Abstract
Purpose of Review
Proton pump inhibitor (PPI) use in gastroesophageal reflux disease (GERD) has been redefined, in light of recent advances highlighting GERD phenotypes that respond to PPIs, and fresh revelations of potential risks of long-term PPI therapy.
Recent Findings
Erosive esophagitis predicts excellent response to PPI therapy, but non-erosive reflux disease (NERD) with abnormal reflux parameters on ambulatory reflux monitoring also demonstrates a similar response. In contrast, response is suboptimal in the absence of abnormal reflux parameters. In this setting, if an alternate appropriate indication for PPI therapy does not coexist, risks may outweigh benefits of PPI therapy. Adverse events from long-term PPI therapy continue to be reported, most based on association rather than cause-and-effect.
Summary
Appropriate indications need to be established before embarking on long-term PPI therapy. Future research will define true risks of long-term PPI therapy, and develop alternate management options for acid peptic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proton pump inhibitors (PPIs) were first introduced into clinical use in the 1980s, and have remained the mainstay for acid suppression ever since [1]. Omeprazole was the first agent discovered in 1979, and introduced into the market in 1989. Since then, several additional PPIs have been introduced, including lansoprazole, rabeprazole, and pantoprazole. Two of the original PPIs have been reintroduced with altered chemical formulation: esomeprazole and dexlansoprazole. Omeprazole has been marketed in combination with sodium bicarbonate, thought to increase bioavailability. However, at their core, PPIs suppress gastric acid secretion, and a meta-analysis of studies evaluating various formulations of PPIs found that there is negligible difference in efficacy between PPIs in the anticipated therapeutic effect [2]. Regardless of formulation, PPIs work by irreversible blockade of activated H+ K+ adenosine triphosphatase proton pump in the gastric parietal cells, which is the mechanism for secretion of hydrochloric acid into the gastric lumen. PPIs are administered orally and need to be absorbed from the small intestine. The active ingredient is carried by the blood stream to the gastric parietal cells. The acid secretory effect is not immediate as the medication needs to concentrate in the acidic secretory canaliculi in the parietal cell before inhibiting the proton pump [1]. Acid production is suppressed until new proton pumps regenerate, and the medication is readministered on a daily basis to ensure continued acid suppression. For optimal efficacy, PPIs need to be dosed before meals, typically 30–60 min prior to the first meal of the day with once daily usage, and the second dose similarly administered before dinner with twice daily usage. There is no clear benefit to additional dosing beyond twice daily.
Over the past three decades, it has become clear that PPIs are extremely effective acid suppressants, and consequently, resolve symptoms as well as improve clinical outcome in acid peptic disorders. In the late 1990s, the use of short-term PPI therapy was explored as a diagnostic test for gastroesophageal reflux disease (GERD) in patients presenting with typical GERD symptoms [3, 4]. The sensitivity of symptom improvement from a 7-day trial of omeprazole in diagnosing GERD was reported to be approximately 80% against a gold standard of erosive esophagitis on endoscopy, or a positive ambulatory pH monitoring study. Despite a modest specificity of 54% [5], an empiric PPI trial is now considered a pragmatic and cost-effective initial step in diagnosing and managing GERD in patients without alarm symptoms. This has led to widespread use of PPIs, often for symptoms that may not be acid peptic in origin. The availability of PPIs over the counter without prescription has further escalated use of these agents, in many instances without instruction from a medical professional, and often in the absence of established indications [6]. Initiation of PPIs by medical personnel involved in cross-sectional care of emergency room or hospitalized patients often leads to continuing chronic use in symptomatic settings where a clear indication is not documented [7]. This widespread use has led to concerns regarding detrimental consequences from chronic use of these agents, based on large association studies, often without clear cause-and-effect relationships [8••].
In this context, this review seeks to define appropriate indications for PPI use in GERD, and describe steps that can be taken when an appropriate indication is not well established. Further, the available data behind current concerns over PPI side effects will be analyzed, and a practical approach to appropriate long-term PPI use will be discussed.
Appropriate Indications for PPI Therapy
Gastroesophageal Reflux Disease
Although gastroesophageal reflux disease (GERD) results from motor or structural disruption of the esophagogastric junction (EGJ) barrier, medical management typically does not target GERD pathophysiology. Instead, acid suppression is utilized to reduce noxious acidic refluxate, thereby improving symptoms and healing eroded mucosa in the distal esophagus [9]. As a consequence, acidic reflux episodes are converted to weakly acidic or non-acidic reflux [10], which tends not to be irritant to the esophageal mucosa in the vast majority of settings. Suppression of gastric acid production therefore allows resolution of reflux symptoms (especially heartburn), and healing of erosive esophagitis.
