Abstract
Purpose of Review
The obesity epidemic is on the rise, and while it is well known that obesity is associated with an increase in cardiovascular risk factors such as type 2 diabetes mellitus, hypertension, and obstructive sleep apnea, recent data has highlighted that the degree and type of fat distribution may play a bigger role in the pathogenesis of cardiovascular disease (CVD) than body mass index (BMI) alone. We aim to review updated data on adipose tissue inflammation and distribution and CVD.
Recent Findings
We review the pathophysiology of inflammation secondary to adipose tissue, the association of obesity-related adipokines and CVD, and the differences and significance of brown versus white adipose tissue. We delve into the clinical manifestations of obesity-related inflammation in CVD. We discuss the available data on heterogeneity of adipose tissue-related inflammation with a focus on subcutaneous versus visceral adipose tissue, the differential pathophysiology, and clinical CVD manifestations of adipose tissue across sex, race, and ethnicity. Finally, we present the available data on lifestyle modification, medical, and surgical therapeutics on reduction of obesity-related inflammation.
Summary
Obesity leads to a state of chronic inflammation which significantly increases the risk for CVD. More research is needed to develop non-invasive VAT quantification indices such as risk calculators which include variables such as sex, age, race, ethnicity, and VAT concentration, along with other well-known CVD risk factors in order to comprehensively determine risk of CVD in obese patients. Finally, pre-clinical biomarkers such as pro-inflammatory adipokines should be validated to estimate risk of CVD in obese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
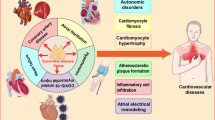
The pathophysiologic link between excess body fat in obesity and inflammation contributes to cardiovascular disease (CVD) pathogenesis and atherosclerotic events in the population. Adipose tissue is an active organ implicated in the synthesis and secretion of inflammatory cytokines that impact the local tissue environment and promote systemic lipotoxicity. Herein, we describe the pathophysiology of inflammation related to adipose tissue and the association of obesity-associated adipokines and CVD. We discuss the available data on heterogeneity of adipose tissue-related inflammation with a focus on the differential pathophysiology of subcutaneous (SAT) versus visceral adipose tissue (VAT), and delve into the clinical manifestations of obesity-related inflammation in CVD across sex, race, and ethnicity. Finally, we discuss the available data on lifestyle modification, medical, and surgical therapeutics on reducing obesity-related inflammation.
Pathophysiology of Inflammation and Adipose Tissue
M1 Versus M2 Macrophages
Mature adipocytes reside in a milieu composed of preadipocytes, immune cells (including macrophages), and endothelial cells [1]. Early human studies showed that weight gain was associated with an increase in macrophages from 10 to 40% in the stroma of abdominal SAT [2]. We now know that obesity leads to an increase in pro-inflammatory M1 macrophages with CD11c (integrin aX chain) markers relative to anti-inflammatory M2 macrophages with CD206 (mannose) receptors [1]. Hence, is has been speculated that the increase in M1 to M2 macrophage ratio might be responsible for inducing inflammation in obesity [3, 4]. VAT in particular accumulates more M1 macrophages, which produce inflammatory cytokines like tumor necrosis factor (TNF)-α and interleukin (IL)-6 and lead to expansion of effector T cells like Th1 cells and CD8+ cytotoxic T cells which produce interferon-γ to further add to the severity of inflammation [5]. In contrast, SAT enlargement leads to an enhancement in the content of anti-inflammatory M2-like macrophages [1]. Thus, the relative burden of VAT compared with SAT in an individual may directly underpin an excess of pro-inflammatory secretory factors that contribute to CVD pathogenesis.
Obesity-Related Adipokines and CVD
Obesity contributes to a state of chronic inflammation by several mechanisms. These include inducing an increase in inflammatory cell lines such as macrophages [2] and reducing adipocyte capillarization which in turn limits nutrient delivery [6] and adipose tissue fibrosis [7]. This promotes upregulation of pro- inflammatory adipokines and the downregulation of anti-inflammatory adipokines. Pro-inflammatory adipokines have direct effects on the cardiovascular system (as discussed below) and indirectly lead to CVD due to metabolic alterations in the liver, skeletal muscle, and heart [6]. Some common adipokines are discussed below and are also summarized in Table 1.
Leptin
Leptin is a pro-inflammatory hormone secreted from adipocytes that suppresses appetite and increases energy expenditure [8]. Obesity leads to increase in 5 levels and also leptin resistance [9]. Hyperleptinemia promotes the expression of MMP-2, a metalloproteinase linked to atherosclerotic plaque vulnerability, and facilitates cholesterol accumulation in macrophages [10,11,12]. Studies have shown that exogenous leptin administration leads to worsening atherosclerosis in mice [13] and promotes plaque calcification [14].
Interleukin-6
This is a pro-inflammatory cytokine with a 2–3 times higher secretion in VAT than SAT [15]. It has direct actions on a variety of immune cells, promoting cell adhesion, differentiation, antibody production by B cells, and recruitment of T-cells to sites of injury [6]. However, some studies on rodents have generated conflicting data suggesting that it may actually prevent atherogenesis by promoting cholesterol removal from the vessel walls [16].
Resistin
Mouse models have shown that resistin, a protein secreted by mature adipocytes, can lead to metabolic dysfunction by inducing insulin resistance [17]. Elevated levels of resistin have been associated with coronary artery calcification and coronary artery disease [18, 19].
Adiponectin
Adiponectin an anti-inflammatory adipokine with levels inversely related to the amount of VAT [20]. Plasma adiponectin levels are decreased in patients with acute coronary syndrome [21], whereas higher plasma concentrations are thought to decrease the risk of CVD in healthy and diabetic men [22, 23].
Omentin-1
This is a soluble lectin produced by VAT which is reduced in obesity and type 2 diabetes mellitus [6]. It has protective effects on the cardiovascular system which may be mediated by attenuation of TNFα-induced inflammatory responses [24].
