. This paper discusses methods of increased water secretion into the colon via decrease in tight junction and increased paracellular movement and an increase in aquaporin channels.
Ma T, Verkman AS. Aquaporin water channels in gastrointestinal physiology. J Physiol. 1999;517(Pt 2):317–26. https://doi.org/10.1111/j.1469-7793.1999.0317t.x.
Zimmerman TW, Binder HJ. Serotonin-induced alteration of colonic electrolyte transport in the rat. Gastroenterology. 1984;86(2):310–7.
Bardhan PK, Rahman AS, Islam S, Rahman M, Gyr K. Effects of tropisetron, a 5-hydroxytryptamine type 3 receptor blocker, on intestinal secretion induced by cholera toxin or deoxycholic acid in rabbits in vivo. J Intl Med Res. 1993;21(6):323–33. https://doi.org/10.1177/030006059302100603.
Camilleri M, Murphy R, Chadwick VS. Pharmacological inhibition of chenodeoxycholate-induced fluid and mucus secretion and mucosal injury in the rabbit colon. Dig Dis Sci. 1982;27(10):865–9. https://doi.org/10.1007/BF01316567.
Duboc H, Tolstanova G, Yuan PQ, Wu V, Kaji I, Biraud M, et al. Reduction of epithelial secretion in male rat distal colonic mucosa by bile acid receptor TGR5 agonist, INT-777: role of submucosal neurons. Neurogastroenterol Motil. 2016;28(11):1663–76. https://doi.org/10.1111/nmo.12866.
Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol Motil. 2013;25(8):708–11. https://doi.org/10.1111/nmo.12148.
• Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol. 2014;592(14):2943–50. This paper identifies that BAs target the TGR5 receptors which activate colonic motility and directly on the enteric neurons. https://doi.org/10.1113/jphysiol.2014.271155.
Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig Dis Sci. 1979;24(7):545–50. https://doi.org/10.1007/BF01489324.
Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144(1):145–54. https://doi.org/10.1053/j.gastro.2012.09.055.
Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Phys. 2002;282(3):G443–9.
Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139(5):1549–58, e1. https://doi.org/10.1053/j.gastro.2010.07.052.
Dior M, Delagrèverie H, Duboc H, Jouet P, Coffin B, Brot L, et al. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2016;28(9):1330–40. https://doi.org/10.1111/nmo.12829.
Ward JBJ, Lajczak NK, Kelly OB, O'Dwyer AM, Giddam AK, Ni Gabhann J, et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am J Phys. 2017;312:G550–8.
Biagioli M, Carino A, Cipriani S, Francisci D, Marchiano S, Scarpelli P, et al. The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. J Immunol. 2017;199(2):718–33. https://doi.org/10.4049/jimmunol.1700183.
Yoneno K, Hisamatsu T, Shimamura K, Kamada N, Ichikawa R, Kitazume MT, et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology. 2013;139(1):19–29. https://doi.org/10.1111/imm.12045.
Ji CG, Xie XL, Yin J, Qi W, Chen L, Bai Y, et al. Bile acid receptor TGR5 overexpression is associated with decreased intestinal mucosal injury and epithelial cell proliferation in obstructive jaundice. Transl Res. 2017;182:88–102. https://doi.org/10.1016/j.trsl.2016.12.001.
Svane MS, Bojsen-Moller KN, Madsbad S, Holst JJ. Updates in weight loss surgery and gastrointestinal peptides. Curr Opin Endocrinol Diab Obes. 2015;22(1):21–8. https://doi.org/10.1097/MED.0000000000000131.
Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281(8):397–402. https://doi.org/10.1056/NEJM196908212810801.
Akerlund JE, Reihner E, Angelin B, Rudling M, Ewerth S, Bjorkhem I, et al. Hepatic metabolism of cholesterol in Crohn’s disease. Effect of partial resection of ileum. Gastroenterology. 1991;100(4):1046–53. https://doi.org/10.1016/0016-5085(91)90281-O.
Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62(5):918–34.
Davie RJ, Hosie KB, Grobler SP, Newbury-Ecob RA, Keighley MRB, Birch NJ. Ileal bile acid malabsorption in colonic Crohn’s disease. Br J Surg. 1994;81(2):289–90. https://doi.org/10.1002/bjs.1800810246.