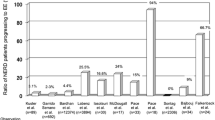
An empiric trial of PPI therapy is considered the initial approach to typical GERD symptoms (heartburn, acid regurgitation), utilized in lieu of diagnostic testing in patients without alarm symptoms [11•, 12•] despite suboptimal specificity of a PPI trial in GERD diagnosis [5]. An 8-week trial of a PPI results in symptom improvement in as many as 70% of uninvestigated heartburn in meta-analysis, with a number needed to treat (NNT) of 2.2 for symptom improvement [13••]. Healing of endoscopic esophagitis is noted in 86%, with NNT of 1.8 [14]. With erosive esophagitis, complete relief of typical reflux symptoms with PPI therapy is reported in 72% (95% confidence intervals 68–74%) [15••]. In the absence of erosive esophagitis on endoscopy (non-erosive reflux disease, NERD), similar results are achieved if the diagnosis of NERD is based on abnormal ambulatory reflux monitoring (73.5% complete symptom relief, 95% CI 69.1–77.6%) [15••]. In contrast, the likelihood of symptom resolution is only 50.5% (95% CI 43.5–57.5%) if NERD diagnosis is based on heartburn and normal endoscopy, without independent documentation of abnormal esophageal acid exposure [15••]. This implies that non-GERD mechanisms, particularly functional esophageal disorders (functional heartburn, reflux hypersensitivity) contribute to perception and presentation of heartburn [16••], and symptoms identical to NERD can be reported with functional heartburn despite lack of acid triggering of symptoms [17]. In recognition of these factors, the modern diagnosis of NERD requires documentation of abnormal esophageal acid exposure in addition to absence of erosive esophagitis and other mucosal processes (especially eosinophilic esophagitis) on endoscopy [16••]. PPIs are also prescribed in patients with confirmed intestinal metaplasia on histopathology (Barrett’s esophagus), particularly long-segment Barrett’s esophagus, which is a reliable predictor of abnormal esophageal acid burden [18, 19••]. Peptic strictures in the distal esophagus are another indicator of pathologic GERD, and are managed with long-term PPI therapy in addition to endoscopic dilation [19••, 20••].
Acid suppression is much less effective in resolving regurgitation-predominant GERD, and atypical GERD symptoms (non-cardiac chest pain, pulmonary symptoms, laryngeal symptoms) [21,22,23,24]. Regurgitation as a GERD symptom responds to PPI therapy in only 26–44%, with only a 17% therapeutic gain over placebo [21, 25]. Similarly, improvement of chronic cough is noted in <25% with PPI therapy and resolution of cough is rare [22, 26]. Response of laryngeal symptoms in the absence of heartburn is similar to that seen with placebo [24]. Asthmatics may improve their nocturnal symptoms and pulmonary function parameters in settings where asthma coexists with heartburn, erosive esophagitis or Barrett’s esophagus; improvement is not consistently seen in the absence of these select circumstances [27]. Non-cardiac chest pain is the only atypical GERD setting where PPI response can be substantial. If abnormal reflux parameters can be demonstrated on objective testing, PPI response can be seen in as many as 56–85%, in contrast to 0–17% when such evidence is absent [23]. This probably reflects the fact that patients interpret their retrosternal sensations as either heartburn or chest pain, and heartburn may be reported as chest pain or vice versa. Consequently, use of a PPI trial has a sensitivity of 84% and specificity of 74% in predicting a reflux etiology when cardiac etiologies have been excluded [28, 29], similar to that initially reported with heartburn.
Beyond the initial 8 weeks, PPIs need to be maintained over the long term in patients with well characterized GERD to avoid recurrence of symptoms or esophagitis [30•, 31]. Maintenance of healing of esophagitis is seen in 93% with the use of continuous PPI therapy for 6 months, in contrast to 29% with placebo [32]. Compared to the full PPI dose typically used to initiate healing, a lower PPI dose can suffice for maintenance of healing. However, use of alternate day dosing or intermittent PPI use can be associated with recurrence of symptoms and dissatisfaction with therapy [30•], which can be attributed to higher esophageal acid exposure on days when the PPI is not taken in patients with proven GERD [31]. On demand therapy, therefore, is best reserved for patients with infrequent symptoms, in the absence of esophageal mucosal manifestations such as erosive esophagitis or Barrett’s esophagus. Acid suppression can sometimes be stepped down to histamine-2 receptor antagonists (H2RA) [33], an approach that can be successful in a third of patients with symptomatic GERD without erosive esophagitis or GERD complications; however, therapy will need to be reverted back to PPIs if symptoms recur on H2RA therapy.