C-reactive Protein (CRP)
CRP promotes monocyte adhesion and transmigration into blood vessels which is a critical step in promoting atherosclerosis. A meta-analysis showed that high sensitivity-CRP level > 3 mg/l was independently associated with a 60% excess risk in incident CVD as compared with levels < 1 mg/l [25]. Studies have shown that statin-treated patients with high sensitivity-CRP levels higher than 2 mg or more per liter at baseline stand a risk of future major adverse cardiovascular event rates that are at least as high as, if not higher than, those among statin-treated patients with a residual risk due to low-density lipoprotein (LDL) cholesterol levels [26••]. Certain individuals may have a genetic locus near gene ATG5 on chromosome 6 which is associated with higher CRP levels [27]. This genetic locus may also modulate adipocyte size and macrophage polarization leading to preferential accumulation of VAT. Loss of body fat has been shown to lower CRP levels [28••] making it a potentially useful inflammatory marker with prognostic value.
Clinical Impact of Inflammation on CVD
Atherosclerosis
Several prospective epidemiologic studies demonstrated that obesity is associated with higher incidence of CVD [29]. A meta-analysis of > 300,000 adults with 18,000 coronary artery disease events demonstrated that body mass index (BMI) in the overweight and obese ranges was associated with elevated coronary artery disease risk [30]. There is conflicting data to suggest the degree to which the association between obesity and atherosclerotic CVD is independent of other metabolic risk factors such as diabetes mellitus and dyslipidemia [29], but inflammation may play a key role in CVD pathogenesis.
The process of atherosclerosis begins in early childhood with macrophage ingestion of cholesterol esters and their deposition in the vascular intima which gradually thickens [31]. Obesity induces inflammation by promoting LDL oxidation [32] as well as endothelial dysfunction causing diminished bioavailability of nitric oxide [33]. This accelerates the process of atherosclerosis from initial fatty streak development all the way to atherothrombosis [34, 35] in major arteries and remodeling of microvascular blood vessels [36]. Results from the Pathobiological Determinants of Atherosclerosis in Youth Study showed that this association is present only for those with a thick abdominal panniculus [37], in other words, those with a high density of abdominal adipose tissue.
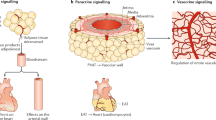
Studies suggest that ectopic fat depots in epicardial and pericardial spaces may further increase atherogenesis [35] by producing adipocytokines that alter blood vessel biology through paracrine signaling or through vasa vasorum [29]. Obesity causes an upregulation in pro-inflammatory cytokines (e.g., IL-6) and lipid peroxidation products (e.g., 4-hydroxynonenal) which force the perivascular adipose tissues to modify its composition (decreased adipocyte size and lipid content) as well as biology (increased production of adiponectin) [38]. Efforts are being made to measure perivascular adipose tissue using computed tomography (CT) or magnetic resonance (MR)-guided imaging and so, it may function as a marker of coronary inflammation in the future [38].
Heart Failure
The association between heart failure and obesity is mediated through both direct effects on the myocardium as well as indirect effects through obesity-related comorbidities [39]. In a study of 5881 Framingham Heart Study participants, heart failure incidence increased by 5% in men and 7% in women for every 1-unit BMI increase after adjustment for other risk factors [40]. Recent work has also suggested that a higher BMI is more strongly associated with risk for heart failure with preserved ejection fraction (HFpEF) than for heart failure with reduced ejection fraction [41]. In fact, in a pooled analysis using data from 3 large longitudinal studies, Pandey et al. demonstrated this strong association, with overweight and class 1 obese participants having 38% and 56% higher risk of HFpEF, respectively, independently of other cardiovascular risk factors [42]. Pulmonary hypertension from long-standing sleep apnea in obesity can lead to right heart failure [39]. Recent data has demonstrated that the associations between BMI and right ventricular systolic dysfunction in HFpEF are most likely driven by increased plasma volumes [43].
Excess adipose tissue affects vasculature by activation of the renin–angiotensin–aldosterone system [44] and leads to hemodynamic alterations by plasma volume expansion [43]. Myocardial fat accumulation and subsequent fibrosis lead to left ventricular diastolic dysfunction and subsequently, HFpEF [29]. At a cellular level, metabolic shifting between glycolysis and mitochondrial oxidative phosphorylation disrupts M1/M2 homeostasis and exacerbates inflammation which promotes adverse cardiac remodelling [45]. Detailed phenotyping of patients with HFpEF with and without obesity compared with controls depicted that patients with obesity and HFpEF had greater concentric LV remodeling, right ventricular dilatation, and right ventricular dysfunction [46, 47]. VAT, in particular, is associated with greater cardiac remodeling and diastolic dysfunction [43]. Patients with obesity-related HFpEF also have significantly lower exercise capacity compared with patients without obesity with HFpEF and control subjects [46].
Atrial Fibrillation (AF)
Obesity is strongly associated with AF with every 5-unit increment in BMI conferring a ≈29% greater risk of incident AF [48] and the Atherosclerosis Risk In Communities (ARIC) study estimating that almost 1 in 5 cases of AF can be attributable to being overweight or obese [49]. Higher BMIs in the ranges of 30 to 34.9 kg/m2 are associated with progression of disease with a 54% likelihood of evolving from paroxysmal to permanent AF [50]. Interestingly, the association between obesity and AF has been shown to be independent of obstructive sleep apnea, a common comorbid condition in obese individuals [51]. From a practical perspective, obese AF patients who undergo ablation have a higher chance of disease recurrence than the general population. A meta-analysis of 16 studies involving 5864 individuals showed a 13% greater excess risk of AF recurrence after ablation for every 5-unit increase in BMI [52] which poses a high burden on healthcare.