Jahnel J, Fickert P, Hauer AC, Hogenauer C, Avian A, Trauner M. Inflammatory bowel disease alters intestinal bile acid transporter expression. Drug Metab Dispos. 2014;42(9):1423–31. https://doi.org/10.1124/dmd.114.058065.
•• Jung D, Fantin AC, Scheurer U, Fried M, Kullak-Ublick GA. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 2004;53(1):78–84. This study details the unique finding of decreased transcription of the ASBT receptor in patients with Crohn’s disease, identifying a new pathway of BAM in patients without overt ileal disease. https://doi.org/10.1136/gut.53.1.78.
• Iwamoto J, Saito Y, Honda A, Miyazaki T, Ikegami T, Matsuzaki Y. Bile acid malabsorption deactivates pregnane X receptor in patients with Crohn’s disease. Inflamm Bowel Dis. 2013;19(6):1278–84. PXR is important in regulating hepatic BA and intestinal inflammation. In Crohn’s disease patients who have BAM, PXR may be low and can result in diarrhea with mucosal inflammation without evidence of active Crohn’s disease. https://doi.org/10.1097/MIB.0b013e318281f423.
Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, et al. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2006;130(2):341–8; quiz 592. https://doi.org/10.1053/j.gastro.2005.12.008.
Prantera C, Lochs H, Campieri M, Scribano ML, Sturniolo GC, Castiglione F, et al. Antibiotic treatment of Crohn’s disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther. 2006;23(8):1117–25. https://doi.org/10.1111/j.1365-2036.2006.02879.x.
Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, et al. Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther. 2010;335(1):32–41. https://doi.org/10.1124/jpet.110.170225.
Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014;5(1):15–29. https://doi.org/10.1177/2040622313510730.
White KL, Henson CC, Jenner K, Burden S, Lal S, Davidson SE, et al. PTH-247 Modern pelvic chemoradiotherapy techniques continue to cause bile acid malabsorption. Gut. 2015;64(Suppl 1):A519.
Phillips F, Muls A, Lalji A, Andreyev H. Are bile acid malabsorption and bile acid diarrhoea important causes of loose stool complicating cancer therapy? Colorect Dis. 2015;17(8):730–4. https://doi.org/10.1111/codi.12932.
Richardson PG, Blood E, Mitsiades CS, Jagannath S, Zeldenrust SR, Alsina M, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108(10):3458–64. https://doi.org/10.1182/blood-2006-04-015909.
Pawlyn C, Khan MS, Muls A, Sriskandarajah P, Kaiser MF, Davies FE, et al. Lenalidomide-induced diarrhea in patients with myeloma is caused by bile acid malabsorption that responds to treatment. Blood. 2014;124(15):2467–8. https://doi.org/10.1182/blood-2014-06-583302.
Farahmandfar MR, Chabok M, Alade M, Bouhelal A, Patel B. Post cholecystectomy diarrhoea: a systematic review. Surg Sci. 2012;3:7.
• Yueh T-P, Chen F-Y, Lin T-E, Chuang M-T. Diarrhea after laparoscopic cholecystectomy: associated factors and predictors. Asian J Surg. 2014;37(4):171–7. Rates of BAM in post-cholecystecomy patients are lower than previously expected with rates decreasing as patient’s get further from their surgical date. https://doi.org/10.1016/j.asjsur.2014.01.008.
Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7(11):1189–94. https://doi.org/10.1016/j.cgh.2009.04.024.
Johnston IM, Nolan JD, Pattni SS, Appleby RN, Zhang JH, Kennie SL, et al. Characterizing factors associated with differences in FGF19 blood levels and synthesis in patients with primary bile acid diarrhea. Am J Gastroenterol. 2016;111(3):423–32. https://doi.org/10.1038/ajg.2015.424.
Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, et al. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. Am J Phys. 2014;307:G508–16.
Camilleri M, Klee EW, Shin A, Carlson P, Li Y, Grover M, et al. Irritable bowel syndrome-diarrhea: characterization of genotype by exome sequencing, and phenotypes of bile acid synthesis and colonic transit. Am J Phys. 2014;306:G13–26.