The most appropriate indications for PPI therapy in GERD, therefore, consist of erosive esophagitis, Barrett’s esophagus or peptic strictures on endoscopy, and evidence of abnormal esophageal acid exposure on ambulatory reflux monitoring [19••, 34•, 35]. These circumstances are termed “proven GERD”, and represent clinical settings where there is strong evidence for improved symptomatic and disease outcomes with PPI therapy compared to placebo or lesser degrees of acid suppression (e.g., H2RA). All other clinical settings are termed “unproven GERD”, where invasive investigation (endoscopy, ambulatory reflux monitoring, esophageal manometry) can determine if the presence of GERD can be proven [19••], or if an alternate mechanism for symptoms can be determined [36].
The designations of proven and unproven GERD are important when esophageal symptoms fail to respond to seemingly adequate PPI therapy (Fig. 1). In both proven and unproven GERD, endoscopy with biopsy could uncover non-GERD mucosal processes (e.g., eosinophilic esophagitis, infectious esophagitis, lichen planus, pill esophagitis) that could be contributing to symptoms [37•, 38, 39•]. When GERD is previously unproven, ambulatory reflux monitoring is performed off acid suppression [16••, 19••], where findings either document pathologic GERD (abnormal reflux parameters), refute GERD (physiological reflux parameters) or are borderline for GERD. When borderline parameters are encountered, alternate investigative evidence (distal esophageal biopsies showing reflux related findings, disruption of the esophagogastric junction on manometry, endoscopy or barium studies) or novel reflux parameters on pH-impedance testing (mean nocturnal baseline impedance, post-reflux swallow-induced peristaltic wave index, numbers of reflux episodes) may support evidence for reflux disease [19••, 40•]. With physiological reflux parameters, motor disorders (especially achalasia and major motor disorders), and functional disorders (functional heartburn, reflux hypersensitivity, supragastric belching, rumination syndrome) need to be considered as mechanisms for symptoms [16••, 36]. Functional esophageal disorders can coexist with proven GERD. Therefore, when symptoms persist while on maximal acid suppression in proven GERD, ambulatory pH-impedance monitoring is performed on PPI therapy, to determine if reflux metrics are abnormal despite PPI therapy, or if overlapping functional syndromes coexist and participate in symptom generation [16••, 19••]. An organized approach to investigation of persisting symptoms, therefore, can determine if reflux disease exists at all, if reflux disease is truly refractory to PPI therapy, or if alternate processes coexist with reflux disease.
A scheme for appropriate use of acid suppressive therapy in GERD. Empiric PPI therapy is appropriate in the absence of alarm symptoms, but documentation of reflux disease is worthwhile prior to long term PPI therapy. Persisting symptoms despite PPI therapy prompts further evaluation with esophageal function tests (endoscopy, esophageal manometry, ambulatory reflux monitoring). Unproven GERD is evaluated with ambulatory pH or pH-impedance monitoring off PPI, while PPIs are continued in proven GERD, and the test employed is pH-impedance monitoring. PPIs can be utilized as the mainstay of management where there is documentation of GERD evidence, when benefits outweigh risk. If there is no GERD evidence on testing in unproven GERD, alternative diagnoses are pursued. In proven GERD, PPIs are continued despite negative GERD evidence, but overlapping diagnoses are considered as a mechanism for symptoms
In summary, within the realm of proven or suspected GERD, PPI therapy has immense value when gastric acid is documented to be the instigator of esophageal symptoms or esophageal mucosal damage (Table 1). True refractoriness to PPI therapy does exist, but is rare. Persisting esophageal symptoms are often termed “refractory” to PPI therapy when in fact non-GERD mechanisms may be driving symptoms, and esophageal function testing can be useful in making this determination [19••]. If GERD cannot be documented, the need for ongoing or chronic PPI therapy depends on whether an alternate appropriate indication exists for PPI therapy. If there is no appropriate indication for PPI therapy, the risk:benefit ratio does not favor continuation of PPI, and the medication should be weaned off and discontinued.
Other Appropriate Indications for PPI Use
Peptic Ulcer Disease
Acid suppressive therapy was first introduced for the management of peptic ulcer disease. Acid suppression targets the two most common causes of peptic ulcer disease (PUD), Helicobacter pylori and nonsteroidal anti-inflammatory drugs (NSAIDs) [1]. Soon after recognition of an infectious etiology for most PUD in the form of H. pylori infection, it became evident that successful eradication of this organism led to accelerated healing of ulcers, and more importantly, a significant reduction in recurrence rates of PUD [41]. By suppressing gastric acid, PPIs allow H. pylori to get into a growth phase where the organism is most susceptible to antibiotics; further, PPIs are directly bacteriostatic against H. pylori [42]. Therefore, PPIs remain an integral part of H. pylori eradication regimens [43]. Beyond H. pylori eradication, acid suppression with a PPI heals PUD, and prevents ulcers and ulcer complications in the setting of chronic aspirin or NSAID use [44]. By extension of this indication, chronic PPI therapy is also employed in high-risk patients on antiplatelet therapy, or anticoagulants combined with antiplatelet agents. In hypersecretory states associated with uncontrolled secretion of gastrin (Zollinger-Ellison syndrome), high doses of PPIs are used at diagnosis, and indefinitely if a curative resection cannot be performed [45].