The pathophysiology of obesity-induced AF can be explained by its direct effects the myocardium as well as indirect effect of inducing a chronic state of inflammation in the body [53]. Obesity increases expression of endothelin receptors and fibrosis which ultimately impairs contractility and increases myocardial arrhythmogenicity [29]. Studies utilizing cardiac MRI and electroanatomic mapping of the left atrium have demonstrated conduction slowing, areas of low voltage and ECG fractionation prior to AF ablation [54] in these individuals. More recently, epicardial adipose tissue located between the visceral pericardium and the epicardial layer of myocardium has emerged as a promising proarrhythmogenic substrate. It secretes metabolic factors (free fatty acids and uncoupling protein-1), angiogenic factors (angiotensin, endostatin, vascular endothelial growth factor-1, thrombospondin-2, angiopoetin), growth and remodeling factors (activing A, follistatin, transforming growth factors 1–3, matrix metalloproteinases 1–13), adipocytokines (adiponectin, leptin, resistin, visfatin, omentin), inflammatory cytokines and chemokines and various interleukins (including IL1b and IL-6), plasminogen activator inhibitor-1, TNF-α, monocyte chemotactic protein 1, chemokine ligands, adrenomedullin, and phospholipase A2 [55, 56]. Multiple studies employing cardiac imaging techniques have shown an association between AF and obesity with each standard deviation increase in pericardial fat volume associated with a 28% increase in the prevalence of AF [57]. Not surprisingly, central obesity is associated with a higher density of pericardial fat depots than other forms of obesity [53].
Heterogeneity of Adipose Tissue-Related Inflammation
Abdominal Subcutaneous versus Visceral Adipose Tissue
Recent human data has suggested that fat tissue distribution may be a more important indicator of CVD than total adipose tissue volume, independent of BMI. This is due to an increased atherogenic gene expression profile in patients with visceral fat [6] and is interesting since VAT accounts for only 5–20% of total body fat volume but a much higher risk of CVD [58]. Results from the Jackson Heart Study have supported this notion with higher fasting glucose levels, triglycerides, and comorbidity burden in patients with VAT than SAT even after accounting for BMI [59]. The Dallas Heart Study enrolled 972 obese participants and followed them for 9 years with a primary outcome of a first or subsequent cardiovascular event. Results showed that the prevalence of CVD event rate increased from 5.3% in lower VAT quartiles to 10.0% in higher quartiles [60].
The exact mechanisms of how VAT leads to CVD are still under investigation but different theories have been proposed relating to the metabolic properties of VAT and its ability to induce inflammation. VAT exposes the liver to high concentrations of free fatty acids and glycerol which leads to impaired glucose metabolism and hypertriglyceridemia [61]. Overtime, these excess triglycerides deposit in ectopic, normally lean tissues such as the liver, the heart (pericardial, epicardial, and intramyocardial), and skeletal muscle [62, 63]. Lastly, the hypertrophied adipocytes become inflamed by secreting pro-inflammatory cytokines such as TNF-α and IL-6 while decreasing production of anti-inflammatory cytokines such as adiponectin as discussed in detail in the section on adipokines above.
Adiposity Across Sexes
Traditionally, men have been identified as a high-risk population due to increased prevalence of CVD in men compared to premenopausal women [64]. This is because fat in men is predominantly distributed around abdominal organs as VAT creating an “apple shape” body habitus while women have more SAT, creating a “pear-shaped” fat distribution [65,66,67] (Fig. 1). The higher VAT leads to increased lipolysis with excessive fatty acid deposition in the liver [68] which ultimately contributes to higher postprandial insulin, free fatty acids, and triglyceride levels in males compared to females [64]. The factors and mechanisms that govern this sexual dimorphism in body fat are critical to the understanding of obesity and its complications. Some theories are discussed as follows:
-
Differential expression of sex steroid receptors: SAT has a higher concentration of estrogen receptors and progesterone receptors compared to androgen receptors in females [69, 70], whereas VAT has a higher concentration of androgen receptors [69, 71]. Studies in mouse models have highlighted the protective benefit of estrogen with increased weight gain and impaired metabolism demonstrated in female mice who underwent ovariectomy, but when estrogen was replaced, there was less weight gain and improved metabolism [72].
-
Role of sex chromosomes and gene expression: The fundamental difference between males and females is the presence of two X chromosomes in females while males possess an X and a Y chromosome. X chromosomes appear to have a higher influence on body weight/fat while Y chromosomes have close to no influence [73]. Furthermore, gene expression is regulated epigenetically by miRNAs that interact with mRNAs. Some miRNAs with distinct sex-biased patterns in expression have been implicated in adipogenesis [74, 75].
-
Adipose tissue inflammation: Men have a more hypertrophic type of adipose tissue [76], while females have more small adipocytes [77, 78] as well as more efficient triglyceride fatty acid uptake from the circulation leading to better insulin sensitivity [79]. On the other hand, obese females can adversely affect their offspring from as early as the prenatal period by releasing proinflammatory cytokines into the systemic circulation [80].
Variation of Adiposity Across Race/Ethnicities
People from different parts of the world have considerable variability in body composition with an interplay from various modifiable factors such as climate, diet, and infectious diseases [81]. The impact of SAT and VAT on the risk of CVD varies also considerably across various across these different ethnic groups [82]. One possible reason for this is racial variability in androgenic sex steroids [83] with high levels of free testosterone leading to increased obesity in females but decreases obesity in males [84].
South Asians may have the most deleterious fat distributions of all with lower SAT and higher VAT despite lower absolute BMIs [85]. In a cross-sectional analysis of 796 participants in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study utilizing CT scans to measure various fat depots, the investigators found that higher pericardial fat volume, visceral fat area, and intermuscular fat area were significantly associated with a higher pooled cohort risk score for ASCVD [86].