Einarsson K, Eusufzai S, Johansson U, Lofberg R, Theodorsson E, Veress B. Villous atrophy of distal ileum and lymphocytic colitis in a woman with bile acid malabsorption. Eur J Gastroenterol Hepatol. 1992;4:585–90.
Marteau P, Lavergne-Slove A, Lemann M, Bouhnik Y, Bertheau P, Becheur H, et al. Primary ileal villous atrophy is often associated with microscopic colitis. Gut. 1997;41(4):561–4. https://doi.org/10.1136/gut.41.4.561.
Fernandez-Banares F, Esteve M, Salas A, Forne TM, Espinos JC, Martin-Comin J, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci. 2001;46(10):2231–8. https://doi.org/10.1023/A:1011927302076.
Giardiello FM, Bayless TM, Jessurun J, Hamilton SR, Yardley JH. Collagenous colitis: physiologic and histopathologic studies in seven patients. Ann Intern Med. 1987;106(1):46–9. https://doi.org/10.7326/0003-4819-106-1-46.
Kingham JG, Levison DA, Ball JA, Dawson AM. Microscopic colitis-a cause of chronic watery diarrhoea. BMJ. 1982;285(6355):1601–4. https://doi.org/10.1136/bmj.285.6355.1601.
Eusufzai S, Löfberg R, Veress B, Einarsson K, Angelin B. Studies on bile acid metabolism in colagenous colitis: no evidence of bile acid malabsorption as determined by the SeHCAT test. Eur J Gastroenterol Hepatol. 1992;4:317–21.
Ung K, Gillberg R, Kilander A, Abrahamsson H. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut. 2000;46(2):170–5. https://doi.org/10.1136/gut.46.2.170.
Galatola G, Jazrawi RP, Bridges C, Joseph AE, Northfield TC. Direct measurement of first-pass ileal clearance of a bile acid in humans. Gastroenterology. 1991;100(4):1100–5. https://doi.org/10.1016/0016-5085(91)90288-V.
Brunner H, Northfield T, Hofmann A, Go V, Summerskill WH. Gastric emptying and secretion of bile acids, cholesterol, and pancreatic enzymes during digestion: duodenal perfusion studies in healthy subjects. Mayo Clin Proc. 1974;49(11):851–60.
Peters AM, Walters JR. Recycling rate of bile acids in the enterohepatic recirculation as a major determinant of whole body 75SeHCAT retention. Eur J Nucl Med Molecul Imag. 2013;40(10):1618–21. https://doi.org/10.1007/s00259-013-2466-z.
Scheurlen C, Kruis W, Bull U, Stellaard F, Lang P, Paumgartner G. Comparison of 75SeHCAT retention half-life and fecal content of individual bile acids in patients with chronic diarrheal disorders. Digestion. 1986;35(2):102–8. https://doi.org/10.1159/000199353.
Sciarretta G, Fagioli G, Furno A, Vicini G, Cecchetti L, Grigolo B, et al. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut. 1987;28(8):970–5. https://doi.org/10.1136/gut.28.8.970.
•• Peleman C, Camilleri M, Busciglio I, Burton D, Donato L, Zinsmeister AR. Colonic transit and bile acid synthesis or excretion in patients with irritable bowel syndrome–diarrhea without bile acid malabsorption. Clin Gastroenterol Hepatol. 2017;15(5):720–7, e1. This paper describes that the increased presence of primary bile acids may be sufficient to increase colonic transit. This may support diagnosis of BAM in patients with total fecal BA < 2,337μmol/48h but have a high proportion of primary fecal BAs or secretory BAs. https://doi.org/10.1016/j.cgh.2016.11.012.
Sauter GH, Munzing W, Ritter CV, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea diagnostic value of 7α-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44(1):14–9. https://doi.org/10.1023/A:1026681512303.
Donato LJ, Lueke A, Kenyon SM, Meeusen JW, Camilleri M. Description of analytical method and clinical utility of measuring serum 7-alpha-hydroxy-4-cholesten-3-one (7aC4) by mass spectrometry. Clin Biochem. 2017; https://doi.org/10.1016/j.clinbiochem.2017.10.008.
Vijayvargiya P, Camilleri M, Carlson P, Lueke A, O'Neill J, Burton D, et al. Performance characteristics of serum C4 and FGF19 measurements to exclude the diagnosis of bile acid diarrhoea in IBS-diarrhoea and functional diarrhoea. Aliment Pharmacol Ther. 2017;46(6):581–8. https://doi.org/10.1111/apt.14214.