Intravenous PPI administration has now become the mainstay of management of acute peptic ulcer bleeding [46, 47•]. This is introduced as either IV bolus or infusion at presentation, and particularly after endoscopic intervention for bleeding ulcers, where this has been demonstrated to reduce rebleeding, need for surgery and mortality from bleeding PUD [47•]. IV administration is typically continued for 72 h, following which the medication is transitioned to the oral route.
Eosinophilic Esophagitis
Within the eosinophilic esophagitis (EoE) spectrum, a PPI responsive phenotype (PPI responsive eosinophilic esophagitis, PPI-REE) exists where symptom improvement and mucosal healing can be expected with chronic PPI use [39•]. On meta-analysis, as many as 61% report clinical improvement of EoE symptoms, and half have histologic healing [48•]; this is not directly associated with abnormal esophageal acid burden on ambulatory pH monitoring. While it is not completely known why EoE patients benefit from PPI use, the high response rate warrants an initial PPI course as the first line management of EoE [39•, 49]. Reduction in mucosal eosinophil counts with PPI is now considered a diagnostic test for segregation of PPI-REE from EoE requiring steroid or dietary therapy [49]. Chronic PPI use is generally indicated for PPI-REE, and potentially as an adjunct even when eosinophil counts do not return to baseline with PPI therapy alone.
Stress Ulcer Syndrome
Mucosal erosive disease is often encountered in the stomach and duodenum in critically ill patients admitted to intensive care units (ICU), particularly in the setting of mechanical ventilation, coagulopathy, stroke, multiple organ failure, and burns [50]. Improved ICU care has significantly reduced the likelihood of stress ulcer bleeding, estimated at 1% in the present day [50]. H2RAs are considered the agents of choice for prophylaxis of stress ulcer bleeding in patients with risk factors [20••, 51, 52]. However, in the setting of stress ulcer bleeding, PPI therapy is often employed, and has been demonstrated to be of value in this setting [53]. Unfortunately, many patients admitted to ICUs are prescribed PPIs in the absence of risk factors, and the medications are maintained at discharge without a clear ongoing indication [54]. Therefore, when PPIs are employed for stress ulcer syndrome while hospitalized, reevaluation of need for continuation is determined at hospital discharge.
Symptomatic Foregut Syndromes
PPIs are often prescribed in uninvestigated dyspepsia, but predictability of an acid peptic process with symptom relief is neither sensitive nor specific (54, 65%, respectively) [55]. This is because there is significant overlap with functional dyspepsia, where symptom resolution is not likely with PPI use alone [56••]. Symptom benefit is most likely when there is a component of GERD, or NSAID associated dyspepsia, both of which can be PPI responsive. In contrast, functional heartburn (normal ambulatory pH or pH-impedance monitoring off PPI), and reflux hypersensitivity (physiologic acid burden with positive symptom-reflux association on ambulatory pH or pH-impedance monitoring) do not respond adequately to acid suppression [16••]. In settings where H. pylori prevalence is high, a “test and treat” approach is often utilized, followed by PPI therapy if symptoms persist despite eradication [57], or if the organism is not found—in these settings, symptom resolution is noted in 34% with NNT of 10 [58]. When symptoms improve, the medications can be tapered off over time, with long-term therapy utilized only in patients with prompt symptomatic recurrence off PPI [59].
Other miscellaneous indications for PPI therapy include patients with persistent steatorrhea from chronic pancreatitis on pancreatic enzyme replacement therapy where acid suppression prevents gastric acid degradation of pancreatic enzymes, foregut mucositis from chemotherapy or radiation, ulcerations from therapy of esophageal varices (band ligation or sclerotherapy), and medication-induced dyspepsia that responds to PPI therapy (Table 1) [20••].
Adverse Effects of PPI Therapy
PPIs have been clinically available for almost three decades and are considered safe in the setting of appropriate use. PPI intolerance is noted in 1–3% of the population, typically in the form of headache, abdominal pain, diarrhea, flatulence, dyspepsia, and rarely, rash and allergy [20••]. In recent years, there has been increasing concern regarding long-term PPI use (Table 2), mainly based on large association studies evaluating prescription databases, and suggesting caution in long-term users [8••, 20••, 60••, 61•]. Using data from association studies, often with relatively low-risk estimates (odds ratios, ORs) for the adverse effect in question, is problematic, as low ORs are subject to potential confounding and bias, both unintended and undetected bias [62]. These low OR associations therefore do not necessarily indicate causality. It is believed that the ORs in association studies need to be >3.0 for the association to be considered clinically relevant [60••, 63]. There are very limited studies specifically designed to evaluate the effect of acid suppression on gut function and overall health, which makes evaluation of the available literature difficult and cumbersome [62]. Because of widespread transmission of available information in nonmedical press, there is confusion, leading to panic among patients, concern among treating physicians, and potential discontinuation of appropriate PPI use.