Earlier studies evaluating CVD largely enrolled patients who predominantly belonged to one ethnicity. A cross-sectional analysis comparing African Americans enrolled in the Jackson Heart study and European Americans in the Framingham Heart Study showed that even though participants in the Jackson Heart study had a higher co-morbidity burden including higher BMIs, they had about half the VAT than their European counterparts [87]. Despite this, African Americans have higher mortality than Caucasians from CVD (156 versus 95 women/100,000 person years) largely due to social injustices from income inequality and racial discrimination [88]. Similarly, a study evaluating the association of VAT and subclinical atherosclerosis in US-born Mexican Americans showed an increased prevalence of carotid intima media thickness in those in the highest quartile for VAT (57.4% versus 15.4% for the lowest quartile; P < 0.001). What was interesting was that this association was only seen among second or higher generation US-born Mexican Americans but not among first generation immigrants [89].
Therapeutic Strategies to Reduce Inflammation
Diet
Studies have shown that a negative energy balance induced by diet or exercise is associated with reduction in VAT depots [90]. However, no studies so far have shown a clear reduction in CVD or mortality with weight loss through lifestyle modification [29]. Look AHEAD, one of the largest clinical trials on lifestyle modification for obesity treatment in patients with type 2 diabetes, failed to show a significant reduction in MACEs (major adverse cardiovascular events) or cardiovascular mortality after 9.6 years of follow-up [91]. Diet-induced weight loss does seem to reduce systemic inflammation by upregulating anti-inflammatory adipokines (e.g., omentin-1) [92] and downregulating pro-inflammatory cytokines (e.g., osteopontin) [93]. A study in Denmark on 60 participants showed a favorable reduction in weight and inflammatory markers such as IL-6 (p = 0.009) and CRP (p = 0.003) with a whole grain diet compared to a diet comprising refined grain [94].
Many diet-fads have come and gone but the Mediterranean diet continues to be considered the most heart-healthy. A randomized control trial in Israel that assigned sedentary participants to a Mediterranean and low-carbohydrate diet or an isocaloric low-fat diet showed that a Mediterranean diet did lead to a significant decrease in VAT (mean difference − 6·67 cm2, 95% confidence interval (CI) − 14·8 to − 0·45), independent of weight loss [95]. Another randomized control trial comparing a high-fat, low-carbohydrate diet with or without exercise to a low glycemic index diet in obese, diabetic patients showed that all three interventions inhibited the JNK pathway in peripheral blood mononuclear cells, which if activated, results in the production of proinflammatory cytokines and chemokines [96]. This supports the notion that any form of lifestyle modification might be better than a sedentary non-healthy lifestyle. Consumption of a low-fiber, high-fat, Western-style diet may actually induce inflammation by way of lipid-overloaded adipocytes and from dissemination of gut bacterial products, resulting in activation of innate immune signaling. Studies on mice involving ablation of gut microbiota have shown dramatic reductions adipose inflammation along with reductions in hepatic steatosis, transaminase levels, and cholesterol levels [97].
Among other diets, a ketogenic diet which comprises 15% protein, 5% carbohydrate, and 80% fat showed mixed results with higher levels of pro-inflammatory CRP as well as higher levels of anti-inflammatory adiponectin seen in the 17 male patients enrolled in the study [98]. One of the biggest limitations of dietary management is the relatively small sample size of all the studies so far which significantly limits generalizability of these findings.
Exercise
Exercise has been shown to reduce VAT even in the absence of weight loss due to increase in both skeletal muscle mass and cardiorespiratory fitness [99,100,101]. On a cellular level, exercise inhibits leptin production which is normally the main driver for inflammation via proliferation of hematopoietic stem and progenitor cells [102]. However, there is a lack of consensus and currently no currently published guidelines suggesting the perfect “exercise prescription” to reduce VAT and hence CVD.
A meta-analysis of 3,602 participants from 17 randomized control trails showed a significant reduction in VAT with exercise compared to use of pharmacological treatments. Aerobic exercise results in greater VAT reduction compared to resistance training but its benefit is governed by the intensity as well as the amount of exercise per unit time [103]. A trial in Denmark which randomized 70 patients with established coronary artery disease and BMI 28–40 kg/m2 to a combination of weight loss and interval training or interval training alone showed that both interventions led to improvements in peak oxygen capacity as well as total cholesterol, non-high-density lipoprotein cholesterol, and triglycerides. However, the combination of low energy diet (800–1000 kcal/day) and aerobic interval training was associated with a greater reduction in body weight and waist circumference. Furthermore, even though exercise alone obtained a significant 35% (p = 0.019) decrease in CRP, a combination of diet and exercise showed significant decreases in TNF-α (13%, p < 0.001), soluble urokinase-type plasminogen activator receptor (11%, p < 0.001), and CRP (33%, p = 0.040) [104]. This suggests that a program including both caloric restriction and exercise should be recommended to reduce cardiovascular and metabolic risk factors.
Pharmacotherapy
Various medications have been in use over the years to help with weight loss when lifestyle modification is sufficient. A meta-analysis of 28 randomized controlled trials showed that pharmacotherapy for obesity was associated with a 3.3 cm (95% CI, − 3.5 to − 3.1, SMD) decline in waist circumference [105].
Obesity-associated inflammation is a key factor towards the pathogenesis of insulin resistance and type 2 diabetes mellitus. Thus, anti-diabetic drugs that help with weight loss are increasingly looked upon as important options. Sodium-glucose cotransporter (SGLT)-2 inhibitors increase urinary glucose excretion by inhibiting renal glucose reabsorption, thereby reducing body weight and blood sugars [106]. Rodent studies have confirmed their beneficial effects on alleviating obesity induced inflammation [107] by reducing the accumulation of pro-inflammatory T cells/M1 macrophages and increasing the concentrations of anti-inflammatory M2 macrophages [106]. Furthermore, empagliflozin increases fatty acid oxidation by altering the expression of adiponectin and leptin, indirectly improving insulin sensitivity. Other medications such as glucagon-like peptide (GLP)-1 receptor agonists that induce weight loss are being considered potential “cures” for VAT. A single-center, double-blinded, randomized controlled trial in Texas assessed the impact of liraglutide in addition to a 500 kcal deficient diet and guideline-recommended physical activity counseling in non-diabetic patients with a BMI of greater than 30 kg/m2 or 27 kg/m2 with metabolic syndrome. One hundred eighty-five participants, out of whom 64% were white, 37% were black, and 22% were Hispanic, were randomized to receive liraglutide or placebo and followed for 40 weeks of treatment. The mean change in VAT over 36.2 weeks was − 12.49% (standard deviation 9.3%) with liraglutide compared with − 1.63% (standard deviation 12.3%) with placebo. It was interesting to see that the effects of liraglutide were consistent across subgroups of age, sex, and ethnicities [105] and liraglutide concomitantly decreased CRP levels compared with placebo.