Gothe F, Beigel F, Rust C, Hajji M, Koletzko S, Freudenberg F. Bile acid malabsorption assessed by 7 alpha-hydroxy-4-cholesten-3-one in pediatric inflammatory bowel disease: correlation to clinical and laboratory findings. J Crohn's & Colitis. 2014;8(9):1072–8. https://doi.org/10.1016/j.crohns.2014.02.027.
Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, Dixon PH, et al. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Phys. 2013;304:G940–8.
Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol. 2017; https://doi.org/10.1016/j.cgh.2017.06.039.
Borup C, Syversen C, Bouchelouche P, Damgaard M, Graff J, Rumessen JJ, et al. Diagnosis of bile acid diarrhoea by fasting and postprandial measurements of fibroblast growth factor 19. Eur J Gastroenterol Hepatol. 2015;27(12):1399–402. https://doi.org/10.1097/MEG.0000000000000476.
•• Borup C, Wildt S, Rumessen JJ, Bouchelouche PN, Graff J, Damgaard M, et al. Chenodeoxycholic acid stimulated fibroblast growth factor 19 response - a potential biochemical test for bile acid diarrhoea. Aliment Pharmacol Ther. 2017;45(11):1433–42. This study demonstrated that stimulated FGF19 may be a better marker of diagnosing BAM. Patients with BAM will not have a significant increase in FGF19 post meal + CDCA as compared to healthy controls. https://doi.org/10.1111/apt.14056.
Jackson A, Lalji A, Kabir M, Muls A, Gee C, Vyoral S, et al. PTU-128 The efficacy of using low-fat dietary interventions to manage bile acid malabsorption. Gut. 2017;66(Suppl.2):A114.
Beigel F, Teich N, Howaldt S, Lammert F, Maul J, Breiteneicher S, et al. Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn’s disease: a randomized, double-blind, placebo-controlled study. J Crohn's & Colitis. 2014;8(11):1471–9. https://doi.org/10.1016/j.crohns.2014.05.009.
Fernandez-Banares F, Rosinach M, Piqueras M, Ruiz-Cerulla A, Modolell I, Zabana Y, et al. Randomised clinical trial: colestyramine vs. hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment Pharmacol Ther. 2015;41(11):1132–40. https://doi.org/10.1111/apt.13193.
Brydon G, Ganguly R, Ghosh S. The effect of hydroxypropylcellulose on bile acid induced watery diarrhoea. Gut. 2003;52(Suppl. 1):A9.
Camilleri M, Acosta A, Busciglio I, Boldingh A, Dyer RB, Zinsmeister AR, et al. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41(5):438–48. https://doi.org/10.1111/apt.13065.
Orekoya O, McLaughlin J, Leitao E, Johns W, Lal S, Paine P. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med. 2015;15(3):252–7. https://doi.org/10.7861/clinmedicine.15-3-252.
Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Medicinal Chem. 2004;47(18):4559–69. https://doi.org/10.1021/jm049904b.
Mroz MS, Keating N, Ward JB, Sarker R, Amu S, Aviello G, et al. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut. 2014;63(5):808–17. https://doi.org/10.1136/gutjnl-2013-305088.
Walters J, Johnston I, Nolan J, Vassie C, Pruzanski M, Shapiro D. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41(1):54–64. https://doi.org/10.1111/apt.12999.
Baghdasaryan A, Fuchs CD, Österreicher CH, Lemberger UJ, Halilbasic E, Påhlman I, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol. 2016;64(3):674–81. https://doi.org/10.1016/j.jhep.2015.10.024.
Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. 2014;12(1):164. https://doi.org/10.1186/1477-7819-12-164.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michael Camilleri and Priya Vijayvargiya declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Large Intestine
Rights and permissions
About this article
Cite this article
Vijayvargiya, P., Camilleri, M. Update on Bile Acid Malabsorption: Finally Ready for Prime Time?. Curr Gastroenterol Rep 20, 10 (2018). https://doi.org/10.1007/s11894-018-0615-z
Published:
DOI: https://doi.org/10.1007/s11894-018-0615-z