Idiosyncratic Reactions
Rare idiosyncratic effects on the kidneys can lead to acute interstitial nephritis (AIN) and acute kidney injury [64, 65]; recurrent AIN can potentially result in chronic kidney disease [66,67,68]. A rare form of profound hypomagnesemia has been reported with chronic PPI use, which does not resolve without discontinuation of PPI [69,70,71]. Microscopic colitis has been reported, particularly with lansoprazole, with resolution upon discontinuation of the agent [72, 73].
Infections
Reduction in gastric acidity is thought to reduce the destruction of vegetative forms of Clostridium difficile, and increase likelihood of C difficile colitis [74,75,76]. Other enteric infections (salmonella, campylobacter) are also reported, with OR for enteric infections of 3.3 (Table 2) [74, 77, 78]. From a similar mechanism, small bowel bacterial overgrowth [79], and spontaneous bacterial peritonitis (in cirrhotics) [80] have been reported in the setting of long-term PPI use. While community-acquired pneumonia has been suggested, robust clinical data supporting this is scant [81,82,83].
Nutrient Deficiencies
Acid suppression has been suggested to cause calcium and iron malabsorption, although clinical deficiency is rare [84, 85]. Calcium malabsorption with achlorhydria can be reversed if calcium is administered in an acidic milieu, e.g., calcium citrate or an acidic meal. Numeric reduction in vitamin B12 levels have been reported, with rare instances of vitamin B12 deficiency [86].
Bone Fracture
PPIs have been linked to increased bone fracture risk based on association or observational studies with very low ORs (pooled OR 1.25, 95% CI 1.14–1.37 in meta-analysis) [87, 88], thought to be based on calcium or vitamin B12 malabsorption, and a direct action of PPIs on osteoclasts [84]. However, cohort studies have not demonstrated reduction in bone mineral density, and therefore, routine monitoring is not recommended [8••].
Dementia
PPIs have been reported to block enzymes in microglial cells that break down amyloid-β, build-up of which is seen in Alzheimer’s dementia. Association studies report higher risk of dementia in PPI users [89, 90], but baseline differences between study cohorts, particularly in rates of depression, stroke and polypharmacy in PPI users, could explain the findings. A more recent report did not demonstrate a higher likelihood of dementia in PPI users [91].
Drug Interactions
PPIs were once thought to decrease the antiplatelet effect of clopidogrel by competing with the cytochrome P450 enzyme system that metabolizes PPIs, but activates clopidogrel. While this effect was plausible in vitro, the COGENT study, a large randomized controlled trial, demonstrated similar cardiac events between patients on just clopidogrel (5.7%) and both omeprazole and clopidogrel (4.9%), suggesting that PPIs in the setting of clopidogrel use do not increase clinical risk for myocardial infarction [92]. Clinically relevant interactions from induction of the cytochrome P450 system are noted with medications that have a narrow therapeutic window, such as diazepam, warfarin, phenytoin, and methotrexate [20••]. Absorption of levothyroxine, ketoconazole, atazanavir, defpodoxime, enoxacin, and dipyridamole may be decreased, while nifedipine, alendronate, and digoxin levels may increase with concomitant PPI therapy [93].
Miscellaneous
PPI use is associated with parietal cell hyperplasia and fundic gland polyps, which are benign findings [94, 95]. Although neuroendocrine tumors have been noted from hypergastrinemia related to acid suppression in mice, a similar effect has not been reported in humans [96]. While vasoconstriction from blockade of nitric oxide synthase has been suggested as a mechanism for myocardial infarction [97], high quality data is not available to support this claim [97].
PPI use in the Modern era
Extensive availability of PPIs over the counter without a prescription, and record high numbers of prescriptions for PPIs has led to inappropriate use of PPIs worldwide [6, 7]. In the present day, it is important to define what symptom or syndrome is being treated with the PPI before relegating a patient to chronic PPI use. There are reflux-related disorders where long-term PPI will improve symptoms and health-related quality of life; PPIs reduce life threatening complications like gastrointestinal bleeding in patients on chronic NSAIDs, antiplatelet drugs and anticoagulation. Many of the inappropriate indications consist of gastro-protection when this is not truly needed [98], or functional disease where acid suppression is not expected to help anyway [16••, 99••]. Further, some prescriptions are continued following short-term indications such as stress ulcer prophylaxis and limited peptic ulcer disease [54].