Newer advancements in medicine are now studying drugs that decrease atherosclerosis by attenuating inflammation without any impacting lipids. One such drug worth mentioning is canakinumab, a therapeutic monoclonal antibody that targets interleukin-1β. The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) Trial randomized 10,061 patients with previous myocardial infarction and a high-sensitivity CRP level of 2 mg or more per liter to three doses of canakinumab or placebo. At 48 months, 150 mg of canakinumab every 3 months led to a significantly lower rate of recurrent cardiovascular events than placebo, independent of lipid-level lowering suggesting that immunotherapy may be the way of the future [26••].
Surgery
Weight loss following bariatric surgery has been shown to decrease morbidity and mortality from CVD due to its restrictive and malabsorptive effects [108]. It is also successful in reducing inflammation by altering adipokine-induced macrophage infiltration [109], but there are no studies to our knowledge that have determined the most anti-inflammatory type of bariatric surgery out of all the options available. A prospective study that followed 19 patients for 4 years after they underwent bariatric surgery (sleeve gastrectomy, biliopancreatic diversion, Roux-en-Y gastric bypass, and laparoscopic adjustable gastric banding) showed not only a reduction in inflammatory markers but also an enhancement in the total antioxidant status of up to 35% in the fasting state [110]. A prospective study in the UK that followed 55 participants who underwent laparoscopic sleeve gastrectomy for 6 months postoperatively showed significant reductions in 6L-6, CRP, leptin, and thiobarbituric acid reactive substances, along with an increase in adiponectin[8].
Roux-en-Y gastric bypass which involves connecting a small gastric pouch to the proximal jejunum allowing for transit of nutrients directly to the small intestine is the longest-standing bariatric procedure with the best evidence for sustained weight loss and improvement in cardiovascular risk [112,113,114]. It can lead to ≥ 30% mean weight loss due to reductions in appetite and caloric intake with greatest improvements in blood pressure, glycemia, and dyslipidemia compared to any other procedure [108]. Data from MRI quantification of fat showed a decrease in BMI from 45.4 kg/m2 from baseline to 42.4 kg/m2 a month after surgery with a corresponding decrease in VAT from 5.94 and 5.33 L (p < 0.001). Interestingly, there was no significant difference in SAT parameters [115]. Another study showed that bypass causes improvements in ectopic fat size and composition [116] along with significant decreases in levels of IL-6 in diabetic patients a year of surgery [117], all of which may explain the decreased CVD risk in this patient population.
Conclusions
Obesity leads to a state of chronic inflammation which significantly increases the risk for CVD. More research is needed to develop non-invasive VAT quantification indices such as risk calculators which include variables such as sex, age, race, ethnicity, and VAT concentration, along with other well-known CVD risk factors in order to comprehensively determine risk of CVD in obese patients. Finally, pre-clinical biomarkers such as pro-inflammatory adipokines should be validated to estimate risk of CVD in obese patients.
Abbreviations
- CVD:
-
Cardiovascular disease
- VAT:
-
Visceral adipose tissue
- SAT:
-
Subcutaneous adipose tissue
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- CRP:
-
C-reactive protein
- LDL:
-
Low-density lipoprotein
- BMI:
-
Body mass index
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- HFpEF:
-
Heart failure with preserved ejection fraction
- AF:
-
Atrial fibrillation
- CI:
-
Confidence interval
- MACE:
-
Major adverse cardiovascular events
- SGLT-2:
-
Sodium-glucose cotransporter-2
- GLP-1:
-
Glucagon-like peptide-1
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Girón-Ulloa A, González-Domínguez E, Klimek RS, et al. Specific macrophage subsets accumulate in human subcutaneous and omental fat depots during obesity. Immunol Cell Biol. 2020;98(10):868–82. https://doi.org/10.1111/imcb.12380.
Weisberg S, McCann D, Desai M, Rosenbaum M, Leibel R, Ferrante A. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. https://doi.org/10.1172/JCI19246.
Aron-Wisnewsky J, Tordjman J, Poitou C, et al. Human adipose tissue macrophages: M1 and M2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619–23.
Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86.
Zhu F, Wang A, Li Y, Liang R, Li D, Li B. Adipose Tissue-resident regulatory T cells. Adv Exp Med Biol. 2017;1011(153–162). https://doi.org/10.1007/978-94-024-1170-6_4
Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-Induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2017;118(11):1786–807. https://doi.org/10.1161/CIRCRESAHA.115.306885.Obesity-induced.
Khan T, Muise E, Iyengar P, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–91. https://doi.org/10.1128/MCB.01300-08.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. https://doi.org/10.1038/372425a0.
Friedman J, Halaas J. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. https://doi.org/10.1038/27376.
Li L, Mamputu J, Wiernsperger N, Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes. 2005;54:2227–34.
Hongo S, Watanabe T, Arita S, et al. Leptin modulates ACAT1 expression and cholesterol efflux from human macrophages. Am J Physiol Endocrinol Metab. 2009;297:E474–82. https://doi.org/10.1152/ajpendo.90369.2008.
O’Rourke L, Gronning L, Yeaman S, Shepherd P. Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem. 2002;277:42557–62. https://doi.org/10.1074/jbc.M202151200.
Bodary P, Gu S, Shen Y, Hasty A, Buckler J, Eitzman D. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arter Thromb Vasc Biol. 2005;25:e119–22. https://doi.org/10.1161/01.ATV.0000173306.47722.ec.