Therefore, appropriate use of PPIs boils down to risk vs. benefit. If there is a symptomatic benefit or a disease state that is controlled with PPI use, some degree of medication-related adverse events may need to be tolerated. However, it will be important to remain within the bounds of appropriate usage, and the lowest dose necessary to relieve symptoms should be utilized. For instance, double dose regimens have not been demonstrated to improve symptoms, and there may be a plateau effect beyond which additional dosing may not be of value. Lifestyle measures (weight loss and sleeping with the head end of the bed elevated with GERD, cessation of smoking with peptic disease,) may provide adjunctive benefit to PPI therapy, and could allow reduction in the dose of the medication [100]. When it is not completely clear as to what syndrome is being treated, objective testing may be necessary [19••]. For instance, ambulatory pH or pH-impedance monitoring off PPI can define if there is increased esophageal reflux burden, and endoscopy with biopsy can diagnose EoE, both conditions would benefit from chronic PPI therapy (Fig. 1). A trial of weaning down or stopping the PPI can sometimes define if the PPI is truly necessary in nonspecific symptomatic states, although rebound acid hypersecretion can cloud this assessment in the first few days after withdrawal of the PPI.
When symptoms do not improve despite appropriately administered PPI therapy, two possibilities need to be considered: truly refractory states where alternate management options need to be explored, and conditions that do not have an acid peptic basis, which would not be expected to improve with acid suppressive therapy. In the former, surgical or invasive options may need to be considered, while in the latter, management will need to move away from acid suppression. When there is partial benefit from PPI therapy, complementary management approaches may need to be combined with PPI therapy.
Conclusions
PPIs, therefore, can be a friend in appropriately diagnosed GERD and acid peptic disorders, and provide profound benefit in terms of symptom improvement and healing of erosive disease in these situations. However, when used for inappropriate indications, risks can outweigh benefits, and PPIs can be considered a foe. Further research is necessary to define which long-term PPI risks are based on true cause-and-effect relationships, and how these risks can be mitigated when appropriate PPI use needs to continue. Alternate treatment options for GERD and acid peptic disease, both pharmacologic and non-pharmacologic, continue to be studied. Careful consideration of the diagnosis before prescribing long-term PPI therapy can go a long way in improving appropriateness of PPI therapy.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance ••Of major importance
Wolfe MM, Sachs G. Acid suppression: optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology. 2000;118:S9–31.
Gralnek IM, Dulai GS, Fennerty MB, et al. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol. 2006;4:1452–8.
Fass R, Ofman JJ, Gralnek IM, et al. Clinical and economic assessment of the omeprazole test in patients with symptoms suggestive of gastroesophageal reflux disease. Arch Intern Med. 1999;159:2161–8.
Fass R, Ofman JJ, Sampliner RE, et al. The omeprazole test is as sensitive as 24-h oesophageal pH monitoring in diagnosing gastro-oesophageal reflux disease in symptomatic patients with erosive oesophagitis. Aliment Pharmacol Ther. 2000;14:389–96.
Numans ME, Lau J, de Wit NJ, et al. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med. 2004;140:518–27.
Sheikh I, Waghray A, Waghray N, et al. Consumer use of over-the-counter proton pump inhibitors in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2014;109:789–94.
Lanas A. We are using too many PPIs, and we need to stop: a European perspective. Am J Gastroenterol. 2016;111:1085–6.
•• Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706–15. An excellent description of evidence and expert opinion-based best practice recommendations for appropriate long-term use of proton pump inhibitors.
Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383–1391, 1391 e1-5.
Vela MF, Camacho-Lobato L, Srinivasan R, et al. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120:1599–606.
• Shaheen NJ, Weinberg DS, Denberg TD, et al. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808–16. Clinical guidelines for appropriate use of upper endoscopy in gastroesophageal reflux disease.
• Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–28; quiz 329. Detailed description of indications for diagnostic testing, description of esophageal physiologic tests, and use of diagnostic testing in making management recommendations.
•• Sigterman KE, van Pinxteren B, Bonis PA, et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013; CD002095. Evidence on the value of short term use of acid suppression and prokinetic therapy in symptomatic foregut syndromes.
Khan M, Santana J, Donnellan C, et al. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev. 2007; CD003244.
•• Weijenborg PW, Cremonini F, Smout AJ, et al. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil. 2012;24:747–57, e350. Meta-analysis detailing the clinical value of proton pump inhibitors in erosive and well characterized nonerosive reflux disease.
•• Aziz Q, Fass R, Gyawali CP, et al. Functional esophageal disorders. Gastroenterology. 2016;150:1368–79. Current ROME IV diagnostic criteria for functional esophageal disorders and the basis for these criteria.
Weijenborg PW, Smout AJ, Bredenoord AJ. Esophageal acid sensitivity and mucosal integrity in patients with functional heartburn. Neurogastroenterol Motil. 2016;28:1649–54.
Savarino E, Zentilin P, Frazzoni M, et al. Characteristics of gastro-esophageal reflux episodes in Barrett’s esophagus, erosive esophagitis and healthy volunteers. Neurogastroenterol Motil. 2010;22:1061–e280.
•• Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. 2017; Evidence and expert opinion on appropriate utilization of ambulatory reflux monitoring in GERD
•• Scarpignato C, Gatta L, Zullo A, et al. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. A valuable resource detailing state-of-the-art evidence for appropriate use of proton pump inhibitors.
Kahrilas PJ, Jonsson A, Denison H, et al. Regurgitation is less responsive to acid suppression than heartburn in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:612–9.
Chang AB, Lasserson TJ, Gaffney J, et al. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2011; CD004823.
Kahrilas PJ, Hughes N, Howden CW. Response of unexplained chest pain to proton pump inhibitor treatment in patients with and without objective evidence of gastro-oesophageal reflux disease. Gut. 2011;60:1473–8.
Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006;116:254–60.
Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1419–25; quiz 1426.
Hersh MJ, Sayuk GS, Gyawali CP. Long-term therapeutic outcome of patients undergoing ambulatory pH monitoring for chronic unexplained cough. J Clin Gastroenterol. 2010;44:254–60.
Kiljander TO, Harding SM, Field SK, et al. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2006;173:1091–7.
Cremonini F, Wise J, Moayyedi P, et al. Diagnostic and therapeutic use of proton pump inhibitors in non-cardiac chest pain: a metaanalysis. Am J Gastroenterol. 2005;100:1226–32.
Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain?: a meta-analysis. Arch Intern Med. 2005;165:1222–8.
• Boghossian TA, Rashid FJ, Thompson W, et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst Rev. 2017;3:CD011969. Evidence for and against on demand use of acid suppression.
Vasiliadis KV, Viazis N, Vlachogiannakos J, et al. Efficacy of three different dosages of esomeprazole in the long-term management of reflux disease: a prospective, randomized study, using the wireless bravo pH system. Am J Gastroenterol. 2010;105:308–13.
Johnson DA, Benjamin SB, Vakil NB, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol. 2001;96:27–34.
Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;121:1095–100.
• Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the esophageal diagnostic advisory panel. J Am Coll Surg. 2013;217:586–97. Expert opinion on preoperative diagnostic work up prior to antireflux surgery for GERD.
Richter JE, Pandolfino JE, Vela MF, et al. Utilization of wireless pH monitoring technologies: a summary of the proceedings from the esophageal diagnostic working group. Dis Esophagus. 2013;26:755–65.
Herregods TV, Troelstra M, Weijenborg PW, et al. Patients with refractory reflux symptoms often do not have GERD. Neurogastroenterol Motil. 2015;27:1267–73.
• Hershcovici T, Fass R. Step-by-step management of refractory gastresophageal reflux disease. Dis Esophagus. 2013;26:27–36. An approach to evaluation and management of refractory symptoms in GERD.
Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc. 2010;71:28–34.
• Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–92; quiz 693. Evidence based description of the use of proton pump inhibitors in the diagnosis and management of eosinophilic esophagitis.
• Gyawali CP, Roman S, Bredenoord AJ, et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: Conclusions from an international consensus group. Neurogastroenterol Motil. 2017; Characterization of findings on esophageal high resolution manometry in GERD
Leodolter A, Kulig M, Brasch H, et al. A meta-analysis comparing eradication, healing and relapse rates in patients with helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001;15:1949–58.
Scott D, Weeks D, Melchers K, et al. The life and death of Helicobacter pylori. Gut. 1998;43(Suppl 1):S56–60.
Kavitt RT, Cifu AS. Management of Helicobacter pylori infection. JAMA. 2017;317:1572–3.
Hunt RH, Lanas A, Stichtenoth DO, et al. Myths and facts in the use of anti-inflammatory drugs. Ann Med. 2009;41:423–37.
Ito T, Igarashi H, Jensen RT. Zollinger-Ellison syndrome: recent advances and controversies. Curr Opin Gastroenterol. 2013;29:650–61.
Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1–46.
• Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–60; quiz 361. Current management of peptic ulcer bleeding.
• Lucendo AJ, Arias A, Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:13–22 e1. Evidence for PPI use in eosinophilic esophagitis.
Kavitt RT, Hirano I, Vaezi MF. Diagnosis and treatment of eosinophilic esophagitis in adults. Am J Med. 2016;129:924–34.
Bardou M, Quenot JP, Barkun A. Stress-related mucosal disease in the critically ill patient. Nat Rev Gastroenterol Hepatol. 2015;12:98–107.
Alhazzani W, Alenezi F, Jaeschke RZ, et al. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41:693–705.
Gardner TB, Robertson DJ. Stress ulcer prophylaxis in non-critically ill patients: less may be more. Am J Gastroenterol. 2006;101:2206–8.
Alshamsi F, Belley-Cote E, Cook D, et al. Efficacy and safety of proton pump inhibitors for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care. 2016;20:120.