Zeadin M, Butcher M, Werstuck G, Khan M, Yee C, Shaughnessy S. Effect of leptin on vascular calcification in apolipoprotein E-deficient mice. Arter Thromb Vasc Biol. 2009;29:2069–75. https://doi.org/10.1161/ATVBAHA.109.195255.
Fain J, Madan A, Hiler M, Cheema P, Bahouth S. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. https://doi.org/10.1210/en.2003-1336.
Schieffer B, Selle T, Hilfiker A, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–500. https://doi.org/10.1161/01.CIR.0000148135.08582.97.
Steppan C, Bailey S, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. https://doi.org/10.1038/35053000.
Reilly M, Lehrke M, Wolfe M, Rohatgi A, Lazar M, Rader D. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–9. https://doi.org/10.1161/01.CIR.0000155620.10387.43.
Muse E, Feldman D, Blaha M, et al. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239:101–8.
Ryo M, Nakamura T, Kihara S, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68:975–81.
Nakamura Y, Shimada K, Fukuda D, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–33.
Pischon T, Girman C, Hotamisligil G, Rifai N, Hu F, Rimm E. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291(1730–1737). https://doi.org/10.1001/jama.291.14.1730.
Schulze M, Shai I, Rimm E, Li T, Rifai N, Hu F. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–9.
Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–43. https://doi.org/10.1016/j.bbrc.2011.04.039.
Yousuf O, Mohanty BD, Martin SS, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62(5):397–408. https://doi.org/10.1016/j.jacc.2013.05.016.
Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/nejmoa1707914. Results of this study showed that therapies that lower inflammation lead to a decrease in CVD events independent of lipid lowering therapy
Shin J, Syme C, Wang D, et al. Novel genetic locus of visceral fat and systemic inflammation. J Clin Endocrinol Metab. 2019;104(9):3735–42. https://doi.org/10.1210/jc.2018-02656.
Neeland I, Marso S, Ayers C, et al. Effects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Diabetes Endocrinol. 2021;9(9):595–605. https://doi.org/10.1016/S2213-8587(21)00179-0. GLP-1 agonists lead to favorable remodeling of adipose tissue composition with decreases in VAT concentration along with inflammatory markers such as CRP
Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21). https://doi.org/10.1161/CIR.0000000000000973
Bogers R, Bemelmans W, Hoogenveen R, et al. BMI-CHD Collaboration Investigators. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–1728. https://doi.org/10.1001/archinte.167.16.1720
McGill HJ. Fatty streaks in the coronary arteries and aorta. Lab Invest. Published online 1968:560–564.
Couillard C, Ruel G, Archer W, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005;90:6454–9. https://doi.org/10.1210/jc.2004-2438.
Engin A. Endothelial dysfunction in obesity. Adv Exp Med Biol. 2017;960:345–79. https://doi.org/10.1007/978-3-319-48382-5_15.
Rocha V, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. https://doi.org/10.1038/nrcardio.2009.55.
Ross R. Atherosclerosis is an inflammatory disease. Am Hear J. 1999;138(pt 2):S419–20. https://doi.org/10.1016/s0002-8703(99)70266-8.
Schindler T, Cardenas J, Prior J, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–95. https://doi.org/10.1016/j.jacc.2005.10.062.
Zieske A, Malcom G, Strong J. Natural history and risk factors of ath- erosclerosis in children and youth: the PDAY study. Pediatr Pathol Mol Med. 2002;21:213–37. https://doi.org/10.1080/15227950252852104.
Mancio J, Oikonomou EK, Antoniades C. Perivascular adipose tissue and coronary atherosclerosis. Heart. 2018;104(20):1654–62. https://doi.org/10.1136/heartjnl-2017-312324.
Alpert M, Lavie C, Agrawal H, Aggarwal K, Kumar S. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res. 2014;164:345–56. https://doi.org/10.1016/j.trsl.2014.04.010.
Kenchaiah S, Evans J, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(305–313). https://doi.org/10.1056/NEJMoa020245
Pandey A, Patel K, Vaduganathan M, et al. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Hear Fail. 2018;6:975–82. https://doi.org/10.1016/j.jchf.2018.09.006.
Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–42. https://doi.org/10.1016/j.jacc.2016.11.081.
Harada T, Obokata M. Obesity-related heart failure with preserved ejection fraction: pathophysiology, diagnosis, and potential therapies. Hear Fail Clin. 16(3):357–368. https://doi.org/10.1016/j.hfc.2020.02.004
Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. Published online 2018:3407306. https://doi.org/10.1155/2018/3407306
Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction novel roles of immunometabolism in macrophage activation and inflammation. Circ Res. Published online 2020:789–806. https://doi.org/10.1161/CIRCRESAHA.119.312321
Obokata M, Reddy Y, Pislaru S, Melenovsky V, Borlaug B. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. https://doi.org/10.1161/CIRCULATIONAHA.116.026807.
Kitzman D, Lam C. Obese heart failure with preserved ejection fraction phenotype: from pariah to central player. Circulation. 2017;136:20–3. https://doi.org/10.1161/CIRCULATIONAHA.117.028365.
Wong C, Sullivan T, Sun M, et al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol. 2015;1(139–152). https://doi.org/10.1016/j.jacep.2015.04.004
Huxley R, Lopez F, Folsom A, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–8.
Tsang T, Barnes M, Miyasaka Y, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Hear J. 2008;29:2227–33. https://doi.org/10.1093/eurheartj/ehn324.
Gami A, Hodge D, Herges R, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–71.
Mangiafico V, Saberwal B, Lavalle C, et al. Impact of obesity on atrial fibrillation ablation. Arch Cardiovasc Dis. 2020;113(8–9):551–63. https://doi.org/10.1016/j.acvd.2020.03.023.
Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70(16):2022–35. https://doi.org/10.1016/j.jacc.2017.09.002.
Mahajan R, Nelson A, Pathak R, et al. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol. 2018;4:1529–40. https://doi.org/10.1016/j.jacep.2018.08.014.
Hatem S, Redheuil A, Grandjbakhch E. Cardiac adipose tissue and atrial fibrillation. Cardiovasc Res. 2016;109:502–9.
Al-Rawahi M, Proletti R, Thansoulis G. Pericardial fat and atrial fibrillation: epidemiology, mechanisms and interventions. Int J Cardiol. 2015;195:98–103.
Thanasoullis G, Massaro J, O’Donnel C. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Study. Circ Arrhythmia Electrophysiol. 2010;3:345–50.
Ibrahim M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–8.
Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors : the Jackson Heart Study. 2010;95(December):5419-5426https://doi.org/10.1210/jc.2010-1378
Neeland I, Turer A, Ayers C, Al. E. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65(19):2150–2151. https://doi.org/10.1016/j.jacc.2015.01.061
Neeland I, Hughes C, Ayers C, Malloy C, Jin E. Effects of visceral adiposity on glycerol pathways in gluconeogenesis. Metabolism. 2017;67:80–9.
Smith U, Kahn B. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. 2016;280:465–75.
Vasan S, Karpe F. Fat, yet fit. Nat Rev Endocrinol. 2016;12:375–6.
Chang E, Varghese M, Singer K. Gender and sex differences in adipose tissue. Curr Diab Rep. 2018;18(9):69. https://doi.org/10.1007/s11892-018-1031-3.
Fried S, Lee M, Karastergiou K. Shaping fat distribution: new insights into the molecular determinants of depot and sex dependent adipose biology. Obes (Silver Spring). 2015;23(7):1345–52.
Schwartz R, Shuman W, Larson V, Cain K, Fellingham G, Beard J, et al. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism. 1991;40(5):545–51.
Link J, Hasin-Brumshtein Y, Cantor R, Chen X, Arnold A, Lusis A, et al. Diet, gonadal sex, and sex chromosome complement influence white adipose tissue miRNA expression. BMC Genomics. 2017;18(1):89.
Shulman G. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(23):2237–8.
Lu S, McKenna S, Cologer-Clifford A, Nau E, Simon N. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139(4):1594–601.
Locke A, Kahali B, Berndt S, Justice A, Pers T, Day F, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206.
Macotela Y, Boucher J, Tran T, Kahn C. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58(4):803–12.
Ornoy A, Giron S, Aner R, Goldstein M, Boyan B, Schwartz Z. Gender dependent effects of testosterone and 17 beta-estradiol on bone growth and modelling in young mice. Bone Min. 1994;24(1):43–58.
Chen X, McClusky R, Chen J, Beaven S, Tontonoz P, Arnold A et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8(5):e1002709.
Xie H, Lim B, Lodish H. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. 2009;58(5):1050–7.
Trajkovski M, Ahmed K, Esau C, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol. 2012;14(12):1330–5.
White U, Tchoukalova Y. Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta. 2014;1842(3):377–92.
Tchoukalova Y, Koutsari C, Votruba S, Tchkonia T, Giorgadze N, Thomou T, et al. Sex and depot-dependent differences in adipogenesis in normal-weight humans. Obes (Silver Spring). 2010;18(10):1875–80.
Tchoukalova Y, Koutsari C, Karpyak M, Votruba S, Wendland E, Jensen M. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr. 2008;87(1):56–63.
Shadid S, Koutsari C, Jensen M. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56(5):1369–75.
Basu S, Haghiac M, Surace P, Challier J, Guerre-Millo M, Singh K, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obes (Silver Spring). 2011;19(3):476–82.
Wells JCK. Ethnic variability in adiposity, thrifty phenotypes and cardiometabolic risk: addressing the full range of ethnicity, including those of mixed ethnicity. Obes Rev. 2012;13(SUPPL.2):14–29. https://doi.org/10.1111/j.1467-789X.2012.01034.x
Rønn PF, Andersen GS, Lauritzen T, et al. Abdominal visceral and subcutaneous adipose tissue and associations with cardiometabolic risk in Inuit, Africans and Europeans: a cross-sectional study. BMJ Open. 2020;10(9):e038071. https://doi.org/10.1136/bmjopen-2020-038071.
Sutton-Tyrrell K, Wildman R, Matthews K, Chae C, Lasley B, Brockwell S, et al. Sex- hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 2005;111(10):1242–9.
Perry A, Martin L. Race differences in obesity and its relationship to the sex hormone milieu. Horm Mol Biol Clin Investig. 2014;19(3):151–61.
Nazare JA, Smith JD, Borel AL, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-. Am J Clin Nutr. 2012;96(4):714–26. https://doi.org/10.3945/ajcn.112.035758.
Mongraw-chaffin M, Gujral UP, Kanaya AM, Namratha R. Relation of ectopic fat with atherosclerotic cardiovascular disease risk score in South Asians living in the USA (from the Mediators of Atherosclerosis in South Asians Living in America [MASALA] Study). Am J Cardiol. 2018;121(3):315–21. https://doi.org/10.1016/j.amjcard.2017.10.026.Relation.
Liu J, Coady S, Carr JJ, Hoffmann U, Taylor HA, Fox CS. Differential associations of abdominal visceral, subcutaneous adipose tissue with cardiometabolic risk factors between African and European Americans. Obesity. 2014;22(3):811–8. https://doi.org/10.1002/oby.20307.
Cooper RS. Social inequality, ethnicity and cardiovascular disease. Int J Epidemiol. 2001;30(SUPPL. 1):48–52. https://doi.org/10.1093/ije/30.suppl_1.S48.
Gill C, Lee M, Vatcheva KP, et al. Association of visceral adipose tissue and subclinical atherosclerosis in US-born immigrants. Published online. 2020. https://doi.org/10.1161/JAHA.120.017373.
Neeland IJ, Ross R, Després J, et al. Visceral and ectopic fat , atherosclerosis , and cardiometabolic disease : a position statement. :715–725. https://doi.org/10.1016/S2213-8587(19)30084-1
Wing R, Bolin P, Brancati F, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54.