Shin S. Evaluation of costs accrued through inadvertent continuation of hospital-initiated proton pump inhibitor therapy for stress ulcer prophylaxis beyond hospital discharge: a retrospective chart review. Ther Clin Risk Manag. 2015;11:649–57.
Dent J, Vakil N, Jones R, et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond tudy. Gut. 2010;59:714–21.
•• Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med. 2015;373:1853–63. Modern evaluation and management of functional dyspepsia.
Lacy BE, Talley NJ, Locke GR 3rd, et al. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther. 2012;36:3–15.
Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006; CD001960.
Moayyedi P, Delaney BC, Vakil N, et al. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329–37.
•• Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterol. 2017; Detailed discussion of how to evaluate published literature addressing risks of long-term PPI therapy
• Kia L, Kahrilas PJ. Therapy: risks associated with chronic PPI use - signal or noise? Nat Rev Gastroenterol Hepatol. 2016;13:253–4. Expert analysis of risks of proton pump inhibitor use.
Yadlapati R, Kahrilas PJ. When is proton pump inhibitor use appropriate? BMC Med. 2017;15:36.
Johnson DA, Oldfield ECT. Reported side effects and complications of long-term proton pump inhibitor use: dissecting the evidence. Clin Gastroenterol Hepatol. 2013;11:458–64; quiz e37-8.
Sierra F, Suarez M, Rey M, et al. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther. 2007;26:545–53.
Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837–44.
Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238–46.
Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29:611–6.
Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
Epstein M, McGrath S, Law F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med. 2006;355:1834–6.
Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37:1237–41.
Sharara AI, Chalhoub JM, Hammoud N, et al. Low prevalence of hypomagnesemia in long-term recipients of proton pump inhibitors in a managed care cohort. Clin Gastroenterol Hepatol. 2016;14:317–21.
Masclee GM, Coloma PM, Kuipers EJ, et al. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol. 2015;110:749–59.
Capurso G, Marignani M, Attilia F, et al. Lansoprazole-induced microscopic colitis: an increasing problem? Results of a prospecive case-series and systematic review of the literature. Dig Liver Dis. 2011;43:380–5.
Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:132–41.
Zacharioudakis IM, Zervou FN, Pliakos EE, et al. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:381–90; quiz 391.
Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171:33–8.
Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–81.
Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047–56; quiz 2057.
Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483–90.
Xu HB, Wang HD, Li CH, et al. Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res. 2015;14:7490–501.
Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149:391–8.
Estborn L, Joelson S. Frequency and time to onset of community-acquired respiratory tract infections in patients receiving esomeprazole: a retrospective analysis of patient-level data in placebo-controlled studies. Aliment Pharmacol Ther. 2015;42:607–13.
Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448–57.
Lam JR, Schneider JL, Quesenberry CP, et al. Proton pump inhibitor and histamine-2 receptor antagonist use and iron deficiency. Gastroenterology. 2017;152:821–829 e1.
Lam JR, Schneider JL, Zhao W, et al. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–42.
Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209–18; quiz 1219.
Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–53.
Gomm W, von Holt K, Thome F, et al. Association of Proton Pump Inhibitors With Risk of Dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410–6.
Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265:419–28.
Goldstein FC, Steenland K, Zhao L, et al. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;
Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–17.
Lahner E, Annibale B, Delle FG. Systematic review: impaired drug absorption related to the co-administration of antisecretory therapy. Aliment Pharmacol Ther. 2009;29:1219–29.
Cats A, Schenk BE, Bloemena E, et al. Parietal cell protrusions and fundic gland cysts during omeprazole maintenance treatment. Hum Pathol. 2000;31:684–90.
Jalving M, Koornstra JJ, Wesseling J, et al. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1341–8.
Song H, Zhu J, Lu D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst Rev. 2014;12:CD010623.
Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10:e0124653.
Quenot JP, Thiery N, Barbar S. When should stress ulcer prophylaxis be used in the ICU? Curr Opin Crit Care. 2009;15:139–43.
•• de Bortoli N, Martinucci I, Savarino E, et al. Proton pump inhibitor responders who are not confirmed as GERD patients with impedance and pH monitoring: who are they? Neurogastroenterol Motil. 2014;26:28–35. Follow-up and phenotyping of responders and non-responders of short-term PPI therapy administered for heartburn with normal endoscopy.
Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166:965–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Prakash Gyawali reports grants and personal fees from Medtronic, Inc. and personal fees from Torax, Quintiles, Ironwood, and Allergan for consulting and speaking, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neurogastroenterology and Motility Disorders of the Gastrointestinal Tract
Rights and permissions
About this article
Cite this article
Gyawali, C.P. Proton Pump Inhibitors in Gastroesophageal Reflux Disease: Friend or Foe. Curr Gastroenterol Rep 19, 46 (2017). https://doi.org/10.1007/s11894-017-0586-5
Published:
DOI: https://doi.org/10.1007/s11894-017-0586-5