Moreno-Navarrete J, Catalan V, Ortega F, et al. Circulating omentin concentration increases after weight loss. 2010, 7, 27. Nutr Metab. 2010;7:27
Lancha A, Moncada R, Valenti V, et al. Effect of sleeve gastrectomy on osteopontin circulating levels and expression in adipose tissue and liver in rats. Obes Surg. 2014;24:1702–8.
Roager HM, Vogt JK, Kristensen M, et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome : a randomised. Published online 2019:83–93. https://doi.org/10.1136/gutjnl-2017-314786
Gepner Y, Shelef I, Schwarzfuchs D, Al E. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137:1143–57.
Myette-côté É, Durrer C, Neudorf H, et al. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes : a randomized trial. 2021;(47):1210–1219. https://doi.org/10.1152/ajpregu.00240.2018
Tran HQ, Bretin A, Adeshirlarijaney A, et al. “Western Diet’’’-induced adipose inflammation requires a complex gut microbiota.” Cell Mol Gastroenterol Hepatol. 9(2):313–333. https://doi.org/10.1016/j.jcmgh.2019.09.009
Rosenbaum M, Hall KD, Guo J, et al. Glucose and lipid homeostasis and inflammation in humans following an isocaloric ketogenic diet. 2019;27(6):971-981https://doi.org/10.1002/oby.22468.GLUCOSE
Ross R, Bradshaw A. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol. 2009;5:319–25.
Despres J. Obesity and cardiovascular disease: weight loss is not the only target. Can J Cardiol. 2015;21:216–22.
Janiszewski P, Ross R. Physical activity in the treatment of obesity: beyond body weight reduction. Appl Physiol Nutr Metab. 2007;32:512–22.
Frodermann V, Rohde D, Courties G, et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. 2019;25(11):1761–71. https://doi.org/10.1038/s41591-019-0633-x.Exercise.
Rao S, Pandey A, Garg S, et al. Effect of exercise and pharmacological interventions on visceral adiposity: a systematic review and meta-analysis of long-term randomized controlled trials. Mayo Clin Proc. 94(2):211–224. https://doi.org/10.1016/j.mayocp.2018.09.019
Pedersen LR, Olsen RH, Anholm C, et al. Effects of 1 year of exercise training versus combined exercise training and weight loss on body composition, low-grade inflammation and lipids in overweight patients with coronary artery disease: a randomized trial. Cardiovasc Diabetol. 2019;18(1):1–13. https://doi.org/10.1186/s12933-019-0934-x.
Khera R, Pandey A, Chandar AK, et al. Effects of weight-loss medications on cardiometabolic risk profiles: a systematic review and network meta-analysis. Gastroenterology. 2018;154(5):1309-1319.e7. https://doi.org/10.1053/j.gastro.2017.12.024.
Xu L, Ota T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: focus on fat browning and macrophage polarization. Adipocyte. 2018;7(2):121–8. https://doi.org/10.1080/21623945.2017.1413516.
Xu L, Nagata N, Nagashimada M, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet induced obese mice. EBioMedicine. 2017;20:137–49. https://doi.org/10.1016/j.ebiom.2017.05.028.
Heffron S, Parham J, Pendse J, Alemán J. Treatment of obesity in mitigating metabolic risk. Circ Res. 126(11):1646–1665. https://doi.org/10.1161/CIRCRESAHA.119.315897. Epub 2020 May 21. Erratum in: Circ Res. 2020 Jul 3;127(2):e79.
Labrecque J, Laforest S, Michaud A, Biertho L, Tchernof A. Impact of bariatric surgery on white adipose tissue inflammation. Can J Diabetes. 2017;41(4):407–17. https://doi.org/10.1016/j.jcjd.2016.12.003.
Min T, Prior SL, Dunseath G, Churm R, Barry JD, Stephens JW. Temporal effects of bariatric surgery on adipokines, inflammation and oxidative stress in subjects with impaired glucose homeostasis at 4 years of follow-up. Obes Surg. 2020;30(5):1712–8. https://doi.org/10.1007/s11695-019-04377-3.
Stephens J, Min T, Dunseath G, Churm R, Barry J, Prior S. Temporal effects of laparoscopic sleeve gastrectomy on adipokines, inflammation, and oxidative stress in patients with impaired glucose homeostasis. Surg Obes Relat Dis. 15(12):2011–2017. 1https://doi.org/10.1016/j.soard.2019.04.006
Schauer P, Bhatt D, Kirwan J, et al. Bariatric Surgery versus intensive medical therapy for diabetes - 5-year outcomes. New Engl J Med. 2017;376:641–51.
Sjostrom C, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36:20–5.
Maciejewski M, Arterburn D, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151:1046–55.
Lehmann S, Linder N, Retschlag U, et al. MRI assessment of changes in adipose tissue parameters after bariatric surgery. PLoS One. 13(11):e0206735. https://doi.org/10.1371/journal.pone.0206735
Adami G, Carbone F, Montecucco F, Camerini G, Cordera R. Adipose tissue composition in obesity and after bariatric surgery. Obes Surg. 29(9):3030–3038.https://doi.org/10.1007/s11695-019-04030-z
Rossi I, Omotosho P, Poirier J, Spagnoli A, Torquati A. Roux-en-Y gastric bypass decreases serum inflammatory markers and cardiovascular risk factors in obese diabetics. Surgery. 169(3):539–542. https://doi.org/10.1016/j.surg.2020.09.039
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
Dr. Neeland reports receiving consulting fees/honoraria from Nestle Health Sciences, Boehringer Ingelheim/Lilly Alliance, and Merck & Co. The other authors declare no competing interests.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Macrovascular Complications in Diabetes
Rights and permissions
About this article
Cite this article
Rana, M.N., Neeland, I.J. Adipose Tissue Inflammation and Cardiovascular Disease: An Update. Curr Diab Rep 22, 27–37 (2022). https://doi.org/10.1007/s11892-021-01446-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-021-01446-9